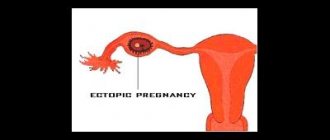
Miscarriage means the termination of pregnancy before the beginning of the 22nd week, when the weight of the fetus has not yet reached 500 grams. Sometimes an interruption occurs in the first weeks after conception, when most women are not yet aware of their situation. There is a need for treatment if there is a threat of miscarriage in the early stages, especially if spontaneous abortion occurs repeatedly. Two failed pregnancies are a reason to start searching for the cause that leads to the development of this pathology.
According to statistics, about 15-20% of confirmed pregnancies end in miscarriages. Most of them occur in the early stages of embryonic development (up to 12 weeks). The most common cause is chromosomal abnormalities.
Compound
One tablet of Prajisan contains 100 or 200 mg of the active ingredient progesterone + excipients (soy lecithin, glycerol , peanut oil, water, sorbitol , gelatin, titanium dioxide).
Vaginal gel contains 80 mg of progesterone per 1 gram of the drug + sorbic acid, guar gum, liquid paraffin, hypromellose K100M, hydrogenated palm oil glyceride, water.
Pharmacodynamics and pharmacokinetics
The drug is a hormone of the corpus luteum . The hormone is able to penetrate into the nucleus of target organs and stimulate RNA and activate DNA .
Prajisan transfers the uterine mucosa to the secretion phase from the proliferative phase . Thus, specific conditions are created for the implantation of a fertilized egg to occur. The muscles of the uterus relax, the organ becomes less sensitive to the action of Oxytocin .
The active substance stimulates lipoprotein lipases , increases fat reserves, and activates the process of inducing Insulin Aldosterone production .
When the gel is applied to the mammary gland, there is a decrease in the proliferation and mitotic activity of the epithelium in the ducts. When taken orally, the permeability of the capillaries of the mammary gland tissue decreases, and the swelling of the connective stroma subsides.
The medicine is quickly and almost completely absorbed. Maximum concentration is achieved within two hours. The degree of binding of the hormone to blood plasma . The drug undergoes metabolic in liver tissue. The drug is excreted with bile and kidneys, with feces.
When using the drug vaginally, the active component accumulates in the uterus; an hour after administration, the maximum concentration of the substance is already blood
After a miscarriage - medical care tactics
Typically, a miscarriage is accompanied by increasing bleeding from the vagina and the release of clots, severe pain in the lumbar region. As a rule, treatment is not required after early spontaneous abortion. The failed mother is provided with assistance in the hospital upon her request and sent home. Sometimes the embryo dies in the uterus, but its death does not cause alarming symptoms. The doctor learns about his death only after an ultrasound and other procedures.
Treatment is aimed at the exit of the formed biological materials from the uterus, if this does not happen immediately and naturally. Several methods can be used here:
- Waiting tactics under the supervision of a specialist.
- Drug therapy.
- Surgical intervention (curettage, vacuum aspiration).
Waiting tactics involve the body naturally ridding itself of the embryo. Ideally, the amniotic sac should be released entirely, with the embryo placed inside it and the amniotic fluid discharged, although a phased release is also possible. From the moment the embryo dies in the uterus until it leaves the organ naturally, several weeks can pass. However, the medical community does not recommend setting a waiting period of more than a month. Otherwise, there is a high risk of developing sepsis due to an infection formed inside.
Treatment can also be carried out with medications, the action of which is aimed at the release of biological material from the uterine cavity as a result of its contractions. The method works well when a frozen pregnancy occurs before the 8th week.
Surgical intervention is a radical solution to the problem. To reduce the risk of an inflammatory process, experts suggest performing gynecological curettage, which involves cleaning the inner lining and inner cavity of the uterus. The procedure takes no more than 45 minutes and is performed using general anesthesia. The patient does not feel pain or discomfort, as she is immersed in a state of deep sleep. A more gentle method is vacuum aspiration, when the contents of the uterus are removed with a special suction that does not damage the tissue.
Indications for use
The drug is prescribed for various disorders associated with a lack of the hormone progesterone in the body.
Capsules taken:
- for premenstrual syndrome ;
- if there is a disturbance in the menstrual cycle due to anovulation and ovulation ;
- for fibrocystic myopathy ;
- during perimenopause ;
- when using hormone replacement therapy for perimenopause and postmenopause .
The medicine is prescribed locally:
- as hormone replacement therapy for progesterone ;
- during preparation for in vitro fertilization to prolong the luteal phase ;
- upon the onset of early menopause ;
- for the treatment of infertility due to failure to enter the luteal phase ;
- to prevent abortion due to hormonal imbalance;
- as a prophylactic for uterine fibroids or endometriosis .
Features of treatment in case of threat
Treatment for threatened miscarriage in the early stages depends on many circumstances. It is determined individually and depends on the results of the examination, ultrasound, blood and urine tests. The examination reveals hormonal levels, structural features of the uterus and organs of the reproductive system, and infectious processes in the body. The set of measures taken is aimed at identifying an unfavorable condition and its subsequent correction using the most effective methods.
The main goal of treatment for the threat of miscarriage in the early stages is to relieve the tone of the uterus, relax the organ, stop bleeding and prolong pregnancy if possible. However, in European countries this treatment is not considered appropriate. Experts argue that the death that occurred is the result of natural selection, when a weak and non-viable organism is destroyed, which can be facilitated by genetic abnormalities and chromosomal rearrangements.
In Russia, a different approach is practiced. A woman who is in danger of losing her fetus is prescribed bed rest, sexual rest, and physical and emotional stress is prohibited. A complete, balanced diet is recommended; in most cases, maintenance medications are indicated.
