TRICHINOSIS
(
trichinellosis
; synonym:
trichinosis, “puffy”
) - helminthiasis from among the nematodes, characterized by fever, muscle pain, facial swelling, skin rashes, eosinophilia, and in severe cases - damage to the myocardium, lungs, and central nervous system.
For the first time, Trichinella larvae were discovered in the muscles of a corpse by an Englishman. medical student J. Paget in 1835. The first observation of human T. with a fatal outcome was described by FA Zenker in 1860.
Geographical distribution
T. is ubiquitous in nature. In the last 20-30 years, the distribution of human T. incidence in the world has changed. The incidence has decreased significantly in Eastern European countries and the USA. Thanks to the improvement of the pig breeding system, T. is practically not registered in Romania, Hungary, and Bulgaria. A stable focus still remains in the east. parts of Poland. Individual diseases and outbreaks are recorded in northern Italy, on the island. Sicily, in France, Spain, the Netherlands. The incidence of trichinosis has increased in Asian countries, on the Pacific Islands, Mexico, and Chile. T.'s diseases began to be recorded in Africa. In Asia, the greatest prevalence of T. is noted in Lebanon; individual diseases are recorded in Iran, Iraq, and India; outbreaks of T. are observed in Indonesia, Thailand, and Laos.
In the USSR, T. has been registered in 65 species of wild animals. By 1978, the incidence of T. in pigs had sharply decreased. The incidence of trichinosis in the BSSR, which was the main focus of T. in our country, has decreased 38 times over the past 15 years. In connection with the development of the territories of the North and East of the country, the number of T. diseases in people who eat wild animal meat increased, so in 1975 in the RSFSR it accounted for up to 96% of all T. diseases, while in 1946-1967 - only 24.4%.
Diagnostics
Methods for intravital diagnosis of trichinosis continue to be improved. They are testing allergic and serological diagnostic methods, as well as biopsy methods. For this purpose, a piece of muscle is cut out from the pinna of the ear in pigs, and from the biceps brachii muscle in humans.
Posthumously, trichinosis is detected by trichinoscopy. To do this, 24 sections (each the size of a grain of oat) are made from a sample of the legs of the diaphragm or the intercostal and abdominal muscles and squeezed between two glass slides or in a compressorium until clear, and then examined under a low magnification microscope or under a trichinelloscope. Trichinella capsules must be distinguished from misher bags.
The latter have different sizes and shapes, from barely noticeable under a microscope to 2 mm long. The outside of the misher bags is covered with a thin shell. Their contents are delimited by partitions into a number of small chambers filled with banana-shaped trophozoites.
The most accurate method of post-mortem diagnosis is considered to be the method of digesting skeletal muscle samples from several animals in artificial gastric juice. Weighed samples of MUSCLE of 5-50 g are used. Muscle samples are ground in a meat grinder with a fine mesh (2 mm), the resulting minced meat is placed in a jar or flask with a capacity of 300-500 ml and filled with 25 times the amount of artificial gastric juice prepared according to the recipe: pepsin 1 ml of hydrochloric acid per 1 liter of water. The mass is mixed well and left in a thermostat for 4-5 hours at a temperature of 42-47 ° C (the minced meat in the flask is mixed several times).
After the specified time has elapsed, the sample is removed from the thermostat, the top layer of liquid is removed (drained or sucked off), and the remainder is examined under a low magnification microscope. Trichinella larvae are found in a decapsulated state. Usually they are weakly mobile; when they are alive, they curl up into a spiral when cooled, while the dead ones remain in an unfolded state.
Etiology
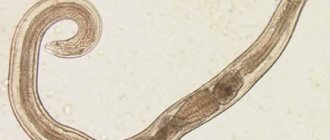
The causative agent of T. is the nematode Trichinella spiralis (Owen, 1835). New species of trichinella, T. nativa (Britov, Boev, 1972) and T. nelsoni (Britov, Boev, 1972), identified in 1972 by S. N. Boev and V. A. Britov, were recognized by the International Commission on Trichinosis in 1980 varieties of T. spiralis.
