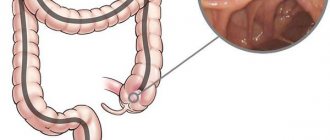
Ileitis is a disease that occurs spontaneously and is quite difficult to cure due to the symptomatic features of the disease. However, it is necessary to treat ileitis, otherwise the disease can take horrific forms.
Treatment of ileitis in clinics in Germany, Israel, Austria, the USA, Finland and Switzerland is aimed not only at eliminating the inflammatory process in the ileum of the small intestine, but also at combating such consequences of the disease as ulcers, polyps, purulent fistulas and destruction of the mucosa.
A special feature of the disease is its early manifestation, because in most cases it is young people aged 15-25 years who suffer from the disease. Chronic ileitis is very dangerous, does not allow you to eat normally and causes constant discomfort. Therefore, it is important and necessary to treat ileitis from real professionals and as soon as possible.
Ileitis - what is it?
Mostly, ileitis is diagnosed in people aged 20 to 40 years, more often in men.
It occurs two times less often in rural populations than in residents of large cities. According to scientific data, in 70% of cases, pain in the right iliac region is a symptom of chronic ileitis. In general, the described pathology accounts for 6% of all intestinal inflammation. Classification of ileitis
According to the criterion of damage to the small intestine, the following are distinguished:
- isolated ileitis;
- ileitis, combined with inflammatory processes in the stomach and colon;
- ileitis affecting the entire intestine.
Due to the occurrence of ileitis, it happens:
- parasitic;
- medicinal;
- infectious;
- enzymatic;
- nutritional;
- postoperative;
- toxic.
It can also be primary or secondary (resulting from another gastrointestinal disease).
Taking into account enzymatic activity, doctors distinguish:
- atrophic ileitis;
- non-atrophic ileitis.
According to the characteristics of the course, inflammation can occur with or without complications in three forms:
- light;
- moderate;
- heavy.
Types of ileitis
Depending on the location of inflammation, ileitis is divided into two types:
- First type. These include: - damage to only one part of the small intestine; - damage only to the ileocecal region (the zone of transition of the small intestine to the large intestine); - damage to only one segment of the colon.
- The second type includes: - damage to several parts of the small and large intestine; - simultaneous damage to the intestines and stomach, esophagus, and oral mucosa.
Forms of ileitis
Taking into account the characteristics of the course of ileitis, gastroenterologists classify it into three forms:
- Primary attack (acute symptoms dominate).
- Chronic intestinal ileitis - there are no acute symptoms, the disease lasts more than six months.
- Recurrent ileitis - symptoms often recur, exacerbations alternate with remission for more than six months.
Depending on the characteristics of the clinical picture, it happens:
- acute ileitis (inflammation of the ileum);
- chronic jejunoileitis, accompanied by impaired absorption of nutrients;
- jejunoileitis, complicated by small intestinal obstruction syndrome (the jejunum and ileum become inflamed, the process of passing feces through the intestines is disrupted);
- granulomatous proctitis (multiple small tumor-like formations form on the wall of the rectum),
- granulomatous colitis (small tumor-like granulomas form on the wall of the large intestine).
Crohn's disease (granulomatous esophagitis, gastritis, colitis; ileocolitis, terminal ileitis)
The anatomy and clinical picture of the disease in 14 patients was described in detail by the American physician Crohn in 1932, and the disease was named after him.
The author observed limited nonspecific inflammation of the terminal part of the small intestine, granulomatous ileitis, affecting all layers of the intestine, including the peritoneum. Later it turned out that with this disease, all parts of the digestive canal are involved in the process - the stomach, small intestine and large intestine. Hence the name: granulomatous esophagitis, gastritis, ileocolitis, terminal ileitis. M.X. Levitan (1974) observed 83 cases of Crohn's disease in 1000 patients with diseases of the stomach and intestines. Crohn's disease (granulomatous esophagitis, granulomatous gastritis, granulomatous colitis, ileocolitis, terminal ileitis) is a chronic nonspecific disease of the intestinal tract, characterized by inflammatory and granulomatous-ulcerative lesions of its various parts.
Terminal ileitis is a lesion of the terminal ileum.
Granulomatous colitis is a lesion of parts of the colon.
Etiology. The etiology of granulomatous enteritis has not been fully studied. It is believed that the disease is determined by infectious inflammation of intestinal tissues caused by streptococci, enterococci, and saprophytes. Infectious agents become aggressive or become more active due to a decrease in immunological reactivity in the intestine. Recently, there have been reports that granulomatous enterocolitis develops as a result of yersinia infection. In such patients, the causative agents of yersiniosis were isolated from the feces, and serological reactions with yersiniosis antigens were found to be positive. However, the role of yersinia infection in the etiology of Crohn's disease has not been definitely established.
Pathogenesis. The bacteria appear to enter the intestinal tissue through the intestinal mucosa. Bacteria can be introduced into the intestine by hematogenous route. Injuries and circulatory disorders contribute to intestinal inflammation. The occurrence of inflammation and the course of the disease is associated with immunity disorders and is accompanied by hyperergic reactions, damage to the lymphatic tract and lymph nodes. The generalization of the inflammatory reaction is evidenced by the frequent involvement in the process of not only the small intestine, but also the large intestine and esophagus, and in some patients, joint damage. Such reactions are observed more often with yersinia infection.
Pathological anatomy . Nonspecific granulomatous terminal ileitis or ileocolitis is accompanied by a nonspecific inflammatory reaction. It is found in various parts of the gastrointestinal tract.
The morphological picture depends on the localization of the process, the stage of development of the disease and the presence of complications:
- The area of the affected large intestine turns into a thick tube, the mucosa of which can be hyperemic, the tissues are infiltrated, thickened, the lumen is narrowed, and the walls are severely deformed. Ulcerative defects on the mucous membrane in Crohn's disease are deep, reach the subserous layer, are usually localized transversely, and have a slit-like shape. Their surface is covered with mucus. The remaining areas of the mucous membrane are covered by inflammatory granulomas located in the submucosa, as a result of which the inner surface of the small intestine resembles a cobblestone street. Various areas of the colon are also affected, most often the anular region. Ulcers, cracks, and fistulas are revealed here.
