Introduction
In the second half of our century, significant discoveries were made in the field of mineral metabolism and its endocrine regulation. The new knowledge gained about the regulation and disorders of calcium metabolism has made it possible to understand the pathogenesis of various clinical syndromes caused by these disorders. Such syndromes include rickets, osteochondrosis, osteoporosis, especially postmenopausal with a high risk of vertebral fractures, visceral and skeletal forms of hyperparathyroidism, osteodystrophy, urolithiasis, atherosclerosis, Fahr's disease with calcium deposition in the brain, calcification, dermatomyositis and others.
Calcium is a phosphatophil by nature and is found in various tissues, in particular in the mitochondria of cells in the form of phosphate salts (calcium hydroxyapatite). If apatites are the most sparingly soluble calcium salts, then compounds with inorganic bisphosphates (pyrophosphates) can become insoluble (in bones) or soluble (in soft tissues), depending on the conditions, i.e. inorganic bisphosphates are natural regulators of calcium metabolism in the body. With a gradual decrease in the activity of numerous regulatory mechanisms with age, calcium metabolism is disrupted and insoluble calcium salts are deposited in soft tissues. The question arises, is skin an exception? To answer this question, a study was conducted on changes in calcium content in the cells of various layers of the skin depending on age.
Calcium deficiency
The main factors leading to calcium deficiency in the body:
- hypovitaminosis of vitamin D;
- poor nutrition (calcium deficiency in incoming food);
- Ca absorption disorders;
- excessive intake of substances that interfere with the absorption of calcium or promote its rapid excretion (phosphorus, oxalic acid, lead, zinc, magnesium, cobalt, iron);
- diseases of the thyroid gland and parathyroid glands;
- uncompensated increased need for calcium (growth in children, pregnancy and lactation, postmenopause);
- increased excretion of calcium when using diuretics and laxatives.
Consequences of calcium deficiency:
- reduced bone density, fractures, vertebral deformity, osteoarthritis, osteoporosis;
- increased fatigue, general weakness as the body’s reaction to calcium deficiency;
- cramps, muscle pain;
- growth disorders;
- Kashin-Beck disease, the list of main causes for the development of which includes a deficiency of microelements entering the body with food and water, including calcium;
- urolithiasis disease;
- blood clotting disorders, bleeding.
The role of calcium in the body and its natural regulation
The biological functions of calcium are extremely complex and diverse. Calcium plays a structural role, forming, together with phosphorus, the mineral basis of the skeleton - hydroxyapatite crystals. The skeleton contains 99% of all calcium and 85% of all phosphorus contained in the body. We can say that due to the significant reserves of calcium in the skeleton, it belongs to the macroelements, but at the level of the cytoplasm, where calcium is involved in regulatory processes, its concentration does not exceed 10-7 M, which makes it possible to classify it as a microelement.
The special role of calcium is the regulation of intracellular processes, the development of which is determined by changes in the intracellular calcium concentration:
- motor activity of cells (muscle contraction, chemotaxis, etc.);
- excitability of cells capable of generating an electrical action potential (muscle tone, heartbeat, etc.);
- secretion of hormones and neurotransmitters;
- phagocytosis, pinocytosis;
- cell reproduction (mitoses, etc.).
The system of intracellular regulation and “threshold action” of calcium is able to function only if the cell has effective mechanisms for maintaining a low concentration of calcium inside the cell and a huge electrochemical gradient in the plasma membranes. Maintaining a low calcium concentration inside the cell (10-7 M) in an environment with a relatively high calcium concentration (10-2 -10-3 M) is ensured by special biochemical mechanisms:
- the ability of the cytoplasmic membrane to resist the penetration of calcium ions into the cytoplasm;
- the presence in the membrane of a special Ca++ATPase, which actively pumps calcium ions out of the cell using ATP energy;
- the action of exchange transport in the membrane (removal of calcium ions from the cell in exchange for sodium entering the cell);
- high calcium-binding ability of a number of intracellular organelles (mitochondria), as well as specific calcium-binding proteins, which play the role of a buffer providing a low concentration of free calcium ions even in the case of an increased calcium flow into the cell (calmodulin - vitamin D, Ca-binding protein , troponin C and others)1.