Sexually mature Trichinella (females 1-3 mm long, males 1-2 mm long) live in the mucous membrane of the small intestine. Males die after fertilizing females; females stay in the small intestine for 3-4 weeks, with intense infestations - up to 5-6 weeks. On the 3rd day after invasion, females give birth to larvae, which spread throughout the body with the flow of lymph and blood and, penetrating under the sarcolemma of the striated muscles, settle in them. At 3-4 weeks after invasion, a capsule forms around the larvae. The encapsulated larva has an oval shape, size 0.5 X 0.2-0.6 XX 0.3 mm. The capsule is gradually impregnated with calcium salts, but the larvae remain viable for many years.
Pathogen
The causative agent of trichinosis is Trichinella spiralis (Trichinella). The body length of a female sexually mature parasite before fertilization is 1.4-1.7 mm; after fertilization, its length increases to 4.5 mm. The length of a sexually mature male is 1.1-2 mm. The width of the female and male is approximately the same. The size of the parasite is largely related to the physiology of the host's digestion. The cylindrical body of Trichinella tapers towards the head. It is covered with a transparent cuticle that has a ring-like structure. In females, the genital opening is located in the front of the body at a distance of 1/3 of the body length from the head end. Males have two lobes in the tail, designed to secure the female during copulation.
Epidemiology
The source of pathogens of invasion (see) for humans are pigs, wild boars, brown, black and polar bears, marine mammals (whales, walruses, seals), as well as horses and dogs. Human infection with T. occurs when eating untested raw, salted, smoked or insufficiently heat-treated meat, as well as lard and sausages. Domestic animals become infected by eating meat waste or the corpses of dead animals; wild animals become infected as a result of predation, as well as by eating animal carcasses. Marine animals become infected through water contaminated with the feces of carnivorous birds, which may contain undigested invasive Trichinella larvae.
Depending on the range of hosts, three types of T. foci are distinguished: natural, synanthropic and mixed. In natural foci (see Natural focality), the circulation of Trichinella is supported by food connections between wild animals of different species. In synanthropic foci, circulation of the pathogen occurs between domestic animals (pigs, cats, dogs) and synanthropic rodents (rats, mice). When the connection between natural and synanthropic foci is maintained, mixed, natural-synanthropic foci arise. This is facilitated by free grazing of pigs, feeding of hunting waste to domestic animals, etc.
An increase in the incidence of T. in synanthropic foci usually occurs in the autumn-winter period, which corresponds to the period of slaughter of pigs and procurement of meat products. T.'s diseases are usually group in nature.
Prevention and control measures
Prevention mainly comes down to a veterinary and sanitary examination of all pork carcasses with mandatory trichinoscopy. Household slaughter of pigs is prohibited. Slaughterhouse waste, as well as carcasses of carnivores and rodents, are prohibited from being fed to pigs. Rats and other rodents are destroyed on farms. Carcasses of various animals can be fed to pigs only after rendering harmless by boiling or recycling into meat and bone meal.
Employees of veterinary and meat processing institutions are required to immediately notify the relevant veterinary and medical organizations of all cases of detection of trichinosis, indicating the address. Pigs delivered to meat processing plants must be tagged by the relevant farms.
Pathogenesis
There are three phases of disease development: enzymatic-toxic, immunological and immunopathological. The enzymatic-toxic phase of the disease is clinically detected only with intensive infection. During the process of penetration into the mucous membrane of the small intestine, the larvae secrete metabolites that have enzymatic, toxic, sensitizing effects, as well as the ability to suppress immune reactions. A specific sensitization of the body occurs. By the end of the 2nd and 3rd weeks, a sufficient amount of antibodies accumulates in the body, the immunosuppressive activity of intestinal trichinella weakens and a violent allergic reaction occurs (immunological phase), accompanied by impaired blood clotting, increased permeability of capillary walls, and tissue edema. Damage to internal organs and damage to c. n.s., systemic vasculitis, developing in the third, immunopathological, phase of the disease, is associated with damage to body cells by the resulting immune complexes (see Immunity).