Contraindications
The medicine is not prescribed:
- patients with disturbances in the blood circulation of the brain, thromboembolic disorders, including a history;
- with thrombophlebitis ;
- patients with porphyria ;
- persons suffering from liver or kidney diseases;
- if you suspect or have malignant neoplasms of the mammary glands or genital organs;
- persons with allergies to the components of the product;
- with bleeding from the genitals of unknown origin;
- after an incomplete abortion .
The solution in oil is not prescribed in the 2nd and 3rd trimester of pregnancy , as there is a tendency to form blood clots .
Treatment of threatened miscarriage in the first trimester
At the current stage of scientific research, many researchers come to the conclusion that there is a close relationship and mutual regulation between the endocrine and immune systems in the early stages of implantation [4,5,8,10,11,12]. It is an undeniable fact that progesterone plays a very important role in a woman’s body. Even before pregnancy, it causes decidual transformation of the endometrium, preparing it for implantation of a fertilized egg, and during gestation it promotes the growth and vascularization of the myometrium, reduces the excitability of the uterus by neutralizing the action of oxytocin, suppresses tissue immunological reactions, etc. [1,5,6,13 ]. It has been proven that progesterone promotes the full secretory transformation of the endometrium, necessary for the implantation of the blastocyst. In addition, during pregnancy, gestagens ensure the growth and development of the myometrium, its vascularization and relaxation by neutralizing the effect of oxytocin and reducing the synthesis of prostaglandins [3,7,8,14]. Complications in the initial stages of gestation can be a consequence of both defective steroidogenesis and insufficiency of the endometrial receptor apparatus. For successful implantation of an embryo, it is necessary to coordinate the readiness of the endometrium for implantation with the development of the embryo (the so-called “implantation window”) [3,6,8]. In such situations, the therapeutic approach should take into account the etiology of the formation of a defective luteal phase and neutralize unfavorable predisposing factors. In case of chronic inflammatory process in the uterus and appendages, in addition to prescribing individually selected etiological therapy, immunomodulatory therapy, correction of hormonal levels is necessary, which allows normalizing the state of the endometrium and ensuring adequate blastogenesis and placentation. Progesterone plays a fundamental role in preparing the uterine mucosa for implantation. It is generally accepted that for a normal pregnancy outcome, a woman’s immune system must recognize her. During a normal pregnancy, progesterone receptors are present in peripheral blood lymphocytes, and the proportion of cells containing such receptors increases as the gestational age increases. In the event of a threatened miscarriage, the proportion of cells containing progesterone receptors is significantly lower than in healthy women at the same stage of pregnancy. A number of scientists believe that an increase in the number of progesterone receptors during pregnancy may be caused by the presence of an embryo, which acts as a chorionic alloantigen (foreign) stimulator [6,9,11]. According to AR Genazzani [10], about 15% of all pregnancies end in spontaneous abortion, which is one of the most common complications of pregnancy. According to statistics, approximately one in four pregnant women experience one or more spontaneous miscarriages. Recurrent miscarriage is said to occur if there have been three or more repeated spontaneous miscarriages. This pathology, according to V.M. Sidelnikova [5], occurs in approximately 1–3% of all women. At the same time, the risk of miscarriage after three repeated spontaneous miscarriages reaches 55%. In most cases (50–60%), the cause of miscarriages is hormonal disorders, structural abnormalities of the embryo's chromosomes, infections, endocrine disorders, anatomical defects in the mother, etc. Many researchers [1,3,5,7,14] believe that most Miscarriages of unknown etiology may be caused by an abnormal immune reaction of the mother's body to the paternal antigens of the fetus. There is now increasing evidence that progesterone appears to play an important role in normalizing the immune response in the early stages of pregnancy. During a normal pregnancy, the corpus luteum and later the placenta produce sufficient amounts of progesterone. In its presence, activated lymphocytes produce a special protein - progesterone-induced blocking factor (PIBF), which has an anti-abortion effect. As is known, if pregnancy continues against the background of luteal insufficiency, primary placental insufficiency subsequently develops. To prevent it, proper preparation for pregnancy and proper management of patients with threatening and habitual miscarriage are necessary. For the treatment of threatening and habitual miscarriage, it is practical and highly effective to directly influence progesterone receptors by replenishing the lack of endogenous progesterone with the help of drugs - progestogens. A modern effective gestagenic drug is Duphaston (dydrogesterone), in the structure of which the methyl group at position 10 is located in the a-position, the hydrogen at carbon 9 is in the b-position, in addition, there is a double bond between carbohydrates 6 and 7. A change in the configuration of the molecule results in Duphaston being easily absorbed when administered orally. Dydrogesterone at a dose of 20–30 mg causes a full-fledged secretion phase in the endometrium. Animal studies confirm the high ability of dydrogesterone to support pregnancy. Duphaston is a potent gestagen, effective when taken orally, which in its molecular structure and pharmacological action is close to endogenous progesterone and, as a result, has a high affinity for progesterone receptors. Unlike many progestogens, it is not a testosterone derivative; its structure differs from the structure of most synthetic progestogens, as a result of which it does not cause any of the side effects characteristic of most of these drugs. The advantages of the chemical structure of dydrogesterone are the higher bioavailability of the drug after oral administration and the absence of metabolites with androgenic or estrogenic activity. The main metabolite of Duphaston is 20 a-dihydroxydydrogesterone, which also has progestogenic activity. Recent evidence has shown that the anti-abortion effects of progesterone during early pregnancy are also due to modulation of the maternal immune response. It has been proven that in the presence of Duphaston, activated lymphocytes synthesize protein (progesterone-induced blocking factor). The latter prevents inflammatory and secondary thrombotic reactions of trophoblast rejection by increasing asymmetric