- Ulcers and inflammatory infiltrates are also found in the small intestine. The inflamed area turns into a tube that has lost the shape of a healthy intestine. Microscopic histological examination of such tissue reveals signs of nonspecific inflammation: inflammatory cellular infiltration, distributed to all layers of the intestinal wall. Often these inflammatory infiltrates are covered by normal mucous membrane. The infiltrates contain a large number of lymphocytes, plasma cells, histiocytes with an admixture of eosinophils. Most lymphocytes belong to the T system population. In some cases, there are clusters of epithelioid cells, some of which resemble Langhans cells. Granulomas, however, do not undergo caseous decomposition. Granulomatous tissue is found around the edges of the ulcers. Adhesions are observed in the area of the affected area of the intestine. Interintestinal and external fistulas have been described. Perforation into the abdominal cavity is rare due to the development of adhesive serositis. Lymphatic vessels are dilated, the tissues around them are rebuilt (lymphangitis). Inflammation is observed in the regional lymph nodes (mesadenitis).
Clinical picture. The leading signs of Crohn's disease are fever, moderate abdominal pain, inflammatory infiltrates found in the right iliac region, or mesogastrium, or in other parts of the large intestine. There is diarrhea. The clinical picture is variable. In some, the disease proceeds with pronounced inflammatory activity and is severe, in others it is sluggish and latent. The acute form of the disease is accompanied by diarrhea, intestinal bleeding, fever, flatulence, and abdominal pain. Palpation in the mesogastrium (with damage to the small intestine) reveals signs of peritoneal irritation (positive Shchetkin-Blumberg sign), painful tumor-like formations in the abdomen. In some patients, the acute inflammatory process that begins quickly becomes chronic. In other patients, the inflammatory process develops slowly, imperceptibly and is chronic. Diarrhea in such patients is replaced by constipation, low-grade fever is often not noticed. Palpation reveals dense inflammatory infiltrates localized in the mesogastrium or right iliac region.
Some patients have granulomatous formations:
- in the esophagus - granulomatous esophagitis,
- in the stomach – granulomatous gastritis,
- in the large intestine - granulomatous enterocolitis.
In some patients, anal fissures, pararectal fistulas, granulomas and dense infiltrates in the pararectal area are the first signs indicating damage to the small intestine.
A slow-moving inflammatory process often leads to damage to the peritoneum. Acute manifestations of the inflammatory reaction are often perceived as signs of appendicitis, and therefore surgical treatment is used. During surgery, inflammatory changes in the intestines are detected. The same changes are described in yersiniosis (B.E. Strelnikov, 1977). The reason for surgical intervention may be intestinal perforation in the area of ulcers and the occurrence of peritonitis, intestinal bleeding and abdominal adhesions, which cause intestinal obstruction or volvulus and its paralytic dilatation.
Crohn's disease is accompanied by heart damage (tachycardia, extrasystoles, ECG reveals signs of impaired myocardial repolarization, which are caused by intoxication, an allergic reaction or autoallergic myocardial lesions). Liver lesions are often regarded as reactive hepatitis, which usually occurs without jaundice. Anemia is caused by several mechanisms: a decrease in the intensity of iron absorption in the intestine and the occurrence of its deficiency in the body as a result of chronic blood loss, bacterial intoxication leading to hypoplasia of the erythropoietic germ of the bone marrow, and deficiency of vitamins, especially vitamin B12. Arthralgia or polyarthritis are observed, which generally do not lead to ankylosis of the joints. Keratitis, corneal ulcers, iridocyclitis, purulent and granulomatous skin lesions, eczema, furunculosis, erythema nodosum have been described. Most patients exhibit an increase in body temperature in the range of 37-37.6°C. For some, the fever reaches 38-40°C, accompanied by chills and sweats. Incorrect type of temperature curve.
Neutrophilic leukocytosis is observed during exacerbation of inflammation, in most cases it lasts a long time. ESR is increased. Blood clotting is increased. In the blood - dysproteinemia with an increased content of gamma, alpha-1 and alpha-2 globulins, sialic acids, seromucoids and a slightly reduced cholesterol content.
The course of Crohn's disease is long: there are periods of improvement and exacerbation. Deterioration is provoked by infection and nutritional disorders.
Diagnosis . The diagnosis of Crohn's disease is usually made late. In the early period, with the acute onset of terminal ileitis, we are talking about the diagnosis of acute appendicitis.
In chronic cases, endoscopic examination - colonoscopy and laparoscopy - provides significant assistance in diagnosis.
Colonoscopy reveals ulcers, severe intestinal destruction, and tumor-like granulomatous formations.
Laparoscopy reveals signs of limited peritonitis, inflammatory bowel disease and adhesions.
Granulomatous tumor-like formations in Crohn's disease are detected in the esophagus and stomach.
X-ray examination reveals changes in the intestine characteristic of Crohn's disease - the affected part of the intestine takes on the appearance of a “cord” or “rope”. If the terminal ileum and cecum are affected, it is difficult to establish the area of the bauhinian valve - its contours disappear. The altered fragment is usually not filled with a tightly contrasting mass. The relief of the mucous membrane is polypoid in nature, the haustra smooth out or disappear. The contours of the affected area of the intestine are often marked finely or coarsely toothed, often with pointed protrusions characteristic of this disease, which are a reflection of rough slit-like ulcers. Sometimes ulcers, located deep in the intestinal wall, cause on radiographs a peculiar picture of “nail heads” running in regular rows along the contour of the affected intestine. The boundaries with healthy areas are often sharply defined. Granulomatous changes in the intestine are characterized by alternation of affected areas with normal ones. On an x-ray, the mucous membrane acquires a coarse or fine-mesh structure, reminiscent of a cobblestone pattern. Against the background of the reconstructed relief of the mucous membrane, persistent contrasting spots of different sizes are found - longitudinal and transverse erosions and ulcers. With tight contrasting of the intestine, narrowing of the affected area, pseudodiverticulous protrusions, rigidity of the walls, sharpness of the boundaries of the affected and normal areas are revealed, the cellular structure of the relief of the mucous membrane is well defined. Crohn's disease is differentiated from chronic secondary enteritis with gastritis, pancreatitis, hepatitis, chronic ulcerative colitis, intestinal tumors, intestinal polyposis. Each of these diseases is characterized by characteristic clinical, endoscopic and radiological signs. Thus, ulcers in chronic ulcerative colitis are located randomly, have an irregular shape and unclear outline, and are often localized in the distal part of the intestine. In the cicatricial-stenotic form of intestinal tuberculosis, significant wrinkling of the affected area of the intestine is noted, the presence of pronounced adhesions is recorded, the mesenteric lymph nodes are enlarged, the mucous membrane of the colon is restructured without structure during the pathological process.