Intracellular calcium homeostasis is closely related to its extracellular levels, which are also carefully controlled in the body. Plasma calcium concentration averages 2.5 mM. Approximately 40% of this amount is bound to proteins and 10% is bound to other ions, so that only about half of serum calcium is normally present in the form of ionized calcium, the concentration of which is carefully controlled not only inside the cell, but also in the extracellular environment. The constancy of the concentration of ionized calcium is explained by its important role in many biological processes, mainly in three target organs: the small intestine, bones, and kidneys. In bones, the processes of bone resorption and remodeling (osteoclasts)2 depend on the level of ionized calcium. The movement of calcium is controlled by three hormones: parathyroid hormone (PTH), the hormonal form of vitamin D-1,25(OH)2D3 - calcitriol and calcitonin, produced by the thyroid gland. All three hormones directly regulate blood calcium concentrations through a feedback mechanism. Of all three hormones, the most important role is played by parathyroid hormone, which increases the level of calcium in the blood, acting on all three target organs. Vitamin D metabolites are also involved in the regulation of bone mineralization. Calcitonin – suppresses bone destruction. This ability is used in the treatment of diseases with increased bone resorption - such? such as Paget's disease, osteoporosis and others. The hormone is secreted in response to high calcium concentrations in the blood (positive feedback).
What foods contain calcium?
Main sources of calcium: dairy products, soy and rice milk, dried fruits, nuts, seafood, carrots, garlic, kale, kale, dill, celery, green leafy vegetables, spinach, sesame seeds, sardines, salmon, soybeans, green beans, berries .
shutterstock.com
For better absorption, calcium should be consumed together with other microelements: phosphorus, as well as vitamins - C, D, B9. To do this, you need to plan your diet in a certain way, or take a complex of vitamins, but only after consulting a doctor. The specialist will determine the required dosage and tell you which microelements you need to take calcium with.
A combination of calcium, phosphorus and vitamin D is found in beef and fish liver, mackerel, herring, crab, shrimp, seaweed, yolks, and butter.
A cardiologist named foods that affect life expectancy
Pathologies of calcium metabolism
Pathogenetic mechanisms of calcium metabolism disorders can be divided into two groups: endocrine and energy-dependent. The first group includes conditions caused by dysfunction of the parathyroid glands, which play a role in regulating the level of calcium in the extracellular fluid. For parathyroid hormone, the target organs are bone tissue and kidneys. The second most important regulator of calcium metabolism is the thyroid hormone calcitonin, which maintains intracellular calcium homeostasis3. But estrogen and vitamin D are most directly involved in the metabolism of calcium in skin cells. With a decrease in the production of estrogen in women after menopause, the skin quickly ages and serious problems arise with calcium metabolism in the bones. On the other hand, limiting skin exposure to UV rays with age reduces the accumulation of vitamin D in the skin, which is formed through photobiogenesis that occurs in the skin under the influence of UV rays. Vitamin D also ensures skin resistance to UV rays. The hormonal form of vitamin D - 1.25 dihydroxycholecalciferol (calcitriol) stimulates the absorption of calcium and phosphates in the intestines and their reabsorption in the kidneys, stimulates bone remodeling, calcium transport through cell membranes, cell differentiation, and the development of the immune system. In addition to the hormones that regulate calcium metabolism (group I factors), the second group of factors produced in cells as a result of ATP transformation is of great importance in its regulation. This group of factors includes inorganic poly- and bisphosphates, which are products of normal energy metabolism in cells. With age, ATP production decreases significantly and, as a result, the formation of poly- and bisphosphates decreases, which leads to disturbances in calcium metabolism4.
Calcium: methods of production and chemical properties
Calcium Ca is an alkaline earth metal, silvery-white, ductile, and fairly hard. Reactive. Strong reducing agent.
Relative molecular weight Mr = 40.078; relative density for solid and liquid states d = 1.54; tmelt = 842º C; tboil = 1495º C.