non-toxic blocking antibodies, as well as blocking degranulation of natural killer cells and by inducing T-lymphocyte-2 (Th2) dependent cytokines, shifting the balance towards Th2 cells, i.e. cytoprotective immune response [4,7]. Compensating for the insufficiency of the luteal phase in the event of a threatened or habitual termination of pregnancy, Duphaston also has a relaxing effect on the muscles of the uterus. Unlike other synthetic progestogens, Duphaston does not cause feminization of the male fetus and does not have side effects on liver function and blood clotting, such manifestations as acne, deepening of the voice, hirsutism and masculinization of the genital organs of the female fetus, and has no metabolic effects (for example , changes in the lipid spectrum of the blood and glucose concentration), and also does not affect the activity of the pituitary-ovarian system and does not cause atrophy of the adrenal glands. One tablet of Duphaston contains 10 mg of dydrogesterone. After oral administration of dihydrogesterone, 63% of the administered dose is eliminated in the urine, with 85% of this amount excreted within 24 hours. Almost complete renal excretion of the administered dose ends after 72 hours. In case of threatened miscarriage, it is recommended to include in the treatment complex 40 mg of this drug at a time, then 10 mg every 8 hours until the symptoms of miscarriage disappear. For habitual miscarriage, 10 mg of Duphaston is prescribed 2 times a day until the 18th–20th week of pregnancy. There is growing evidence that the immunomodulatory effects of hormones are important for maintaining normal endometrial function. The results of studies conducted by AR Genazzani [10] show the role of the immune system during pregnancy and, in particular, an increase in the number of progesterone receptors on lymphocytes as pregnancy progresses. In the presence of progesterone or its analog Duphaston, lymphocytes produce progesterone-induced blocking factor. As a result of the immunological effects of PIBP, the activity of type II T helper cells (Th2), which contribute to the normal course of pregnancy, increases, and the activity of type I T helper cells (TM), which have an adverse effect on pregnancy, decreases. The authors showed that dydrogesterone (Duphaston) is also able to shift the Th2/Th1 ratio in a favorable direction and thereby increase the likelihood of a successful pregnancy. This effect was confirmed in two clinical studies showing that dydrogesterone reduces the incidence of spontaneous abortion in women with a history of threatened or recurrent miscarriage. In-depth studies on the role of PIBP in maintaining pregnancy were carried out by J. Szekeres-Bartho et al. [13,14]. It is generally accepted that for a normal pregnancy outcome it is necessary that the immune system be able to recognize it. During a normal pregnancy, progesterone receptors are present in peripheral blood lymphocytes, and the proportion of cells containing such receptors increases as the gestational age increases. However, in women at high risk of premature termination of pregnancy, the proportion of cells containing progesterone receptors is significantly lower than in healthy women at the same stage of pregnancy. After a transplant or blood transfusion, the number of cells containing progesterone receptors is comparable to that in pregnant women. This suggests that in pregnant women, an increase in the number of progesterone receptors on lymphocytes may be caused by the presence of the fetus, which acts as an alloantigen stimulator. In the presence of progesterone, these lymphocytes produce a special 34-kD mediator protein, or PIBP. The concentration of PIBP increases with increasing gestational age, but disappears after 40 weeks during a normal pregnancy. Thanks to the immunological influence of PIBP, pregnancy is maintained. PIBP alters the balance of cytokines in the immune system. There are two types of cytokines: cytokines produced by Th1 T helper cells, which have an adverse effect on pregnancy, and cytokines produced by Th2 T helper cells, which contribute to the normal course of pregnancy. In the presence of PIBP, a shift occurs towards the predominance of Th2 cytokines. A simultaneous decrease in the production of Th1 cytokines entails a decrease in the activity of natural killer cells (NKC) and a normal pregnancy outcome. While taking Duphaston, there is a significant increase in the concentration of PIBP-positive lymphocytes, which entails the above-described changes in the immune system aimed at maintaining pregnancy. In cases where there is no PIBP, the concentration of TM cytokines increases and at the same time natural killer cells are activated, which increases the likelihood of abortion. When there is a threat of miscarriage or premature birth, the level of PIBP is significantly lower than during a normal pregnancy. With a lack of PIBP, the activity of natural killer cells increases approximately 4 times. It is currently believed that increased NK activity is one of the factors responsible for early termination of pregnancy. MY El-Zibdeh [9] publishes data on the results of two studies, the purpose of which was to determine whether dydrogesterone could improve pregnancy outcomes in women suffering from recurrent miscarriage. 114 women with a history of recurrent miscarriage (average number of previous miscarriages 3.3) were randomly divided into three groups and received: dydrogesterone (Duphaston) orally 10 mg twice a day; or human chorionic gonadotropin (hCG) intramuscularly at 5000 IU every 4 days; or did not receive any treatment. Therapy began immediately after confirmation of pregnancy and stopped at 12 weeks of gestation. In the group of women taking Duphaston, the frequency of abortions significantly (p<0.05) decreased compared to the control group by 27%, in the hCG group - by 16.6% (p<0.05). In the group taking Duphaston, the abortion rate was 14.6%, in the hCG group - 16.6%, in the control group - 20%. Duphaston was well tolerated by patients. The incidence of pregnancy complications was approximately the same in all three groups. In another study, MY El-Zibdeh [9] studied the effectiveness of the drug Duphaston in early miscarriage. Bleeding in early pregnancy is common and occurs in 30–50% of all pregnancies. In the author's opinion, the cause of bleeding should be determined before starting treatment. It is necessary to know which of their patients is at risk. An increased risk of miscarriage is observed in the following situations: maternal age over 35 years; father's age over 53 years; the presence of genetic defects in one of the parents; history of spontaneous miscarriages or stillbirths; birth of children with congenital developmental anomalies. A total of 146 pregnant women with mild or moderate bleeding were included in the study. The patients were divided into two groups using random sampling. In one group, in addition to