Symptoms of Trichinosis
Pigs can get trichinosis by eating small rodents, raw waste or food scraps, and the insides of sick animals. Often the disease goes unnoticed. With severe infection, the following symptoms are observed on days 3–5:
- heat;
- diarrhea;
- vomit;
- rapid weight loss;
- muscle pain (pigs lie motionless with their limbs outstretched);
- shallow breathing;
- swelling.
If the animal does not die, the painful state continues for 1–1.5 months until the larvae form capsules. Then the symptoms of the lesion disappear and the pig looks healthy. The disease enters the chronic stage.
Pathological anatomy
With moderately intense invasion, by the end of the first week after infection, female Trichinella are found in the small intestine (to a lesser extent in other parts of the gastrointestinal tract), partially immersed in the mucous membrane and hanging into the lumen. In the mucous membrane, there is acute inflammation with hemorrhages and desquamation of the villous epithelium, in the deeper layers of the intestinal wall there is infiltration of lymphoid cells, macrophages and leukocytes with an increasing content of eosinophils. With intense invasion, the inflammatory reaction in the mucous membrane develops in the first days, and sometimes even in the first hours after the invasion, spreading to all parts of the intestines and stomach. In this case, the process in the 2-3rd week can take on an ulcerative-necrotic character with bleeding and even perforation.
Rice. Microslides of striated muscles in trichinosis: a - encapsulating Trichinella larva (1), surrounded by an inflammatory infiltrate (2), spreading between the muscle fibers; hematoxylin-eosin staining; X200; b — encapsulated Trichinella larvae (dark areas in the area of the poles of the capsules are deposits of calcium salts); hematoxylin-eosin staining; x50.
In the 2nd week, with moderately intense invasion due to the migration of parasites in striated muscle tissue, myocardium, lungs, kidneys, and brain, rod-shaped larvae can be found, surrounded by inflammatory infiltrates containing a significant number of eosinophils, which leads to the development of myocarditis (see .), meningoencephalitis, pneumonia (see), etc. At the 3rd week after such an invasion, the larvae acquire an S-shape; they are surrounded by massive inflammatory infiltrates (Fig., a). The muscle fibers along the periphery of the larvae are thickened, with basophilic sarcoplasm, which has lost its cross-striations. In the myocardium, lungs, brain and other organs, granulomas form around the larvae, in which they undergo lysis and resorption. With intense invasion of the myocardium during these periods, pronounced focal-diffuse inflammatory infiltration and degenerative changes (trichinosis myocarditis) are noted. Similar changes are observed in the lungs and other organs. Systemic vasculitis is possible.
By the end of the 3rd and 4th weeks, with moderately intense and intense invasion, the inflammatory reaction in the wall of the small intestine and in the muscle tissue reaches its maximum development, and a sharp swelling of the mucous membrane of the small intestine is noted. The larvae in the muscles acquire a spiral shape, and a fibrous capsule is formed around them. Subsequently, the internal hyaline layer differentiates in the capsule, and calcium salts are deposited along the poles of the larva or diffusely in the capsule (Fig., b). Its complete calcification occurs after a few years.
5-6 weeks after infection, with intense invasion, inflammatory processes are replaced by dystrophic ones (dystrophic skin changes with hair loss, fatty liver). During recovery, inflammatory changes in organs and tissues, as a rule, disappear without a trace; elimination of changes associated with dystrophic processes occurs slowly - within 6-12 months.
Why are parasites dangerous?
Trichinella larvae enclosed in capsules are dangerous because they are resistant to high and low temperatures and other destructive processes. When cooking a piece of meat weighing about 1 kg, the destruction of the larvae can be achieved only after two and a half hours. The freezing process at -25 degrees must be carried out for at least 4-5 days. In rotting remains, the larvae remain alive for 4-6 months.
