© Author: A. Olesya Valerievna, candidate of medical sciences, practicing physician, teacher at a medical university, especially for SosudInfo.ru (about the authors)
Ebstein's anomaly (EA) is a very rare cardiac defect that develops during fetal development. With pathology, the normal arrangement of the tricuspid valve leaflets is disrupted, resulting in its insufficiency and circulatory disorders. According to statistics, Ebstein's anomaly accounts for no more than 1% of cases of all congenital heart anomalies.
In general, congenital malformations of organs are becoming more common. Constantly deteriorating environmental conditions, unfavorable factors affecting the expectant mother, and the pathology of pregnancy are considered to be to blame. Heart anomalies constitute a large group of defects, which, even if compatible with life, very often lead to serious hemodynamic disorders requiring surgical correction.
When signs of a heart defect are detected in the fetus during an ultrasound examination of a pregnant woman, the expectant mother, of course, begins to worry and look for information about the disease in order to be ready to help her baby after birth. Let's try to figure out what Ebstein's anomaly is and what possible ways to correct this rare defect.
Causes and essence of Ebstein's anomaly
As with most congenital heart defects (CHD), the causes of the pathology remain unclear. It is believed that genetic changes in the form of spontaneous mutations and the influence of external causes play a role. Thus, in some cases of Ebstein's anomaly, it was found that the pregnant woman took lithium drugs or suffered from various types of infections, which suggested the possible negative impact of taking medications and infectious diseases suffered during pregnancy.
By the time of birth, as a rule, the presence of a defect is already known , because all women must undergo timely ultrasound monitoring of the development of the heart in the fetus. If the expectant mother ignored visits to the doctor, then the presence of pathology in the structure of the heart will be indicated by changes in blood circulation, which are usually noticeable already in the first months of the baby’s life.
The human heart consists of two atria and two ventricles, which work harmoniously and push blood out in only one direction due to the presence of valves. It is the valve apparatus that ensures the one-way movement of blood through the cavities of the heart and blood vessels, therefore, with its anomalies, certain hemodynamic disorders always occur.
Speaking about Ebstein's anomaly, we mean pathology on the part of the tricuspid (three-leaf) valve, which is not where it should be, but lower, that is, displaced towards the right ventricle. The right atrium above the low-lying valve is larger than expected, and that part of the myocardium that is normally part of the right ventricle is called atrialized, which indicates its “atrial” affiliation.
With AE, only that part of the right ventricle that is located under the displaced valve functions. It is clear that it is impossible to accommodate the entire volume of blood pushed there from the atrium. A shrinkage of the right ventricle inevitably leads to excess blood in the atrium. In addition, through the altered valve, part of the blood returns to the already overcrowded atrium. These disorders cause the atrium to be stretched by volume, it cannot cope with the load, and as a result, blood flow in the lungs suffers, and the vessels of the systemic circulation are involved.
Ebstein's anomaly is rarely presented in isolated form. Nine out of ten patients have other changes . Thus, the most common is the combination of AE with a septal defect between the atria or an open foramen ovale. It is clear that hemodynamic disorders are further aggravated in this case. The combination of congenital heart disease causes a discharge of venous blood from the crowded right atrium to the left, while arterial blood going to the internal organs mixes with venous blood, and the result is hypoxia, that is, lack of oxygen.
Defects in the septum between the atria in combination with AE contribute not only to blood mixing, but also to local thrombus formation, so such patients always have a high risk of thromboembolism (stroke, necrosis in internal organs).
Often, with Ebstein's anomaly, additional pathways for conducting electrical impulses to myocardial cells are discovered, which “results” in heart rhythm disturbances - tachyarrhythmias, WPW syndrome and others.
Video: Ebstein's anomaly - medical animation (eng)
Tricuspid valve atresia
With this defect, the structures of the tricuspid valve are not visualized. Since there is no communication between the right atrium and the right ventricle, there is always an atrial septal defect and usually a ventricular septal defect. Other anomalies often associated with tricuspid atresia include transposition of the great vessels and pulmonary stenosis.
There are three types of defect depending on the position of the great vessels, the size of the VSD and the presence of pulmonary artery stenosis (J.Keith, M.Paul).
Type I
- with normal position of the great vessels:
- A. with pulmonary artery atresia (combined with PDA),
- B. with stenosis (hypoplasia) of the pulmonary artery and a small VSD,
- V. with stenosis (hypoplasia) of the pulmonary artery and a large VSD.
Type II
— with D-transposition of the great vessels:
- A. with pulmonary artery atresia (combined with PDA),
- B. with pulmonary artery stenosis,
- V. with a wide pulmonary artery (usually combined with stenosis or coarctation of the aorta),
III type
- with L-transposition of the great vessels (with subpulmonary or subaortic stenosis).