standard treatment, women received Duphaston (dydrogesterone) 10 mg twice a day. The second group was the control group. Treatment with the drug was stopped 1 week after bleeding stopped. Treatment was discontinued if the intensity of bleeding increased, there were signs of discharge of the contents of the membranes, an increase in body temperature, there were no signs of growth of the membranes after a week of observation, the fetal pole was insufficiently pronounced or absent when the size of the membranes was 25 mm or more, or the absence of cardiac activity. The frequency of abortions significantly decreased (p<0.05) in the group of women receiving Duphaston compared to the control group. Pregnancy ended with timely delivery in 75% of subjects in the group taking Duphaston and in 66.6% in the control group. The incidence of pregnancy complications, including preeclampsia, intrauterine growth restriction, hemorrhage or congenital anomalies, was almost the same in both groups. At the X World Congress of Gynecological Endocrinology in Wroclaw (Poland), AR Genazzani [10] reviewed previous studies in which Duphaston was used to treat recurrent miscarriage and threatened abortion. The speaker cited the following statistics. According to published reports, 339 women participated in the studies. In 275 (81%) the pregnancy ended successfully with delivery, miscarriage occurred in 64 women (19%). 19 of the 64 miscarriages occurred within 48 hours of starting therapy, indicating that the miscarriage occurred before treatment began. In 8 of 64 cases the cause of miscarriage was known. According to AR Genazzani, these data indicate an obvious positive effect of therapy. Assessing the effectiveness of Duphaston during pregnancy, the author points out that the drug has a beneficial effect in cases of threatened abortion and recurrent miscarriage. As for the risks when taking Duphaston, it has proven to be an extremely safe drug and is well tolerated by patients. According to statistics, there are 7 million people in the world who have experienced the effect of the drug in the womb, but there are no signs of the drug’s teratogenic effect. Z.S. Zaidieva et al. [7] analyzed the initial clinical characteristics and features of the course of the first trimester of pregnancy in 97 women with a high infectious risk and a burdened obstetric history. Group I - 52 women at high infectious risk who received Duphaston in 3 menstrual cycles before planned pregnancy at a dose of 10 mg 2 times a day, group II - 45 women at high infectious risk who received Duphaston from the first weeks of gestation. The data obtained by the authors indicate that during the course of pregnancy in the first trimester of gestation in patients of group II, early toxicosis and the threat of miscarriage were significantly more common. During ultrasound examination, chorionitis and chorionic detachment were significantly more often observed in the group of women who did not receive Duphaston therapy. Based on the analysis, the authors showed that the prescription of gestagenic support is a necessary component of therapy aimed at preparing for pregnancy and its successful prolongation in women with infectious pathology and a burdened obstetric and reproductive history. O.F. Serovaya [4] studied the therapeutic efficiency of Duphaston with threatening termination of pregnancy in the first trimester. Examination of 54 patients with the help of general clinical, radiimmunological and ultrasound methods of research was carried out. The author showed that the use of duphaston contributes to a rapid improvement in the well -being of patients, normalizing the function of the fetoplacental complex and blood flow in the ovary and uterine arteries. The foregoing shows that therapy with gestagenic drugs must begin in preparation for pregnancy, and with its occurrence - without waiting for the development of clinical signs of the complicated course of the process of gestation. The appointment of this group of drugs from the first weeks of gestation, especially in women who have not undergone preparation for pregnancy, can significantly reduce the risk of developing such complications as the threat of interruption, chorionitis, the detachment of chorion, spontaneous abortion in the early stages, etc. Full -fledged therapy in the first trimester of gestation contributes to the adequate formation of the feto -placental system and will further avoid complications such as placental failure, delayed intrauterine development of the fetus, intrauterine infection, etc. All of the foregoing will be reflected in the improvement of obstetric and perinatal outcomes.
Literature 1. Demidova E.M. Habitual miscarriage (pathogenesis, obstetric tactics): Diss…. doc. honey. Sciences. – M. – 1993. 2. Zaidieva Z.S., Prozorov V.V., Karapetyan T.E. Progesterone support when planning pregnancy in women with a high infectious risk. // Russian Medical Journal. – 2006. – T.11, No. 1. – P.25–28. 3. Kulakov V.I., Ordzhonikidze N.V., Tyutyunnik V.L. Placental insufficiency and infection. M.: 2004.– 494 p. 4. Serova O.F. Experience with the use of duphaston for the treatment of women with threatened miscarriage in the first trimester. // Bulletin of the Russian Association of Obstetricians and Gynecologists.–2000.– No. 3. P.1–2 5. Sidelnikova V.M. Habitual pregnancy loss. – M.; Triad-X, 2002.– 304 p. 6. Sukhikh G.T., Vanko L.V. Immunology of pregnancy. // M.: Publishing House of the Russian Academy of Medical Sciences, 2003.– 400 p. 7. Bick RL, Madden J., Heller KB, Toofanian A. Recurrent miscarriage: causes, evaluation, and treatment. // Medscape Women`s Health.–1998.– Vol. 3, No. 3.– P.2–13. 8. Choi V.S., Polgar K., Xiao L. et al. Progesterone inhibits in–vitro embryotoxic Th1 cytokine production to trophoblast in women with recurrent pregnancy loss. //Hum. Reprod.–2000.–Vol.15.–P.46–59. 9. El–Zibdeh MY Randomized study comparing the efficacy of reducing spontaneous abortion following treatment with a dydrogesterone and human chorionic gonadotrophin (hCG).–Fertil. Steril.–1998.–Vol.70.–P.77–78. 10. Genazzani AR Hormone replacement therapy: the perspectives for the 21st century. // Maturitas.–1999.– Vol.31–32, 1.– P.11–17. 11. Klentzeris LD The role of endometrium in implantation. //Hum. Reprod.–1997.– Vol. 12.– P.170–175. 12. Simoncini T., Caruso A., Giretti MS et al. Effects of dydrogesterone and of its stable metabolite, 20-alpha-dihydrodydrogesterone, on nitric oxide synthesis in human endothelial cells. // Fertil. Steril.–2006.– Vol.37.– P.777–787. 13. Szekeres–Bartho J., Faust Z., Varga P. et al. The immunological pregnancy protective effect of progesterone is manifested via controlling cytokine production. //Am. J. Reprod. Immunol.–1996.–Vol. 35, No. 4.– P.348–351. 14. Szekeres–Bartho J. Progesterone receptor–mediated immunomodulation and anti–abortive effects: The role of PIBF. // Ginecologycal Endocrinology.–2001.– Vol.15, No. 5.– P.43–47.