1. As a result of electrolysis liquid calcium chloride, calcium and chlorine are formed
CaCl2 = Ca↓ + Cl2↑
2. Calcium chloride
interacts
with aluminum at 600 - 700º C forming calcium and aluminum chloride:
3CaCl2 + 2Al = 3Ca + 2AlCl3
3. As a result of the decomposition of calcium hydride at temperatures above 1000º C, calcium and hydrogen are formed:
CaH2 = Ca + H2
4. Calcium oxide reacts with aluminum at 1200º C and forms calcium and calcium aluminate:
4CaO + 2Al = 3Ca + Ca(AlO2)2
Calcium colors the flame of a gas burner brownish-red.
1. Calcium is a strong reducing agent . Therefore it reacts with almost all non-metals :
1.1. Calcium reacts with nitrogen at 200 - 450º C forming calcium nitride:
3Ca + N2 = Ca3N2
1.2. Calcium burns in oxygen (air) at above 300º C to form calcium oxide :
2Ca + O2 = 2CaO
1.3. Calcium reacts actively at temperatures of 200 - 400º C with chlorine, bromine and iodine
.
In this case, the corresponding salts
:
Ca + Br2 = CaBr2
Ca + Cl2 = CaCl2
Ca + I2 = CaI2
1.4. Calcium reacts with hydrogen calcium hydride :
Ca + H2 = CaH2
1.5. As a result of the interaction of calcium and fluorine at room temperature, calcium fluoride is formed:
Ca + F2 = CaF2
1.6. Calcium reacts with sulfur at 150º C and forms calcium sulfide:
Ca + S = CaS
1.7. As a result of the reaction between calcium and phosphorus at 350 - 450º C, calcium phosphide is formed:
3Ca + 2P = Ca3P2
1.8. Calcium interacts with carbon (graphite) at 550º C and forms calcium carbide:
Ca + 2C = CaC2
2. Calcium actively interacts with complex substances:
2.1. Calcium reacts with water . The interaction of calcium with water leads to the formation of calcium hydroxide hydrogen gas :
Ca + 2H2O = Ca(OH)2↓ + H2↑,
2.2. Calcium interacts with acids:
2.2.1. Calcium reacts with dilute hydrochloric acid to form calcium chloride and hydrogen:
Ca + 2HCl = CaCl2 + H2 ↑
2.2.2. Reacting with dilute nitric acid, calcium forms calcium nitrate, nitric oxide (I) and water:
4Ca + 10HNO3= 4Ca(NO3)2 + N2O↑ + 5H2O,
If nitric acid is further diluted, calcium nitrate, ammonium nitrate and water are formed:
4Ca + 10HNO3 = 4Ca(NO3)2 + NH4NO3 + 3H2O
2.3. Calcium reacts with ammonia gas at 600 - 650º C. As a result of this reaction, calcium nitride and calcium hydride are formed:
6Ca + 2NH3 = Ca3N2 + 3CaH2,
if ammonia is liquid, then as a result of the reaction in the presence of a platinum catalyst, calcium amide and hydrogen are formed:
Ca + 2NH3 = Ca(NH2)2↓ + H2↑
Pathological skin conditions caused by the deposition of calcium salts
In order to study the nature of the accumulation of calcium salts (mainly phosphates) in the skin depending on age, we examined histological samples of the skin of the anterior thigh in 8 people of different ages: 4 years, 9 years, 12 years, 30 years, 50 years, 60 years , 65 years old, 70 years old. In children, skin samples were taken incidentally during diagnostic skeletal muscle biopsy. The choice of place for skin biopsy is due to the virtual absence of exposure to the external environment (UV rays, chapping) and cosmetics. To assess the nature of the accumulation of calcium salts in the skin, the Kossa histochemical staining method was used. The biopsy material was fixed in strong alcohol, followed by the preparation of sections from paraffin blocks. Sections were stained with 5% silver nitrate for 60 minutes in the light, followed by treatment with a 5% hyposulfite solution, washing and embedding in paraffin. Analysis of the deposits of calcium salts, colored black, was carried out using a light microscope. The intensity and prevalence of calcium salt deposition in skin cells was assessed (Fig. 1, 2, 3).
Fig.1. Skin of a 12 year old child. Microconglomerates of calcium salts are detected in small quantities, mainly in the area of the basal (germinal) layer of the epidermis. Coloring according to Kossa, uv. 40x.