Treatment . There are no specific treatments for Crohn's disease. The following antibiotics and sulfa drugs are used primarily to combat infection: ampicillin or oxacillin - 2-3 g per day orally or intramuscularly, streptomycin - 1 g per day, neomycin sulfate - 0.1 g 2 times a day, chloramphenicol - 0.5 g 4 times a day, tetracycline - 0.25 g 4 times a day. In Crohn's disease, sulfonamide preparations are advantageous, which are well absorbed in the intestine: biseptol - 2 tablets 2 times a day, sulfalen - 1 g during the first day, and then 0.2 g 1 time per day for 7-10 days and etc. Intestopan (0.24 g) is also used - 2 tablets 3 times a day for 5 days, enteroseptol - 0.25 g 1-2 tablets 3 times a day. If yersiniosis is detected, tetracycline or sulfonamide drugs are used.
For anemia, blood products, plasma, multivitamin preparations, polyglucin, and saline solutions are used. In some cases, in order to influence immunological processes and anti-inflammatory, prednisolone is indicated - 20-40 mg per day or other corticosteroid drugs.
Surgical treatment is used for peritonitis (perforation of an intestinal ulcer), cicatricial narrowing of the intestine and in the presence of abdominal adhesions that stenose the intestines.
Diet . The diet for diseases of the small intestine should be easily digestible, high-calorie, gentle, contain a large amount of vitamins and a sufficient amount of liquid.
Physiotherapy. Reducing the clinical signs of inflammation allows the use of physiotherapy: thermal procedures, inducto therapy, UHF therapy, etc. The procedures have an anti-inflammatory effect and normalize the function of the intestines, stomach, liver and other organs. Salt, carbon dioxide, radon baths are indicated; for constipation - subaqueous, mud intestinal tampons, mud therapy.
Spa treatment is carried out at resorts where there are sources of drinking mineral water and mud therapy: Essentuki, Pyatigorsk, Zheleznovodsk, Borjomi, Truskavets, Morshyn, Druskininkai, Birshtonas, in Belarus - Zhdanovichi, Bobruisk.
If you have any questions, you can get advice from leading clinic specialists.
Causes of ileitis
The most common cause of infectious ileitis is Yersinia infestation. Less commonly, inflammation in the ileum is caused by:
- salmonella;
- coli;
- staphylococci.
Viral acute ileitis is a consequence of the negative effects of rotoviruses and enteroviruses. Giardiasis and helminthic infestations lead to a chronic form of the disease.
Common causes of ileitis include:
- allergic reactions;
- alcoholism, smoking;
- constant consumption of fatty and spicy foods;
- poisoning with heavy metals, poisons, chemical reagents;
- hereditary predisposition;
- intestinal surgery;
- taking certain medications.
Ileitis can also be a symptom of typhoid fever, tuberculosis, and ulcerative colitis.
Choice of treatment tactics for different clinical variants of Crohn's disease
In the clinical picture of CD with intestinal damage, there are four main syndromes: intestinal syndrome, endotoxemia, extraintestinal manifestations and malabsorption syndrome. Intestinal symptoms during the active phase of the disease include diarrhea and abdominal pain. Diarrhea, the most common symptom, occurs in 70–80% of cases of CD of the small intestine, ileum, or colon. Excretion of blood in the stool is not necessary. This symptom appears only when the process is left-sided and distal in the colon. Despite the absence of visible blood in the stool, CD is characterized by progressive iron deficiency anemia. Abdominal pain of a constant or paroxysmal nature usually corresponds to the site of the lesion and the formation of an abdominal infiltrate, but it can also be diffuse and not localized. In some cases, acute attacks of pain may be the only symptom for several years. The cause of the pain is often not identified. Ultimately, patients are operated on with suspected acute appendicitis. During laparotomy, the phenomena of terminal ileitis or typhlitis are detected. Attacks of pain are usually accompanied by a rise in temperature to 38–39°C. Fever, peripheral blood parameters (leukocytosis, increased ESR, toxigenic granularity of neutrophils), an increase in blood C-reactive protein, seromucoid and fibrinogen indicate acute inflammation and reflect endotoxemia syndrome during the attack of the disease [2]. Episodes of fever without abdominal pain may occur for several years before the first intestinal symptoms appear. Persistent diarrhea, an inflammatory process with exudation of protein into the intestinal lumen and increased protein catabolism lead to significant weight loss, metabolic disorders (dehydration, hypokalemia, hyponatremia, hypoproteinemia, etc.) and the development of malabsorption syndrome. CD is often accompanied by extraintestinal systemic organ damage, reflecting an autoimmune component in the pathogenesis of the disease. CD is characterized by extraintestinal manifestations such as erythema nodosum, pyoderma gangrenosum, aphthous stomatitis, various arthropathy, including HLA B27 associated rheumatoid arthritis and ankylosing spondylitis and sacroiliitis (Bechterew's disease), vitiligo, psoriasis. The frequency of various systemic manifestations of the disease is in the range of 5–20%. According to our data, their overall frequency is 62%, of which almost 25% of patients have arthropathy. Rheumatoid arthritis and ankylosing spondylitis occur in equal proportions with a frequency of 8% [2,3]. The nature of histological changes and the depth of damage to the intestinal wall during transmural inflammation determine the range of complications of CD. The most typical and common complications: inflammatory strictures with subsequent development of intestinal obstruction, abdominal infiltrates and abscesses, interintestinal and external (intestinal-cutaneous) fistulas. When the anorectal area is affected, paraproctitis, perianal and rectovaginal fistulas and deep anal fissures are formed. When assessing the severity of CD, the nature and severity of clinical symptoms and complications are taken into account. It is customary to assess the severity of the disease using the CDAI (Crohn's disease activity index), also called the Best index [3,5]. This index includes a number of clinical signs, each of which, depending on their presence or severity, is assessed with a certain number of points. The index is calculated using a special formula. The CD activity index includes the following criteria: frequency of loose stools per week, presence of abdominal pain and its intensity, general health (good, fair, bad, very bad), systemic manifestations and/or anal manifestations (fissures, fistulas, abscesses) (there are , no), use of opiate antidiarrheals (loperamide), fever (> 37.5°C), hematocrit, degree of weight loss, presence of palpable infiltrate in the abdominal cavity. It is accepted that an index of less than 150 is regarded as remission of CD, an index of 150–220 corresponds to low activity and a mild course of the disease, 221–450 characterizes the moderate severity of CD, and above 450 points corresponds to high inflammatory activity and a severe course [1]. Treatment of patients with CD is a very difficult problem, because even with adequate therapy, in most patients the disease is progressive in nature with the development of complications or the formation of continuous forms of the disease. Treatment should be carried out differentially, taking into account the localization and extent of the inflammatory process, the type and nature of complications and the severity of the disease. The goal of treating CD is to relieve exacerbations and achieve and maintain remission. Basic pathogenetic therapy for CD includes three groups of drugs: corticosteroid hormones, 5-aminosalicylic acid (5-ASA) drugs and immunosuppressants [1,2]. Currently, therapy aimed at inducing remission in patients with mild or moderate CD traditionally begins with the prescription of 5-ASA drugs - mesalazine or sulfasalazine. In case of insufficient effectiveness of aminosalicylates, metronidazole and/or ciprofloxacin are prescribed. In the absence of an adequate therapeutic response, corticosteroids are used. In severe cases, steroid hormones are used as first-line therapy, and