Side effects
Of all the possible adverse reactions, the most common is allergies .
Adverse reactions from taking tablets and ointments:
- tension and swelling of the mammary glands;
- the onset of menstruation ahead of time;
- drowsiness, dizziness, nausea;
- headaches, jaundice , swelling and fluid retention in the body;
- decreased performance, skin rashes, anaphylactic shock .
For a solution in oil, the following may additionally develop:
- increased blood pressure , cholecystitis , hepatitis , visual impairment;
- thromboembolism , decreased sexual activity;
- pain at the injection site.
How to maintain pregnancy after IVF
Pregnancy after IVF often occurs with complications. This happens because women with diseases of the endocrine system and reproductive system resort to in vitro fertilization. They often experience miscarriage, toxicosis and other problems that put pregnancy at risk. To prevent this and maintain pregnancy after IVF, you need to be observed in a clinic that has the necessary equipment and qualified specialists working with IVF mothers.
Hormonal disorders during pregnancy after IVF
These problems are caused by the introduction of large doses of hormones during the preparation for IVF, if hormonal stimulation of the ovaries was carried out. For many women, this is a necessity and the only opportunity to become pregnant, since stimulation of the ovaries leads to the maturation of several follicles at once. At the same time, the concentration of estrogen in the blood increases sharply. Hormonal imbalance can cause disturbances in vascular permeability and accumulation of fluid in the lungs and abdominal cavity. Excess moisture prevents a woman from breathing, causes nausea, vomiting and loss of appetite.
Another possible consequence of HS is a slow increase in hCG levels (human chorionic gonadotropin), which is called the “pregnancy hormone”. This hormone should:
- preserve the corpus luteum;
- prepare the woman’s immune system for the upcoming pregnancy;
- stimulate fetal development
With a lack of hCG, fetal growth slows down, which leads to miscarriage and fading of pregnancy.
In this case, pregnancy support after IVF When observed in a specialized clinic, doctors will be able to correct hormonal levels in time, remove swelling and eliminate the threat of miscarriage.
Infectious and inflammatory processes
Women who become pregnant after IVF often suffer from chronic inflammation of the genital organs, which leads to the impossibility of natural conception. Although women are treated as much as possible during the period of preparation for pregnancy, the disease often worsens again. All this affects the functioning of the immune system and reduces the production of hormones responsible for the normal course of pregnancy.
A woman develops a bloody “spot,” indicating a threat of miscarriage. Under these conditions, it is not easy for the fetus to stay in the uterus, so medical support for pregnancy after IVF is required. Medical supervision helps eliminate dangerous inflammatory processes, maintaining pregnancy after IVF.
Maternal problems
IVF is mainly used by women aged 30 years and older. A large percentage of them have various diseases of internal organs (kidneys, heart, liver, gall bladder) and metabolic disorders. All these “sores” are prone to exacerbation during pregnancy, which negatively affects the intrauterine development of the child.
Women who already have health problems may develop gestational diabetes, caused by the pancreas not working properly. The level of glucose in the blood rises, which causes oxygen starvation of the fetus. This complication occurs more often if a woman is carrying twins, which often happens after artificial insemination. If the problems that arise are not treated in time, the pregnancy can be lost. To do this, women, being observed in the clinic after IVF, regularly donate blood for biochemistry and sugar.
Fetoplacental insufficiency
This condition occurs as a result of malformation and or insufficiency of the placenta. Since women who become pregnant after IVF often have problems in the sexual sphere, fetoplacental disorders are not uncommon.
As a result, the fetus suffers, which receives few nutrients and oxygen, which can lead to miscarriage or the birth of a sick child. Periodic Doppler testing, which shows the state of the feto-placental blood flow, can help with this condition. If a pathology is suspected, supporting pregnancy after IVF involves prescribing medications that improve blood circulation in the placenta and help the pregnancy proceed properly.
Multiple pregnancy
During IVF, a woman is often implanted with several embryos at once. If they all take root, a multiple pregnancy occurs. Sometimes twins develop from one egg, as in a normal pregnancy; a woman gives birth to identical twins. Many people consider twins or triplets to be an excellent result after IVF, forgetting that several babies are harder to bear and easier to lose.
Sometimes nature itself regulates the process, and extra embryos stop developing, but often all implanted eggs grow equally. It is possible, of course, to reduce (remove) excess embryos, but parents rarely agree to this.