Fig.2. Skin of a 50 year old woman. A significant amount of microconglomerates of calcium salts is detected in the area of the basal (germ) layer of the epidermis. Coloring according to Kossa, uv. 40x.
Fig.3. Skin of a 70 year old woman. A sharp increase in microconglomerates of calcium salts in the area of the basal (germ) layer of the epidermis is detected. Coloring according to Kossa, uv. 40x.
The results of the analysis showed that the skin of children was characterized by a small amount of microconglomerates of calcium salts. Histological preparations were characterized by the presence of a small amount of diffuse dust-like inclusions, mainly in the basal layer of the epidermis. In older people, both the number and size of microconglomerates of calcium salts in the skin increased. The greatest degree of their accumulation was noted in the basal layer of the epidermis of 65- and 70-year-old people. In addition to accumulation in the basal layer, coarse microconglomerates of calcium salts were detected in the connective tissues of the papillary and reticular layers of the skin. In Fig. Figure 4 shows an increase in the number of calcium deposits in the skin, taking into account the nature of their deposits in the cell. In Fig. Figure 5 shows the total assessment in points of the intensity of filling human skin cells with calcium salts depending on age. When calculating the scores, the proportion and degree of filling of the germ zone cells with calcium deposits was taken into account.
Rice. 4. Changes in the nature and type of deposition of calcium salts in skin cells with age in humans.
Rice. 5. Increase in the amount of insoluble calcium salts with age (estimated in points).
Thus, it has been morphologically shown that with age, the degree of accumulation of calcium salts in the skin of people increases, maximum calcium deposits are found in elderly people, and the deposition of calcium salts mainly in the cells of the germinal layer of the epidermis contributes to the aging process of the skin, which intensifies with age.
Cause of osteoporosis
Calcium and phosphorus together form the mineral basis of the skeleton. In most cases, the cause of osteoporosis is a violation of calcium and phosphorus metabolism.
The most important link in providing the body with calcium is its sufficient absorption in the intestines, which is possible in the presence of at least three prerequisites: sufficient calcium in the diet; the body's supply of vitamin D, including its active metabolites; absence of diseases of the gastrointestinal tract with malabsorption. If there is a lack of calcium in the body, the latter is extracted from its own bone tissue. As a result, bone mass decreases and bones become brittle.
There are many factors for the development of osteoporosis. The division into primary and secondary osteoporosis is relative. Primary osteoporosis includes postmenopausal, senile (senile), and idiopathic osteoporosis. Secondary osteoporosis includes a decrease in bone mass as a result of genetic disorders, certain diseases of the endocrine system, rheumatism, circulatory system, kidneys, excessive alcohol intake, prolonged immobilization, taking medications, especially corticosteroids, immunosuppressants, as a result of mental disorders (anorexia nervosa) , as well as insufficient dietary intake or impaired absorption of certain nutrients in the intestine, primarily calcium and vitamin D.
The role of protein deficiency as an independent factor is debated. The most common causes of the development of osteoporosis are violations of the consumption of certain nutrients from foods, the postmenopausal period, senile syndrome, pathological metabolic disorders as a result of taking glucocorticosteroids, as well as excessive amounts of alcohol.
Recommended norms
Special studies have shown that women of all age groups consume significantly less calcium from food than recommended. Men of all ages consume more calcium than women, possibly due to their higher energy intake in general. Less than 15% of women under the age of 50 and less than 5% of women under the age of 70 consume foods with adequate calcium.
Low dietary calcium intake among adolescents is of particular concern because calcium deficiency coincides with a period of rapid skeletal growth. This is an opportune time to gain maximum peak bone mass and protect yourself from the future risk of osteoporosis. Approximately 90% of women's complete bone mineralization is achieved around age 17, 95% by age 20, and 99% by age 26. Consequently, the period for optimization of peak bone mass by calcium decreases rapidly after adolescence. It should be noted that current dietary recommendations for adequate calcium intake have been increased to 500 mg for children aged 1–3 years, 800 mg for children aged 4–8 years, 1300 mg for adolescents aged 9–18 years, 1000 mg for adults aged 18–60 years and 1200 mg for adults aged 60 years and older. Unfortunately, these dietary recommendations are not being followed.