in case of refractory to steroids (steroid resistance or steroid dependence), reserve drugs - immunosuppressants - are used. Such established approaches are not differentiated and do not take into account the evidence-based medicine accumulated to date, which makes it possible to weigh the relationship between the effectiveness, safety and tolerability of drugs and to develop optimal treatment approaches for different courses and different forms of the disease. The aminosalicylates Sulfasalazine and mesalazine are highly effective basic drugs for the treatment of mild to moderate ulcerative colitis. This suggested that the drugs could be equally effective in CD. Today, they have become firmly established in the treatment regimens of CD. However, the results of controlled trials refute the feasibility of this approach. Several placebo-controlled clinical trials, as well as comparative studies with glucocorticoids, have been devoted to studying the effectiveness and safety of sulfasalazine in CD [5–8]. The results of these studies indicate that although the effectiveness of sulfasalazine was higher than placebo, it was nevertheless inferior to even low doses of systemic corticosteroids (0.25 mg/kg/day). Unlike ulcerative colitis, even with mild CD, sulfasalazine exhibits low activity. A positive result of treatment with sulfasalazine was obtained only in patients with predominant damage to the colon, which is explained by its direct activation by enzymes of the colon flora, and only in cases of mild disease [9]. Only patients who did not receive prior hormonal therapy responded to sulfasalazine therapy, while the drug was not effective in those who received corticosteroids [7]. All of the above suggests that sulfasalazine has limited possibilities for use in patients with only mild CD with predominant localization in the colon. The well-known side effects of sulfasalazine, occurring in 10–30% of patients, also limit its use [9,10]. Thus, the proven low effectiveness of sulfasalazine in combination with a wide range of adverse reactions does not allow it to be recommended for the treatment of patients with CD with terminal ileitis or ileocolitis; there are limited indications for the treatment of mild colon CD. Mesalazine does not have the side effects of sulfasalazine and is not inferior to it in effectiveness. Tablet mesalazine preparations produced in different countries are similar in action and effectiveness and are 5-ASA in a protective coating. They differ in the nature of the enteric coating (eudragit, acrylic or ethylcellulose) and, accordingly, in the location and rate of release of 5-ASA in the intestine [2]. The clinical effectiveness of mesalazine correlates with the intraluminal concentration of 5-ASA, so the location of the lesion must be taken into account when prescribing it. Mesalazine is used as first-line therapy in patients with mild to moderate CD by 75% of physicians [11]. Clinical trial results, however, are conflicting. Mesalazine at various doses has been studied in several double-blind, placebo-controlled studies [12,13,15]. In some of them, the drug at a dose of 2–4 g per day was not superior to placebo in its ability to induce remission in patients with CD. However, when analyzing pooled data from 3 studies using a relatively high dose of mesalazine (4–4.5 g/day), a statistically significant difference from placebo was revealed [11]. Mesalazine at a dose of 2 g per day is significantly inferior to steroids (prednisolone, methylprednisolone and budesonide); increasing the dose to 4.5 g gives comparable results [20,23,24,25]. In a comparative study with budesonide (9 mg/day), mesalazine (4 g/day) was inferior to the glucorticoid in its ability to induce remission in patients with mild and moderate forms of CD [16]. Side effects when using mesalazine are quite rare. Thus, not all research results are equally positive regarding mesalazine. The minimum effective dose for mild CD is 4 g per day, although some studies did not have enough patients to draw meaningful conclusions. Given the low incidence of side effects compared with sulfasalazine and systemic steroids, mesalazine can be recommended as an initial trial treatment for mild CD, but the benefit rate is not high enough [4,11]. The overall effectiveness of 5-ASA drugs does not exceed 30%. The feasibility of their use as anti-relapse therapy has not been proven. Systemic glucocorticosteroids Glucocorticosteroids have been used in the treatment of inflammatory bowel diseases for 50 years and have traditionally been first-line drugs for severe CD [1,2,4]. However, in the context of the above, steroids should be recommended for moderate to severe disease and in case of ineffectiveness of 5-ASA in mild cases [4,11]. The effectiveness of corticosteroids and the severity of side effects depend on the rate of their metabolism in the liver, bioavailability, affinity for receptors and other parameters. Prednisolone and its methylated analogs (methylprednisolone) are considered the drug of choice. The average oral dose of prednisolone during a CD attack can be 40–60 mg, but a dose of 1 mg/kg body weight per day should be considered more effective, and in very severe cases the dose can be increased to 1.5–2 mg/kg per day. The use of high-dose oral steroids for a severe attack of CD competes in effectiveness with intravenous high-dose hormonal therapy (250–500 mg methylprednisolone or hydrocortisone per day for 7 days). Use of prednisolone in a minimum and average dose of 0.25–0.75 mg kg/day. led to improvement in 60% of patients, compared with 30% in the placebo group [7]. More convincing results were obtained with 6-methylprednisolone at a dose of 48 mg [8]. Other studies have confirmed that systemic steroids are more active in CD than placebo, sulfasalazine, antibiotics, and azathioprine, but are associated with a high incidence of side effects [13,15,17,18–23]. Tolerability of corticosteroids largely depends on the route of administration, dose, duration of therapy, gender and individual sensitivity to drugs. Common side effects include moon face, steroid diabetes, osteoporosis, mood changes, insomnia, swelling, weight gain, stretch marks. Thus, despite the proven high effectiveness of systemic steroids in inducing remission in CD, their long-term use is limited by frequent and varied side effects. For the same reason, it is impossible to use steroids to maintain remission. Topical steroids The need to use predominantly steroid hormones in the treatment of CD and the limitations caused by their side effects have led to the creation of a safe alternative to systemic steroids that are not inferior to them in effectiveness. The new group of drugs is called “topical” steroids. These are local hormones that create a high concentration of the drug in the affected area (in the intestine) and have virtually no side effects [11,24–26]. In Russia, in 2004, one of the most effective topical steroids for the treatment of CD was registered - budesonide. Budesonide demonstrated similar activity to systemic corticosteroids, with better tolerability and less influence on the hypothalamic-pituitary-adrenal system. The advantages of budesonide over systemic steroids lie in the peculiarities of its pharmacodynamics. First of all, budesonide has a high affinity for glucocorticoid receptors in target tissues (in this case, receptors of the intestinal mucosa), many times higher than the affinity of traditional drugs: prednisolone and hydrocortisone. Thus, the affinity of budesonide for steroid receptors is 100 times higher than that of hydrocortisone, 50 times higher than that of prednisolone and approximately 18 times higher than that of methylprednisolone. In addition, budesonide has minimal absorption from the gastrointestinal tract, low systemic bioavailability (no more than 10%) compared to prednisolone and hydrocortisone, and high first-pass metabolism. The latter quality ensures the absence of toxic metabolites in the blood after the first passage of the drug through the liver. These properties of budesonide make it possible to certain extent to increase the effectiveness of treatment of patients in need of hormonal therapy. The activity of the drug is