Parasites are very dangerous for humans. Poorly cooked, undercooked meat from infected pigs can become a source of infection. Complete recovery from trichinosis can occur after 6-12 months of complex therapy. In severe cases, it can cause various serious disturbances in the functioning of the heart and central nervous system, which causes the death of the patient.
Immunity
After suffering from T., due to the presence of parasite larvae in the body of the recovered person, non-sterile immunity remains for life. Its intensity depends on the intensity of the invasion, the frequency of infection, the characteristics of the pathogen strains, and the constitutional characteristics of the patient’s body. The local residents of the north. districts of the USSR, Canada, USA, who constantly eat meat of wild animals that have not undergone heat treatment and are affected by T., the disease manifests itself clinically extremely rarely.
Clinical picture
There are various forms of T.—from subclinical to severe with widespread lesions of internal organs. The duration of the incubation period is usually 10-25 days, with an erased and mild course of the disease - 4-5 weeks, with a severe course - 7-10 days (sometimes 1-3 days). All in. In latitudes, the incubation period of T. is described as up to 43 days in severe cases of the disease. When infested meat is repeatedly consumed in the prodromal period, liquid stool often appears, while there is no abdominal pain and the general condition of the patient is not disturbed.
In erased and mild forms, body temperature does not exceed 38-38.5°, the number of eosinophils is within 10-20%; the illness lasts 1-2 weeks. In moderate cases of T., the body temperature reaches 39-40°C within 2-3 weeks. gradually decreases to low-grade fever (see Low-grade fever). Patients complain of severe muscle pain, they develop swelling of the face, conjunctivitis (see), and skin rashes of an exudative or polymorphic nature often appear. In 1IS patients, in the initial period, liquid stools, short-term abdominal pain are noted, with roentgenol. The study sometimes reveals “volatile” infiltrates in the lungs. The number of eosinophils against the background of moderate leukocytosis reaches 25-40%.
In severe cases, the disease often begins with diarrhea and dyspeptic disorders. Feverish reaction, edema, myalgia develop gradually and reach a maximum in the 3rd week of the disease. Muscle pain becomes generalized, accompanied by contractures with immobilization of the patient, swelling spreads from the face and neck to the torso and limbs. Characteristic rashes are erythematous-papular, sometimes hemorrhagic in nature, such as hemorrhagic vasculitis or in the form of pustules. Disorders of c are expressed. n. pp.— insomnia (see), delirium (see), hallucinations (see). Tachycardia (see), cardiac arrhythmias, collapse (see) are observed. From the end of the first week, myocardial lesions of a diffuse nature are detected; in the 3-4th week, the clinical picture of myocarditis, pneumonia, and lesions of c develops. n. With. Sometimes patients experience acute paroxysmal pain, usually in the upper abdomen, accompanied by hemorrhagic rashes and hypereosinophilia up to 80-90%. Dystrophic liver lesions are observed in all cases of severe T., but with recovery they disappear without a trace. The clinic of meningoencephalitis includes general neurol. disorders and is determined by the predominant localization of diffuse focal brain lesions. The development of acute psychoses (see Mental illnesses), hysterical amblyopia (see), epileptiform seizures is possible. The number of eosinophils often does not exceed 30-40%. At the onset of the disease, a decrease in ROE, an increase in hypoproteinemia due to hypoalbuminemia, and an increase in the activity of aldolase and cholinesterase are characteristic. At 4-6 weeks of the disease, there is a tendency to hypercoagulation with the development of thrombohemorrhagic syndrome (see), phlebothrombosis (see Thrombophlebitis).
Children usually tolerate T. relatively easily; pain in the throat, enlargement of the pharyngeal tonsils are often observed, and in some cases a severe course of the disease with a fatal outcome is possible.