In women pregnant with twins, toxicosis occurs more often and is more severe. The uterus, growing much faster, puts pressure on the legs, causing them to swell. Pressure on the diaphragm in recent months interferes with the mother’s ability to breathe properly. In the later stages of pregnancy with twins or triplets, an increase in blood pressure is more often observed, which can lead to convulsive syndrome (eclampsia) and premature birth. Since twins are already born with low weight, this can lead to extreme prematurity and death of newborns.
Overstretching of the uterus and increased stress on the body cause:
- bleeding;
- oxygen starvation of the fruits;
- anemia caused by a high load on the mother’s hematopoietic system;
- feto-fetal transfusion syndrome, when one fetus takes more nutrients, inhibiting the development of the second;
- thrombosis of placental vessels. During multiple pregnancies, one or both “baby places” are located incorrectly, which causes premature placental abruption;
- oblique, transverse or pelvic location in the uterus of one or all babies
A woman who has developed a multiple pregnancy as a result of IVF needs special observation and strict control, so she often undergoes an ultrasound to find out how the children are developing.
The condition of babies carried by IVF mothers is monitored using ultrasound and Doppler. Competent doctors working at the Life Line clinic will provide full support for pregnancy after IVF and will help you successfully endure multiple pregnancies and give birth to healthy children. The conscious attitude of a woman and the supervision of experienced specialists allows us to save the majority of pregnancies that occur after IVF.
Instructions for use of Prajisan (Method and dosage)
The duration of administration and dosage should be determined by the attending physician, depending on the disease.
Instructions for Prajisan capsules
Inside, with plenty of liquid.
As a rule, 200 to 300 mg of the drug are prescribed, divided into 2 doses.
For premenstrual syndrome, dysmenorrhea, fibrocystic mastopathy , during premenopause , the recommended dosage is 200-400 mg per day. The course of treatment lasts from 10 to 12 days.
When introducing a capsule or cream via the vaginal route, they are inserted deep into the vagina.
During preparation for egg and women with removed testicles, 200 mg per day is prescribed, on days 13 and 14 of the menstrual cycle, from days 15 to 26 - 100 mg. In case of pregnancy, the dosage is increased by 100 mg per day every week; upon reaching the level of 600 mg per day, the dosage is divided into 3 doses. The maximum dosage of 600 mg can be taken for no more than 2 months.
In order to maintain the luteal phase in preparation for IVF , 200-600 mg per day is used during the first and second trimesters of pregnancy.
If there is a threat of abortion or for the purpose of preventing abortions due to a lack of the hormone progesterone in the body, 200-400 mg is prescribed every day, in 2 doses, in the 1st and 2nd trimester of pregnancy.
Instructions for the gel
- Open the package with the applicator.
- Remove the cap.
- Place the applicator between your middle and thumb and press the plunger with your index finger.
- The woman needs to take a “lying on her back” position, bend her knees slightly.
- Slowly insert the applicator into the vagina and press the plunger.
- Throw away the used applicator with any remaining gel.
Prajisan caps 200 mg x10
Prajisan caps 200 mg x10
ATX Code: G03DA04 (Progesterone)
Active substance: progesterone (progesterone) Rec.INN registered by WHO
Dosage form
PRAJISAN
caps. 200 mg: 10 or 30 pcs.reg. No.: LP-000698 dated 09/28/11 - Valid
Release form, composition and packaging
Soft gelatin capsules, oval in shape, light yellow in color, the contents of the capsules are an oily suspension of almost white color.
1 caps.
progesterone 200 mg
Excipients: peanut oil - 295 mg, soy lecithin - 5 mg.
Composition of the capsule shell: sorbitol solution 70% (uncrystallized) - 12.8 mg, glycerol - 63.84 mg, gelatin - 121.6 mg, titanium dioxide - 1.5 mg, purified water - 120.26 mg.
Clinical-pharmacological group: Gestagen Pharmaco-therapeutic group: Progestagen
Indications
Disorders associated with progesterone deficiency.
Oral route of administration:
- infertility due to luteal insufficiency,
- premenstrual syndrome,
- menstrual irregularities due to ovulation or anovulation disorders,
- fibrocystic mastopathy,
- premenopause,
- hormone replacement therapy for peri- and postmenopause (in combination with estrogen-containing drugs).
Vaginal route of administration:
— hormone replacement therapy in case of progesterone deficiency with non-functioning (absent) ovaries (egg donation),
— support of the luteal phase during preparation for in vitro fertilization,
- support of the luteal phase in a spontaneous or induced menstrual cycle,
- premature menopause,
- hormone replacement therapy (in combination with estrogen drugs),
- infertility due to luteal insufficiency,
- prevention of habitual and threatened abortion due to progestin deficiency,
- prevention of uterine fibroids,
- prevention of endometriosis.
ICD-10 codes
ICD-10 code Indication
D25 Leiomyoma of the uterus
E28.3 Primary ovarian failure
N60 Benign breast dysplasia (including fibrocystic mastopathy)
N80 Endometriosis
N94.3 Premenstrual tension syndrome
N95.1 Menopause and menopause in women
N95.3 Conditions associated with induced menopause
N96 Recurrent miscarriage
N97 Female infertility
O20.0 Threatened abortion
Z31.1 Artificial insemination
Dosage regimen
The duration of treatment is determined by the nature and characteristics of the disease.
Oral route of administration
The drug is taken orally with water.
In most cases, with progesterone deficiency, the daily dose of Prajisan is 200-300 mg, divided into 2 doses (morning and evening).
For luteal phase deficiency (premenstrual syndrome, fibrocystic mastopathy, dysmenorrhea, premenopause), the daily dose is 200 or 400 mg, taken for 10 days (usually from the 17th to the 26th day of the cycle).
For hormone replacement therapy in peri- and postmenopause while taking estrogen, Prajisan is used at a dose of 200 mg/day for 10-12 days.