The most typical combination: moderate pulmonary artery stenosis, normally located great vessels, small ventricular septal defect.
EchoCG criteria
One-dimensional echocardiography:
- Small right ventricle.
- Enlargement of the left ventricle and left atrium.
- Volume overload of the left ventricle.
- Inability to visualize tricuspid valve structures.
Two-dimensional echocardiography:
- Dense echo signals at the level of the tricuspid ring without visualization of the leaflets.
- Large atrial septal defect.
- The small right ventricle is connected to the left through a ventricular septal defect.
- Hypoplasia of the right ventricular outflow tract.
- Identifies concomitant anomalies of the great vessels.
Doppler EchoCG:
- Lack of communication between the right atrium and the right ventricle.
- Turbulent flow through a ventricular septal defect.
How does AE manifest itself?
The time of onset of AE symptoms depends on the depth of valve damage, the severity of its insufficiency, and combination with other congenital heart defects. In children who have severe changes in valve structures, signs of the defect are diagnosed immediately after birth. Characteristic:
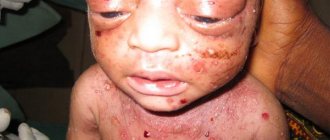
- Blueness of the skin;
- Weak sucking reflex;
- The child gets tired quickly when feeding and slowly gains weight.
The presence of an open foramen ovale in a newborn baby allows, to some extent, to compensate for the load on the right atrium, because part of the blood goes to the left half of the heart. If this hole is absent or very small, then the child’s condition can quickly become critical, and he can die from severe heart failure within the first weeks of life. Thus, the combination of AE with a defect in the septum in this case can even play a positive role, providing at least some unloading of the “right heart”.
With a moderate or small degree of valve displacement into the right ventricle for a long time, the only sign may be cyanosis. Such patients live with it for up to 10-15 years, and sometimes the defect is even diagnosed in adults. Another, no less dangerous manifestation is arrhythmia, which may also require surgical treatment.
Symptoms of AE include:
- Cyanosis of the skin and mucous membranes;
- Shortness of breath;
- Fatigue, weakness;
- Various arrhythmias;
- When heart failure occurs, edema occurs.
The lack of oxygen in the blood delivered to the organs causes not only an external change in the form of cyanosis, but also a metabolic disorder in the tissues caused by hypoxia. The consequence of this is a change in nails in the form of “watch glasses” and fingers in the form of “drumsticks”. These signs accompany many congenital heart diseases and indicate an insufficient concentration of oxygen in the blood or a discharge of venous blood into the arterial bed.
The enlarged right atrium, the volume of which can reach a liter or more, puts pressure on the anterior surface of the chest, which is especially pronounced in a growing child, whose bones are very pliable. This phenomenon leads to the appearance of such an external sign of pathology as a “heart hump” - a bulging of the front part of the chest in the area of the heart.
Complications of AE include fatal arrhythmias, cardiac arrest, and thromboembolism. The cause of death can be a stroke, sudden cardiac death, or an increase in congestive heart failure with an uncompensated defect.
Transposition of the great vessels
In transposition, the aorta is located to the right and in front of the pulmonary artery (D-transposition) or in front and to the left (L-transposition and communicates with the right ventricle; the pulmonary artery is located to the left and behind and communicates with the left ventricle. The connection between the pulmonary and systemic circulation is through the VSD , ASD, PDA or large bronchial arteries (Fig. 119).
Fig. 119.
Transposition of the great vessels (diagram).
Fig. 120.
Transposition of the great vessels in two-dimensional mode.
Fig. 121.
Transposition of the great vessels in one-dimensional mode.
EchoCG criteria
One-dimensional echocardiography:
- Simultaneous recording of two semilunar valves, with the pulmonary valve having a shorter ejection period.
- Dilatation of the right ventricle.
- Right ventricular hypertrophy.
- Increased excursion of the anterior leaflet of the tricuspid valve.
Two-dimensional echocardiography:
- Identification of the anterior and right (D-transposition) or anterior and left (L-transposition) aorta and posterior pulmonary artery. These vessels lie one below the other. The pulmonary artery lies posteriorly and its bifurcation can be seen.
- In the longitudinal section, a parallel orientation of the outflow tracts of both ventricles and both great vessels is visible, while the pulmonary artery does not bend around the aorta.
- The pulmonary artery arises from the left ventricle and forms the mitral-lunate continuation.
- The aorta arises from the right ventricle.
- Detection of associated intracardiac anomalies: large ventricular septal defect, patent ductus arteriosus, common atrioventricular canal, pulmonary stenosis or atresia, hypoplasia (atresia) of the atrioventricular valve.
Doppler EchoCG:
- Turbulent blood flow through a ventricular septal defect.