enhanced due to its high affinity for steroid receptors, microgranular galenic form with the release of high concentrations in the terminal ileum and colon. The absence of side effects characteristic of systemic steroids is due to low absorption from the intestinal lumen in combination with high first-pass metabolism and low systemic bioavailability. The attractive features of budesonide make it possible to take the drug for a long time to stop an attack of CD without the risk of developing side effects. The use of budesonide for anti-relapse therapy of this disease is debated [27,28]. Randomized clinical trials have demonstrated similar efficacy of systemic steroids and budesonide with significantly fewer side effects of the latter [20,23,29,30]. The optimal dose of budesonide was 9 mg per day, the duration of treatment was 12–16 weeks, followed by gradual withdrawal. Budesonide proved to be effective mainly in moderate cases of CD in the form of terminal ileitis and ileocolitis and in these cases should be a first-line drug [4,11,26]. From our point of view, it should also be used for mild CD of the same localization. Budesonide is not indicated for CD with systemic autoimmune manifestations. In severe cases of CD, the effectiveness of the drug at the indicated dose is insufficient. Immunosuppressants In the absence of a response to treatment with hormones, in cases of steroid resistance and steroid dependence, reserve drugs are prescribed - immunosuppressants [32–35]. Azathioprine and 6-mercaptopurine, methotrexate and cyclosporine A are used. Data on the effectiveness of immunosuppressants in CD are very contradictory. The rate of achieving clinical effect and entering remission for both azathioprine and methotrexate does not exceed 70% [32]. Cyclosporine is less effective for CD; its effect is more pronounced in patients with ulcerative colitis. The use of immunosuppressants is limited to some extent by a wide range of side effects. For azathioprine, these are nausea, vomiting, diarrhea, flu-like syndrome with fever and myalgia, bone marrow suppression with leukopenia, neutropenia (less often with agranulocytosis), thrombocytopenia. Taking azathioprine may also be accompanied by hepatotoxic reactions in the form of cytolysis and cholestasis, pancreatitis, and the development of polyneuritis. The drug is believed to increase the risk of malignancy, although many dispute this position [36]. Side effects develop with a frequency of 6–20%. Methotrexate is the treatment of choice in patients with CD who have not responded to treatment with steroids and azathioprine [34,37]. The incidence of side effects when taking methotrexate is 10–20% (including nausea, vomiting, diarrhea, ulcerative stomatitis, leukopenia, thrombocytopenia, anemia, hepatotoxicity, pulmonary fibrosis, alopecia). With long-term use, necrosis of hepatocytes and liver fibrosis are possible. The action of azathioprine and methotrexate develops slowly, improvement can be noticeably earlier than 3-4 weeks, a period of 4-6 months is required to obtain the maximum effect, because immunosuppressors cannot be used in acute situations, they are suitable only for treatment chronic sluggish active forms of BC. Azatioprine is also used to maintain remission, but the ability of methotrexate to maintain prolonged remission is considered doubtful. Remission induced by methotrexate persists for one year only in 40% of patients [38]. Thus, the use of immunosuppressors is limited, on the one hand, by side effects and a slowly developing effect, on the other hand, they often do not give the desired result. In practice, an alternative treatment method for patients with resistant CD is surgical treatment. Infliximab a new direction in the treatment of patients with BCs who did not respond to treatment with steroids (both systemic and topical), provides for the selective suppression of the synthesis and activity of the factor of tumor necrosis (FNO). For this purpose, infliximab is widely used, which is chimeric monoclonal antibodies to FNO -A and consisting of 25% of mouse protein and 75% of human immunoglobulin. Infliximab has been used abroad for 8 years, and in Russia, first of all, it was registered for the treatment of two diseases similar in terms of development of inflammation: BC and rheumatoid arthritis (currently also registered for the treatment of ulcerative colitis). Infliximab is recommended with a non -complicated steroid -resistant form of BC and with a fistulous form, and it is active both with a moderate and in severe course of the disease [33,39,40]. In the case of a complicated course for induction of remission, one intravenous infusion is enough at the rate of 5 mg/kg/day. With a fistulous form, 3 -fold administration of the drug with an interval of 2 weeks is used [41,42]. Ifliximab has a prolonged effect. Successful results were obtained in patients with BC with the development of inflammation in the field of the ileoanal reservoir and with perianal localization of BC [43]. In controlled multicenter tests, the frequency of response to infliximab in steroid -resistant forms of BCs ranges in an interval of 50–82%, the frequency of reaching clinical remission is 25–48% after 4 weeks [44–46]. Long -term treatment with infliximab for 44 weeks turned out to be an effective, well -tolerated method of stopping the symptoms of BC and the achievement of remission in patients who did not respond to traditional treatment, and with maintenance therapy, remission was preserved for 54 weeks in 39% of patients [47]. It is noteworthy that in patients who received infliximab to maintain remission, they managed to completely abandon the use of corticosteroids [47]. Infliximab also turned out to be the only drug at the moment, which made it possible to achieve not only clinical, but also endoscopic remission at BC [47]. The effect of infliximab develops quickly; after 2 weeks, the onset of clinical effect can be observed. The duration of its action is up to 30 weeks after a single infusion, however, after 8–12 weeks, the concentration of antibodies in serum decreases, therefore, in patients with the most stubborn form of the disease, repeated infusions are recommended every 8 weeks to maintain a clinical response [45]. At the Expert Council for the development of new standards for the use of infliximab in the EU and the USA in October 2005 (UC and CD: Treating to New Standarts Expert Round Table. 10.10.2005) it was proposed to include not only steroid refractory forms of BC and ulcerative forms Polititis (yak), but to recommend infliximab as a first -line drug in severe forms of these diseases. Currently, in Russia, infliximab is included in the list of Life preparations for the treatment of BC, included with the treatment standards of BC (MZ RF) and registered for the treatment of Yak. Inflocial therapy The issue of supporting BC therapy after reached remission remains not fully resolved. Sulfasalazine, oral mesalazine and prednisone in low doses are ineffective for maintaining remission. Budsonide at a dose of 6 mg leads to lengthening time before relapse, but does not allow maintaining remission throughout the year and does not prevent BC relapses after operations [31]. Antibiotics have not been studied in clinical research on this indication. Azatioprine, 6 - Merkaptopurin, are also effective as means of supporting therapy, but their use is limited by a large number of side effects. Thus, the data available at the moment do not allow us to recommend any drug for maintaining therapy, as a favorable ratio of effectiveness/safety. In this regard, some authors recommend only therapy to patients with mild and moderate BCs aimed at achieving remission with the subsequent renewal of its relapse [11]. The conclusion of this work pursued two goals: firstly, to demonstrate the insufficient validity of traditional approaches to the treatment of BC, primarily its lungs and moderate forms. Secondly, to show the possibilities of new drugs and their specific niche in the therapy of BC. Based on the above, the choice of treatment tactics with different forms of BC, taking into account the severity, localization and nature of complications, can be represented as shown in table 1.