Vaginal route of administration
Capsules are inserted deep into the vagina.
Absolute progesterone deficiency in women with non-functioning (absent) ovaries (egg donation): against the background of estrogen therapy, 200 mg/day on the 13th and 14th days of the cycle, then 100 mg 2 times/day from the 15th to the 25th day of the cycle, from the 26th day, and if pregnancy is established, the dose increases by 100 mg/day every week, reaching a maximum of 600 mg/day, divided into 3 doses. This dose can be used for 60 days.
Luteal phase support during preparation for in vitro fertilization: it is recommended to take from 200 to 600 mg/day, starting from the day of human chorionic gonadotropin injection during the first and second trimester of pregnancy.
Support of the luteal phase in a spontaneous or induced menstrual cycle, in case of infertility associated with dysfunction of the corpus luteum, it is recommended to take 200-300 mg/day, starting from the 17th day of the cycle for 10 days, in case of delayed menstruation and diagnosis of pregnancy, treatment should be continued.
In cases of threatened abortion or for the purpose of preventing habitual abortions that occur due to progesterone deficiency: 200-400 mg daily in 2 doses in the first and second trimesters of pregnancy.
Side effect
By oral route of administration
“Breakthrough” bleeding or shortening of the normal menstrual cycle, breast tenderness (usually in the first month of treatment).
Drowsiness, transient dizziness (usually 1-3 hours after administration), nausea. These side effects can be reduced by reducing the dose, changing the drug regimen, or switching to the vaginal route of administration. These effects are usually the first signs of an overdose.
Feeling tired, migraine, headache, skin rash, itching, jaundice, fluid retention.
For oral and vaginal routes of administration
Allergic reactions (urticaria, anaphylactic shock).
Contraindications for use
For oral and vaginal use
- thrombophlebitis, thromboembolic disorders, intracranial hemorrhage or a history of these conditions,
- bleeding from the genital tract of unknown origin,
- incomplete abortion,
- porphyria,
- established or suspected malignant neoplasms of the mammary glands or genital organs,
- hypersensitivity to the components of the drug, incl. to peanut butter, soy.
For oral use (optional)
- severe liver disease currently (including cholestatic jaundice, hepatitis, hepatocellular carcinoma, Dubin-Johnson, Rotor syndromes) or in history, if liver function indicators have not returned to normal values.
The drug should be prescribed with caution for diseases of the cardiovascular system, arterial hypertension, chronic renal failure, diabetes mellitus, bronchial asthma, epilepsy, migraine, depression, hyperlipoproteinemia.
Use during pregnancy and breastfeeding
The use of the drug during pregnancy is not contraindicated. However, there is a potential risk to the fetus (especially males) when using progesterone in the first 4 months of pregnancy. The use of micronized progesterone in the II-III trimesters of pregnancy can lead to the development of liver diseases in pregnant women. Numerous epidemiological studies have not revealed cases of fetal abnormalities when using progesterone during pregnancy.
Progesterone passes into breast milk. Data on the use of the drug during lactation are insufficient to assess the potential risk to the infant.
Use for liver dysfunction Oral administration is contraindicated in current severe liver diseases (including cholestatic jaundice, hepatitis, hepatocellular carcinoma, Dubin-Johnson, Rotor syndromes) or in history, if liver function tests have not returned to normal values.
Use for impaired renal function The drug should be prescribed with caution in chronic renal failure.
special instructions
The drug should not be used for contraception.
During long-term treatment with progesterone, periodic medical examinations (including liver function tests) should be carried out, and treatment should be discontinued if there are abnormalities in diagnostic tests of liver function or cholestatic jaundice occurs. When using estrogen and/or progestagen-containing drugs, cases of the development of chloasma have been reported, especially in patients with a history of chloasma during a previous pregnancy. In women prone to developing chloasma, exposure of the skin to natural or artificial UV irradiation may cause or worsen chloasma.
Patients with a history of depression should be monitored, and if severe depression develops, the drug should be discontinued. Patients with concomitant cardiovascular diseases or a history of them should also be observed periodically by a physician.
Fluid retention may occur during treatment with progesterone, which may affect the course of epilepsy, migraine, bronchial asthma, heart failure or renal failure; such patients should be carefully monitored.
Impact on the ability to drive vehicles and operate machinery
When taken orally, caution must be exercised when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Overdose
Symptoms: drowsiness, transient dizziness, shortening of the normal menstrual cycle.
Treatment: dose reduction or correction of the regimen, for example, in case of drowsiness and dizziness - 200 mg is taken at bedtime from the 12th to the 14th day of the cycle or switch to the vaginal route of administration: in case of a shortening of the menstrual cycle - start treatment later in the cycle , for example from the 19th day, instead of the 17th. If necessary, carry out symptomatic treatment.
Drug interactions
When administered orally
With long-term concomitant use, barbiturates, carbamazepine, hydantoin or rifampicin may reduce the effectiveness of progesterone.
Although data are limited, it is suggested that activated charcoal and griseofulvin may also reduce the effectiveness of the drug.
Progesterone may increase the therapeutic, pharmacological or toxic effects of cyclosporine, theophylline and troleandomycin.
For vaginal use
Interaction with intravaginal use has not been assessed. The simultaneous administration of other intravaginal drugs should be avoided to avoid interfering with the release and absorption of progesterone.
Conditions for dispensing from pharmacies The drug is dispensed with a prescription.
Storage conditions and periods
The drug should be stored in a dry place, protected from light, out of reach of children, at a temperature not exceeding 25°C. Shelf life: 2 years.
Interaction
When combining the drug with Carbamazepine , Rifampicin , barbiturates or Hydantoin , the effectiveness of the drug may be reduced.