Diagnosis
The diagnosis is established on the basis of a characteristic wedge, picture, epidemiol. medical history (eating raw or insufficiently heat-treated animal meat, which is a possible source of infection). The most difficult diagnosis is severe forms of T., which appear during epidemic outbreaks earlier than other forms, and often begin atypically - with diarrhea. Confirmation of the diagnosis is the detection of Trichinella larvae in meat, which is considered the cause of infection. In case of single diseases, in the case of an unclear source of invasion, a diagnostic muscle biopsy is performed (see Helminthological research methods). Serological reactions are used - RSK, indirect hemagglutination reaction with antigen (see Serological tests).
Differential diagnosis during outbreaks of T. is carried out with acute respiratory viral diseases (see), typhoid fever (see), paratyphoid fever (see), food toxic infections (see Food toxic infections), in children - with measles (see), rubella (see), scarlet fever (see). The main distinguishing features of T. are pronounced eosinophilia, decreased ROE, and high aldolasemia. In case of single diseases, trichinosis is differentiated from Quincke's edema (see Quincke's edema), an allergic reaction to drugs (see Drug allergy), dermatomyositis (see). Granulomatous changes in muscle tissue during dermatomyositis can be interpreted as trichinosis, but unlike T., the skin itself is affected.
Life cycle
Copulation of dioecious worms occurs in the lumen of the small intestine of the final host. Embryonic development and hatching of larvae from eggs occurs in the female genital tract (ovoviviparity). Trichinella females penetrate the anterior end of the body into the intestinal epithelium and give birth to 1-2 thousand larvae, which are spread through the blood and lymphatic vessels throughout the host’s body.
Only those larvae survive that get into striated muscles with a good blood supply (masticatory, extraocular muscles, muscles of the diaphragm). There, with a stylet that functions only at this stage, they destroy muscle tissue and cause the host to form a spindle-shaped capsule. Subsequently, the capsule is saturated with lime, but the exchange of substances between the parasite and the host does not stop. This stage (muscle trichina) can exist for several years. Infection of muscles with Trichinella larvae can reach 15 thousand per kilogram of tissue.
To complete the life cycle, the host's muscles must be eaten by another mammal. Once in the small intestine, Trichinella undergoes four moults over the course of several days, reaching sexual maturity. Thus, for the development of one generation, a change of host is necessary, which consistently acts as the final one for the parent forms and intermediate for the daughter forms.
Treatment
Treatment of patients with mild and erased forms of T. can be carried out at home. For diseases of moderate and severe forms, patients are hospitalized in hospitals with intensive care facilities. For erased and mild forms, antihistamines, calcium salts, analgesics, ascorbic acid are prescribed, but no specific treatment is carried out. For moderate and severe T., specific treatment is prescribed with vermox (mebendazole) at a dose of 300 mg per day for adults (for children - at a dose of 5 mg/kg) in 3 doses after meals. For moderate forms, the drug is taken for 7 days, for severe forms - 10-12 days. Specific therapy for T. is often accompanied by an increase in allergic manifestations of the disease and an increase in the severity of organ damage in the first 1-3 days of treatment. In this regard, for T. of moderate severity, vermox is prescribed while taking antihistamines, and for severe disease - while taking prednisolone at a dose of 30-40 mg per day (for particularly severe disease - at a dose of 60-80 mg ), preferably inside. Treatment with prednisolone at the indicated dose continues for 5-7 days, then the dose of the hormone is reduced over 7-10 days. Specific therapy is more effective and better tolerated the earlier treatment is started. The most effective is specific treatment of infested individuals during the incubation period (preventive therapy), especially in the first week after invasion. If intensive invasion is suspected, preventive treatment must be carried out with Vermox at a dose of 300 mg per day for 7 days.
Patients are prescribed a complete protein diet with limited salt; according to indications, cardiovascular drugs, drugs that improve metabolic processes (ATP, cocarboxylase, vitamins C, group B, plasma transfusion, albumin, digestive enzymes, anabolic hormones, etc.).