Literature 1. Adler G. Crohn’s disease and ulcerative colitis. – Goetar – Med. – 2001. – 500 p. 2. Belousova E.A. Ulcerative colitis and Crohn's disease. – Triad. – 2002. – 128 p. 3. Morozova N.A., Belousova E.A. Systemic autoimmune manifestations of inflammatory bowel diseases (oral communication) 4. Belousova Pharmacotherapy and algorithm for the treatment of mild and moderate Crohn's disease from the perspective of evidence-based medicine. – Pharmateka. – 2004. – No. 13. – p. 8–18. 5. Anthonisen P, Barany F, Folkenborg O, et al. The clinical effect of salazosulphapyridine (Salazopyrin R) in Crohn's disease. Scand J Gastroenterol 1974; 9:549–54. 6. Van Hees PA, Van Lier HJ, Van Elteren PH, et al. Effect of sulphasalazine in patients with active Crohn's disease: a controlled double–blind study. Gut 1981; 22:404–9. 7. Summers RW, Switz DM, Sessions JT, et al. National Cooperative Crohn's Disease Study: results of drug treatment. Gastroenterology 1979; 77:847–69. 8. Malchow H, et al. European Cooperative Crohn's Disease Study (ECCDS): results of drug treatment. Gastroenterology 1984; 86:249–66. 9. Das KM, Eastwood MA, McManus JP, et al. The relationship between metabolites and the response to treatment in inpatients. Gut 1973; 14: 631–41. 10. Taffet SL, Das KM. Sulfasalazine. Adverse effects and desensitization. Dig Dis Sci 1983; 28:833–42. 11. Sandborn WJ, Feagan BG Mild to Moderate Crohn's Disease – Defining the Basis for a New Treatment Algorithm. Aliment Pharmacol Ther 2003;18:263–77. 12. Tremaine WJ, Schroeder KW, Harrison JM, et al. A randomized, double–blind, placebo–controlled trial of the oral mesalamine (5–ASA) preparation, Asacol, in the treatment of symptomatic Crohn's colitis and ileocolitis. J Clin Gastroenterol 1994; 19: 278–82. 13. Gross V, Andus T, Fischbach W, et al. Comparison between high dose 5–aminosalicylic acid and 6–methylprednisolone in active Crohn's ileocolitis. A multicenter randomized double–blind study. German 5–ASA Study Group. Z Gastroenterol 1995; 33:581–4. 14. Scholmerich J, Jenss H, Hartmann F, the German 5–ASA Study Group. Oral 5–aminosalicylic acid versus 6–methylprednisolone in active Crohn's disease. Can J Gastroenterol 1990;4:446–51. 15. Prantera C, Cottone M, Pallone F, et al. Mesalamine in the treatment of mild to moderate active Crohn's ileitis: results of a randomized, multicenter trial. Gastroenterology 1999; 116:521–6. 16. Thomsen OO, Cortot A, Jewell D, et al. A comparison of budesonide and mesalamine for active Crohn's disease. International Budesonide–Mesalamine Study Group. N Engl J Med 1998; 339:370–4. 17. Rijk MC, van Hogezand RA, van Lier HJ, et al. Sulphasalazine and prednisone compared with sulphasalazine for treating active Crohn disease. A double–blind, randomized, multicenter trial. Ann Intern Med 1991; 114:445–50. 18. Martin F, Sutherland L, Beck IT, et al. Oral 5–ASA versus prednisone in short term treatment of Crohn's disease: a multicentre controlled trial. Can J Gastroenterol 1990; 4:452–7. 19. Yang YX, Lichtenstein GR. Corticosteroids in Crohn's disease. Am J Gastroenterol 2002; 97:803–23. 20. Gross V, Andus T, Caesar I, et al. Oral pH–modified release budesonide versus 6–methylprednisolone in active Crohn's disease. German/Austrian Budesonide Study Group. Eur J Gastroenterol Hepatol 1996; 8:905–9. 21. Rutgeerts P, Lofberg R, Malchow H, et al. A comparison of budesonide with prednisolone for active Crohn's disease. N Engl J Med 1994; 331:842–5. 22. Prantera C, Zannoni F, Scribano ML, et al. An antibiotic regimen for the treatment of active Crohn's disease: a randomized, controlled clinical trial of metronidazole plus ciprofloxacin. Am J Gastroenterol 1996; 91: 328–32. 23. Bar–Meir, S, Chower, Y, Lavy, A, et al. Budesonide versus prednisone in the treatment of active Crohn's disease. Gastroenterology 1998; 115:835. 24. Yang YX, Lichtenstein GR. Corticosteroids in Crohn's disease. Am J Gastroenterol 2002; 97:803–23. 25. Lyckegaard E, Hakansson K, Bengtsson B. Compassionate use of budesonide capsules (ENTOCORT EC) in patients with Crohn's disease. Gastroenterology 2002; 122:T1665. 26. Lofberg, R, Rutgeerts, P, Malchow, H, et al. Budesonide prolongs time to relapse in ileal and ileocecal Crohn's disease: A placebo controlled one year study. Gut 1996; 39:82. 27. Hellers, G, Cortot, A, Jewell, D, et al. Oral budesonide for prevention of postsurgical recurrence in Crohn's disease. Gastroenterology 1999; 116:294. 28. Greenberg GR, Feagan BG, Martin F, et al. Oral budesonide for active Crohn's disease. Canadian Inflammatory Bowel Disease Study Group. N Engl J Med 1994; 331:836–41. 29. Rutgeerts P, Lofberg R, Malchow H, et al. A comparison of budesonide with prednisolone for active Crohn's disease. N Engl J Med 1994; 331:842–5. 30. Kane SV, Schoenfeld P, Sandborn WJ, et al. Systematic review: The effectiveness of budesonide therapy for Crohn's disease. Aliment Pharmacol Ther 2002; 16:1509–17. 31. Greenberg GR, Feagan BG, Martin F, et al. Oral budesonide for active Crohn's disease. Canadian Inflammatory Bowel Disease Study Group. N Engl J Med 1994; 331:836–41. 32. Elson CO The effects of immunosuppressive agents on cytokines. //Aliment. Pharmacol. Ther.– 1996.– v.10.–suppl.2.–p.100–105. 33. Sanborn WJ Steroid–dependent Crohn's disease. // Scand. J. Gastroenterol..– 2000.– v. 14.– (Suppl. C).