- Assessment of the degree of pulmonary blood flow (TMS with increased pulmonary blood flow, TMS with weakened pulmonary blood flow).
- Identification of concomitant congenital anomalies.
How to suspect Ebstein's anomaly?
Suspicion of any congenital defect requires a thorough examination. After analyzing the symptoms and complaints of the patient (or his parents in the case of newborns), the cardiologist prescribes additional diagnostic procedures:
- Chest X-ray to determine the size of the heart;
- Ultrasound is a highly accurate method for detecting AE and associated defects;
- ECG, Holter monitoring and electrophysiological study are necessary for cardiac arrhythmias;
- Atriography – examination of the atrium using a contrast agent;
- Catheterization of the heart cavities to determine the pressure in them.
Video: Ebstein's anomaly on EchoCG and description of the essence of the defect
Corrected transposition of the great vessels
The defect is characterized by atrioventricular and ventricular-arterial discordance, while the blood flow has a physiological direction (Fig. 122).
Fig. 122.
Corrected transposition of the great vessels: Inversion of the ventricles, Transposition of the great vessels, Intact interventricular septum, Left-sided aortic arch (diagram).
EchoCG criteria
One-dimensional echocardiography:
- The posterior great vessel (pulmonary artery) passes into the anterior leaflet of the right-sided (mitral) valve.
- The anterior leaflet of the posterior (tricuspid) valve continues into the anterior great vessel (aorta) and has a large excursion.
- Simultaneous visualization of two atrioventricular valves.
- Paradoxical movement of the interventricular septum (in 60% of cases).
Fig. 123.
Corrected transposition of the great vessels: Left-sided valve - tricuspid.
Two-dimensional echocardiography:
- The anterior great vessel (aorta) arises from the anterior wall of the left ventricle and does not continue into the atrioventricular valve.
- Both main vessels run parallel and do not intersect.
- The right ventricle is located on the left and contains the tricuspid valve (Fig. 123).
- The left ventricle is on the right and contains the mitral valve.
- Detection of associated anomalies: VSD (70%), pulmonary stenosis, arterial (tricuspid) valve insufficiency.
Doppler EchoCG:
- Assessment of arterial (tricuspid) valve function.
- Identification of concomitant congenital anomalies.
Treatment of Ebstein's anomaly
Drug therapy for AE is necessary to correct the heart rhythm when signs of heart failure appear. Antiarrhythmic drugs include beta blockers (atenolol, metoprolol), calcium antagonists (verapamil, diltiazem). For heart failure, diuretics, ACE inhibitors, and cardiac glycosides are indicated. The choice of drug is determined by the patient’s age and the course of the pathology.
AE is one of those developmental defects whose manifestations cannot be corrected only by conservative methods , therefore the vast majority of patients require surgical treatment. The age of the operation and its type depend on the structural abnormalities in the heart itself, the severity of the defect and the nature of the hemodynamic disorders.
The most common types of operations for AE:
- Tricuspid valve repair;
- Valve replacement.
If the valve leaflets and accompanying congenital heart disease are such that they can “correct” hemodynamic disorders with plastic surgery, then preference will be given to this method of treatment. As a rule, plastic surgery is indicated for valve insufficiency, while its narrowing (stenosis) will require a more radical intervention to replace this part of the heart.
During plastic surgery, the excess part of the right atrium is eliminated, a single-leaf valve is created and the diameter of the valve fibrous ring is reduced. If there is a defect in the interatrial septum, the surgeon will also suture it. Both during plastic surgery and prosthetics, “extra” impulse pathways are crossed, which contribute to arrhythmias.
example of reconstructive surgery for Ebstein's anomaly
Newborns with severe overflow of the right atrium and an insufficient size of the oval window may require surgery in the first weeks of life. Its essence is to expand the oval window or a defect in the septum with a special device (balloon) to ensure the movement of “excess” blood to the left half of the heart. This measure is not radical, but it eliminates the risk to the baby’s life, and in the future the valve will still require plastic surgery or its replacement.
If it is impossible to perform plastic surgery on the valve, the only way to cure the defect is prosthetics. Its disadvantage is the presence of foreign material in the organ, but, on the other hand, it is a very reliable method of correcting congenital heart disease. It is advisable to carry it out in adolescence, when the volume of the heart is already as close as possible to that of adults, in order to avoid a discrepancy between the size of the valve and the area of the heart and, as a result, stenosis.
Prosthetics means removing the structures of the damaged valve and replacing it with an artificial analogue. Modern medicine proposes to supplement such operations with the introduction of stem cells, which provide additional material for restoring the missing mass of the myocardium of the right ventricle of the heart.
tricuspid valve for transplantation
When it comes to the need to implant a prosthesis to replace a damaged valve, any parent will want to know what exactly will be installed in their child’s heart. Today, cardiologists can offer either a mechanical valve, consisting entirely of synthetic materials and metal, or a biological one, which is made from elements of human pericardium, or simply transplanting a pig valve corresponding in size to a human one.