Also, Griseofulvin and enterosorbents may reduce the effectiveness of the drug.
The medicine is combined with caution with Troleandomycin , Theophylline and Cyclosporine .
When administered intravaginally, the drug cannot be combined with various suppositories and vaginal tablets.
special instructions
When taking the drug, caution should be exercised by persons suffering from cardiovascular diseases or with depression , including a history of depression.
The medicine should not be used to protect against unwanted pregnancy.
With long-term and systematic use of the drug, it is necessary to monitor blood counts, liver function and kidney function. chloasma increases ; sun exposure and exposure to ultraviolet radiation should be limited.
When using progesterone orally, you should be careful when driving.
Pragisan
Prajisan® should not be used for contraception.
The drug should not be taken with food, since food intake increases the bioavailability of progesterone.
The drug Prajisan® should be taken with caution in patients with diseases and conditions that may be aggravated by fluid retention (arterial hypertension, cardiovascular disease, chronic renal failure, epilepsy, migraine, bronchial asthma); in patients with diabetes mellitus, mild to moderate liver dysfunction, and photosensitivity.
Patients with a history of depression should be monitored, and if severe depression develops, the drug should be discontinued.
Patients with concomitant cardiovascular diseases or a history of them should also be periodically observed by a doctor.
The use of Prajisan® after the first trimester of pregnancy may cause the development of cholestasis.
During long-term treatment with progesterone, regular medical examinations (including liver function tests) are necessary; Treatment should be discontinued if abnormal liver function tests or cholestatic jaundice are present.
When using progesterone, it is possible to reduce glucose tolerance and increase the need for insulin and other hypoglycemic drugs in patients with diabetes mellitus.
If amenorrhea occurs during treatment, pregnancy must be excluded. If the course of treatment begins very early in the menstrual cycle, especially before the 15th day of the cycle, a shortening of the menstrual cycle and/or acyclic bleeding is possible. In case of acyclic bleeding, the drug should not be used until the cause is determined, including a histological examination of the endometrium.
If there is a history of chloasma or a tendency to develop it, patients are advised to avoid UV irradiation.
More than 50% of spontaneous abortions in early pregnancy are caused by genetic disorders. In addition, the cause of spontaneous abortions in early pregnancy can be infectious processes and mechanical damage. The use of the drug Prajisan® in these cases can only lead to a delay in rejection and evacuation of a non-viable ovum. The prescription of Prajisan® for the purpose of preventing and/or treating the threat of miscarriage is justified only in cases of progesterone deficiency.
The drug contains soy lecithin, which can cause hypersensitivity reactions (urticaria and anaphylactic shock).
When conducting MHT with estrogen during perimenopause, it is recommended to use Prajisan® for at least 12 days of the menstrual cycle.
With a continuous MHT regimen in postmenopause, it is recommended to use the drug from the first day of taking estrogen.
When conducting MHT, the risk of developing venous thromboembolism (deep vein thrombosis or pulmonary embolism), the risk of developing ischemic stroke, and coronary heart disease increases.
Due to the risk of developing thromboembolic complications, you should stop using the drug if: visual disturbances such as vision loss, exophthalmos, double vision, vascular lesions of the retina: migraine; venous thromboembolism or thrombotic complications, regardless of their location.
If there is a history of thrombophlebitis, the patient should be closely monitored.
When using Prajisan® with estrogen-containing drugs, you should refer to the instructions for their use regarding the risks of venous thromboembolism.
The results of the Women Health Initiative Study (WHI) clinical study indicate a slight increase in the risk of breast cancer with long-term, more than 5 years, combined use of estrogen-containing drugs with synthetic gestagens. It is unknown whether there is an increased risk of breast cancer in postmenopausal women when undergoing MHT with estrogen-containing drugs in combination with progesterone.
The WHI study also found an increased risk of dementia when starting MHT after age 65 years.
Before starting MHT and regularly during it, a woman should be examined to identify contraindications to its implementation. If clinically indicated, a breast examination and gynecological examination should be performed.
The use of progesterone may affect the results of some laboratory tests, including liver and thyroid function tests; coagulation parameters; pregnanediol concentration.
Analogs
Matches by level 4 ATX code:
Iprozhin
Utrozhestan
Crinon
Progesterone
Progestogel
There are the following analogues of this drug: Krainon, Progesterone, Iprozhin, Prozhestogel, Utrozhestan .
Reviews about Prajisana
There are generally good reviews on the Internet about the medicine if you use it according to your doctor's recommendations.
Reviews of Prajisana on forums:
- “I took the drug to get rid of pain before my period. The medicine significantly relieved all my pain. I advise you to buy...”
- “After I took the first pill, I felt bad, dizzy, nauseous, and there was a vacuum in my ears, I felt unwell all day...”
- “My sister took pills during pregnancy. I carried and gave birth, everything is fine...”
Preparing for a new pregnancy
Unfortunately, sometimes any tricks of doctors and couples to save the child end in failure. What happened becomes a heavy blow for both the failed mother and the father. Not wanting to come to terms with what happened, many strive to immediately conceive again, forgetting that a woman’s body has little resemblance to the incubation system. Full recovery takes time. On average, at least six months should pass before a new pregnancy. During this period, future parents gain balance, regain strength and begin to strive to achieve their goals. Do not forget that pregnancy can occur immediately after the end of menstruation. To prevent the process from becoming spontaneous and occurring unexpectedly, it is important to consult about the prescription of contraceptive drugs.
Health must be treated with special care if the first birth occurs after the age of 30 years. To be sure that things are going well, you should consult a doctor immediately after learning about pregnancy and attend every appointment he recommends.