– 17C. 34. Sands BE Medical therapy of steroid–resistant Crohn's disease. // Scand. J. Gastroenterol.– 2000.– v. 14.– (Suppl. C).– 33C. 35. Steinhart H. Steroid resistant and steroid dependent Crohn's disease. // IBD, salicylates and other relevant therapies–Proceeding of the International IBD Symposium.– London.– 1999.– p.83–90 36. Lewis JD, Sanford–Schwartz J., Lichtenstein GR Azatioprine for maintenance of remission in Crohn's disease: benefits outweigh the risk of lymphoma // Gastroenterology. –2000.– v. 118.– p.1018–1024 37. Lemann M., Zenjari T., Cosnes J., Mesnard B. Methotrexate in Crohn's disease: long–term efficacy and toxicity.– Am. J. Gastroenterol. 2000.–v. 95.– p.1730–1734 38. Feagan BG, Fedorak RN, Irvine EJ, Wild G. et all. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease // N. Engl. J. Med.– 2000.– v.342.– p. 1627–1632 39. Belousova E.A., Morozova N.A., Nikitina N.V. Infliximab (Remicade) in the treatment of refractory forms of Crohn's disease.–Breast cancer.–2005.–vol.7.–No.1.–p.28–32 40. Sands B., Anderson F., Bernstein C. Infliximab Maintenance Therary for Fistulizung Crohn's Disease.– N.Eng.J.Med. 2004.–v.350.– p.876–885. 41. Lichtensteyn G., Yan S., Bala M. et all. Infliximab Maintenance Treatment Reduces Hospitalizations, Surgeries, and Procedures in Fistulizing Crohn's Disease. – Gastroenterology 2005.–v.128.–p.863–869 42. Present DH, Rutgeerts P., Targan S., Hanauer SB et all. Infliximab for the treatment of fistulas in patients with Crohn's disease.– N. Engl. J. Med. – 1999. – v. 340.– 1398–1405 43. Ricart E., Panaccione R., Loftus E., Tremain W. Successful management of Crohn's disease of the ileoanal pouch with infliximab // Gastroenterology.– 1999.– v. 117.– p. 429–432 44. Hanauer S., Feagan B., Lichtenstein G. et all. Maintenanse Infliximab for Crohn's Disease: the ACCENT I Randomised Trial.– Lancet 2002.–v.359.–p.1541–1549. 45. Rutgeerts P. A Critical Assessment of new Therapies in Inflammatory Bovel Diseases. // J. Gastroenterol. Hepatol. 2002.–V.17.–Suppl..–S177 QR. 46. Targan SR, Van Deventer SJH, et all. A short–term study of chimeric monoclonal antibody cA2 to tumor necrosis factor? for Crohn's disease.// N.Engl.J.Med. –1997.–v.337.–p.1029–1035 47. Rutgeerts P., D'Haens G., Targan S. et al. Efficacy and Safety of Retreatment with Anti–Tumor necrosis factor antibody (Infliximab) to maintain remission in Crohn’s disease // Gastroenterology, 1999.– V.117.– P.761–769.
Symptoms of intestinal ileitis
Acute ileitis manifests itself:
- pain in the iliac region;
- bloating and rumbling stomach;
- loose stools (up to 10 times a day);
- nausea, vomiting;
- high temperature;
- weakness;
- severe headaches.
Due to a disorder of the digestive system, dehydration may occur, which can lead to convulsions, blood coagulation disorders, and hypovolemic shock.
Chronic ileitis is characterized by:
- moderate pain in the right iliac region, around the navel;
- bloating and rumbling stomach;
- watery stools containing undigested food;
- weight loss (due to impaired absorption of vitamins, minerals and nutrients supplied with food);
- osteoporosis;
- hypovitaminosis.
If you notice similar symptoms, consult a doctor immediately. It is easier to prevent a disease than to deal with the consequences.
Symptoms of intestinal diseases: when should you see a doctor?
The disease often manifests itself with intimate symptoms, which the majority of patients try to fight on their own, without rushing to a paid appointment with a proctologist or gastroenterologist. In addition, intestinal inflammation is often accompanied by “masking” symptoms: for example, only stomatitis, as in Crohn's disease, or only inflammation around the anus - perianal dermatitis, or lesions of the eyes or joints. Therefore, the patient often comes to the doctor with a severe form, when the path to recovery turns out to be both longer and more financially expensive.
The following signs should alert anyone:
- frequent diarrhea - up to 15–20 times a day;
- pain and/or cramping in the abdominal area;
- weakness, increased fatigue;
- loss of appetite and noticeable weight loss;
- temperature increase;
- the appearance of traces of blood in the stool.
The likelihood of damage to the large and small intestines is increased in people who regularly and in large quantities consume dark varieties of meat and processed products (sausages, sausage, ham, bacon, etc.). But you should know that adherence to a diet with a predominance of vegetables, fruits, and foods made from whole grains is not a panacea.
A genetic factor plays a certain role: the tendency to intestinal inflammation can be inherited. Among the sick people, people who abuse smoking predominate. Also important are the lack of vitamin D in the body and frequent and uncontrolled self-administration of antipyretic medications.
An appointment with a proctologist in Moscow is recommended not only for people with unstable stools. In order not to fill the army of colorectal cancer patients, even a generally healthy person after 45 years of age should visit a doctor at least once every five years for preventive purposes. The absence of visible signs of inflammation does not exclude the possibility of a hidden disease. Symptoms of intestinal cancer detected at an early stage allow doctors to save the patient and achieve stable remission.