These options have both advantages and disadvantages. A mechanical prosthesis requires lifelong use of anticoagulants, but it is durable and reliable. The biological valve does not require anticoagulant therapy, but also has a slightly shorter service life. The choice of the type of prosthesis remains with the cardiac surgeon, who evaluates the real clinical situation.
Tetralogy of Fallot
The vice includes 4 components:
- pulmonary stenosis
- ventricular septal defect
- dextraposition of the aorta
- right ventricular hypertrophy.
The most important 2 components of this defect are pulmonary stenosis and ventricular septal defect. Pulmonary artery stenosis can be infundibular, at the level of the valve, pulmonary trunk, or in the subinfundibular zone. The most typical combination is valvular and infundibular stenosis. Dextraposition of the aorta can be of varying degrees of severity. Conotruncal ventricular septal defect. Right ventricular hypertrophy is secondary and results from outflow tract obstruction. The right-sided aortic arch is observed in 20-30% (Fig. 114).
Fig. 114.
Tetralogy of Fallot: ventricular septal defect. Valvular and infundibular pulmonary artery stenosis. Right ventricular hypertrophy. Atrial septal defect (diagram).
Fig. 115.
Tetralogy of Fallot: the aorta “riding” over the interventricular septum. Large septal defect.
Fig. 116.
Tetralogy of Fallot.
EchoCG criteria
One-dimensional echocardiography:
- Lack of anterior continuation.
- Dilatation of the aorta.
- Dextraposition of the aorta (location of the anterior wall of the aorta and the interventricular septum at different depths).
- Hypertrophy of the anterior wall of the right ventricle.
- Hypertrophy of the interventricular septum.
- Reduction in the diameter of the pulmonary artery.
- Reduction of the left atrium.
Two-dimensional echocardiography:
- Direct visualization of the ventricular septal defect, aortic displacement and dilatation in the parasternal long-axis projection (Fig. 115).
- Direct visualization of pulmonary artery stenosis, its location and severity.
Doppler EchoCG:
- Turbulent flow through a ventricular septal defect.
- Turbulent flow in the pulmonary artery trunk is more than 1.1 m/s.
Differential diagnosis:
- Common arterial trunk.
- Pulmonary atresia with ventricular septal defect.
Double origin of the great vessels from the left ventricle
With this defect, the aorta and pulmonary artery are located side by side, completely departing from the left ventricle, the VSD is an outlet of the right ventricle.
EchoCG criteria
One-dimensional echocardiography:
- Lack of anterior continuation.
- Lack of posterior (mitral-aortic) continuation.
- Interruption of the echo signal from the interventricular septum.
Two-dimensional echocardiography:
- Parallel course of the great vessels.
- Communication of both great vessels with the cavity of the left ventricle.
- Large ventricular septal defect (usually in the subaortic region).
- Detection of associated abnormalities (usually pulmonary stenosis).
Doppler EchoCG:
- Determination of the systolic gradient between the left and right ventricles.
- Determination of the systolic gradient between the left ventricle and the pulmonary artery.
- Assessment of pulmonary hemodynamics.
Differential diagnosis:
Complete transposition of the great vessels with VSD and pulmonary stenosis.
Double origin of the great vessels from the right ventricle
With this defect, the pulmonary artery and aorta communicate with the right ventricle, and the VSD provides exit from the left ventricle. The defect is classified depending on the position of the interventricular defect and the presence or absence of pulmonary stenosis (Fig. 124).
I type DOS
with subaortic VSD
- A. without pulmonary stenosis
- B. with pulmonary artery stenosis
II type DOS
with subpulmonary VSD
- A. without pulmonary artery stenosis (Taussig-Bing anomaly)
- B. with pulmonary artery stenosis.
Fig. 124.
Double origin of the great vessels from the right ventricle, ventricular septal defect.
EchoCG criteria
One-dimensional echocardiography:
- Lack of anterior continuation.
- Lack of posterior continuation.
- Interruption of the echo signal from the interventricular septum.
- Right ventricular hypertrophy.
- Reduction of the left ventricular cavity.
Two-dimensional echocardiography:
- Visualization of two parallel great vessels arising from the right ventricle (the posterior vessel is the pulmonary artery).
- Determination of VSD localization: subaortic, subpulmonary, under both great vessels, distant from the great vessels.
- Confirmation of the presence or absence of pulmonary stenosis.
Doppler EchoCG:
- Determination of the systolic gradient between the left and right ventricles.
- Determination of the systolic gradient between the aorta and the right ventricle.
- Determination of the systolic gradient between the pulmonary artery and the right ventricle.