Diagnosis of ileitis
Since the distal small intestine is almost impossible to examine with an endoscope, the diagnosis of ileitis is based on laboratory methods. The patient is prescribed:
- CBC (increased ESR, leukocyte formula shift to the left, leukocytosis);
- biochemical blood test (lack of microelements and protein is diagnosed);
- bacteriological and virological examination of stool;
- stool occult blood test.
An informative diagnostic method for suspected ileitis is radiography of barium passage through the small intestine. The study makes it possible to detect intestinal motility disorders and intestinal obstruction. The images may also reveal fistulas, strictures, and areas of ileal spasm.
If pancreatitis and cholelithiasis are suspected, the patient is prescribed an abdominal ultrasound.
Terminal ileitis
Share information with your Facebook friends
VK
Question:
Good afternoon.
Please comment on my examinations.
According to colonoscopy from October 2021: terminal ileitis, the ileum is hyperemic, red, the vascular pattern is not visible, hyperplasia. The rest of the intestine is normal.
According to the biopsy, “the picture corresponds to terminal ileitis.”
Since October 2021, treated as Crohn's disease. “Pentas” 4 g per day, the stool returned to normal (before that there was constipation), but as for the other symptoms (pain above the navel, bloating, epigastric pain) - nothing changed, both with and without the diet.
According to the MRI dated January 2021, the conclusion on the sacroiliac joints “the picture may correspond to sacroiliitis.”
The sacrum hurt very badly, the pain was in the left leg and under the shoulder blades. The diagnosis made by a rheumatologist was “Left-sided sacroiliitis FC 1.” Azathioprine was added in March (3 days 50 mg). Then she took tests. There was a norm for ESR, leukocytes, hemoglobin).
On the 4th day I began to feel sick on any food, but especially on meat. The doctor said that this could lead to an exacerbation. I had loose stools with mucus 4 times in a couple of days. I stopped Azathioprine for a week, and after 3 days my condition returned to normal. Then, at the insistence of the attending physician, I began a second round of the same drug. The first analysis after 3 days - everything is normal, except for hemoglobin - 112, lymphocytes - 39.9 (they have been frozen at this indicator for a year already), according to CRP 0.4 (normal up to 5), iron 5.27 (normal 9.0 – 30.4), ferritin – 6 (normal range from 10 to 120). Liver parameters and lipid profile are all normal.
I started taking 100 mg per day on the recommendation of my doctor. On the 10th day of treatment after this analysis, my health began to deteriorate. Nausea, nausea from some smells, severe pain between the navel and stomach and constant bloating. “Duspatalin” does not stop it, “Espumizan” does not help with bloating. Today is the 14th day that I have been taking 2 tablets of Azathioprine and the 17th day of taking it on the second go. My abdominal wall is tense, my stomach hurts and the pain radiates under my shoulder blades - on Mesalazine my stomach didn’t hurt so much, and I ate everything at all. There was only a slight background dull ache, but the joints ached with it. With Azathioprine, as I understand it, until it accumulates, it is useless to wait for the pain in the joints to stop. But I understand that this drug makes me feel bad. The doctor said that if nausea and abdominal pain are difficult to bear, then it should be replaced with Mercaptopurine. In this regard, I have questions.
1) I know that Mercaptopurine is also used in the treatment of malignant tumors. I have a mild form of Crohn's disease, no fever, blood in the stool, diarrhea, fistulas, ulcers, etc. Is it advisable for me to take cytostatics at all due to the addition of sacroiliitis? Maybe you can deal with it somehow locally, considering that on Mesalazine I felt relatively well in the intestines.
2) If yes, does it make sense to replace “Azathioprine” with “Mercaptopurine”, since it has exactly the same side effects? And is it normal to continue taking the drug and ignore the side effects that appear?
3) I have practically no reaction to food (only to very fatty foods, and not always), nothing changes from diets like SUD, FODMAP, tests are mostly normal, CRP, ESR and leukocytes have never increased during the year of illness , according to the biopsy, no one wrote to me that it was Crohn’s disease, only about terminal ileitis, but the doctor told me that these are synonyms, and therefore I would like to ask: this may not be Crohn’s disease based on all the symptoms that I described? I have already been checked for parasites, infections (except for Yersinia) - there is nothing, hidden blood in the stool (no), and sometimes I have doubts whether this is Crohn's disease at all... Thank you!
Answer:
Greetings! I do not comment on treatment; I am not certified to treat gastroenterological pathologies. For endoscopy and biopsy of the small intestine, you need to read the entire pathohistological report. Based on what data is terminal ileitis diagnosed? In the literature, this is indeed equated to Crohn's disease, but there must be inevitable signs in histology. If these signs are not present, but there is no conviction to either make or remove the diagnosis of Crohn’s disease, because They set it not only on the basis of a biopsy, but on the totality of data - endoscopy, CT - enterography, clinic. And tests - you didn’t say anything about specific tests - stool for calprotectin, blood for ASCA IgA/IgG, ANCA and, in rare cases, anti-OmpC antibodies, which increase the likelihood of Crohn’s disease. And in 98% of cases, high calportectin removes the diagnosis of IBS.
print version
TagsCrohn's disease Health ileitis
Treatment of ileitis
All patients who present with symptoms of ileitis should consult a gastroenterologist. The acute form of the disease can only be treated in a hospital. Bacterial inflammation after determining the sensitivity of the microflora to antibiotics is eliminated with the help of antibacterial drugs. For persistent vomiting and diarrhea, infusion therapy with saline solutions and glucose is used. To normalize the absorption of food, enzyme medications are prescribed.
Treatment of chronic ileitis involves following a gentle diet, taking enzymes and medications that help normalize intestinal motility, vitamins, minerals, probiotics, and herbal astringents.
Organization of treatment of ileitis abroad
For several years now it has been helping everyone who wants to receive high-quality diagnosis and treatment of gastroenterological diseases, including treatment of ileitis in leading clinics around the world. We organize and support the treatment of each of our clients on an individual basis.
It is important to understand that each of the following medical institutions has its own characteristics of the range of services provided, as well as location. Therefore, when choosing the best gastroenterology center in your case, you should contact specialists, that is, us!
Diet for ileitis
Patients with ileitis are prescribed diet No. 4 according to Pevzner. They can eat:
- yesterday's wheat bread;
- lean meats;
- soft-boiled eggs;
- cereals, pasta;
- porridge with water;
- meat broths.
The following are prohibited:
- raw, fried and hard-boiled eggs;
- fatty meat, smoked meats;
- grape juice, carbonated drinks;
- cabbage, mustard, horseradish, cucumbers;
- milk, cheese.