Scientific evidence suggests that consuming adequate amounts of calcium or calcium-rich foods (milk and other dairy products) promotes peak bone mass before age 30 and earlier. This slows down age-related bone loss and reduces the risk of fractures later in life.
Individualization of diet therapy
The pathogenetic principle of individualization of diet therapy at the preparatory stage of treatment is as follows:
- Finding out the possible causes of impaired calcium absorption in the small intestine:
- malabsorption syndrome due to enteropathy (enteritis, involutional atrophy of the intestinal mucosa, chronic renal failure, etc.);
- food supply with vitamins D, C;
- the presence of acidic bases in food (citric, ascorbic, oxalic and some other acids);
- analysis of the consistency of hormonal regulation of calcium metabolism (hormones that regulate Ca metabolism in the body: parathyroid hormone, calcitonin, glucocorticosteroids, thyroid hormones, growth hormone, insulin, estrogens).
- Study of possible causes of excess calcium requirements in metabolic processes:
- arterial hypertension;
- increased excretion of Ca from the body (with urine, bile);
- diseases accompanied by an increased need for calcium (for example, colon tumors, hyperparathyroidism).
We should not forget that the etiopathogenetic variants of the development of osteoporosis are different. Therefore, approaches to drug therapy will also be different. This can be either hormone replacement therapy or treatment of the underlying disease. Diet therapy as an independent method of treatment is generally not used, but is used as a reliable support for drug therapy and for prevention.
Areas of diet therapy for calcium metabolism disorders:
- Water mineralization.
- Using foods rich in calcium.
- Activation of calcium absorption in the body.
- Dietary stimulation of gastric secretion, enzymatic activity of the pancreas.
- Restoration of disturbances in the absorption function of the small intestine.
- Prescribing diets taking into account possible enzymopathies (for example, lactase deficiency).
- Dietary correction of food intolerance.
More about calcium
In dietary therapy for osteoporosis, the main role is played by calcium and vitamin D, the use of which can weaken the process of osteoporosis progression, although there are many nutritional factors that affect bone development (proteins, vitamins and other minerals).
Information about dietary sources of calcium and factors affecting its bioavailability is important.
Food Sources of Calcium
The population receives more than half of the amount of calcium consumed from dairy products. Other sources include some green vegetables (broccoli, cabbage), nuts, calcium-precipitated bean curd, bone meal.
Calcium-fortified foods (juices and flours) may make a significant contribution to calcium intake in some people. In food products, calcium is contained mainly in the form of sparingly soluble salts (phosphates, carbonates, oxalates, etc.). The bioavailability of calcium from a number of non-dairy sources is insufficient. The list of foods high in calcium is presented in Table 1
Food Components that Increase the Bioavailability of Calcium
Lactose increases calcium absorption. Absorption also increases after the addition of lactase, which can be explained by the fact that the most metabolized milk sugar increases calcium absorption. These data were obtained for infants. It is unclear whether lactose improves the absorption of calcium from dairy products in adults? The higher prevalence of osteoporosis in people with lactose intolerance is likely due to low dairy intake rather than to the effect of lactose on calcium absorption.
Food components that reduce the bioavailability of calcium
Dietary fiber reduces calcium absorption. Replacing white flour (22 grams of dietary fiber per day) with whole wheat flour (53 grams of dietary fiber per day) in a regular diet causes a negative calcium balance even at higher calcium intakes.
Dietary fiber from fruits and vegetables has a similar effect on calcium absorption. Several fiber constituents bind calcium. Uronic acids bind calcium strongly in vitro. This is probably why hemicellulose inhibits calcium absorption. 80% of pectin uronic acids are methylated and cannot bind calcium. Therefore, pectin does not affect calcium absorption. In theory, a typical vegetarian diet contains enough uronic acids to bind 360 mg of calcium, but most of these acids are digested in the distal intestine, so some calcium is still absorbed. A balanced diet that contains moderate amounts of various fibers does not likely affect calcium absorption.
Table 1. Food sources of calcium
| Products | Volume | Calcium, mg |
| Milk and dairy products Milk (skimmed, whole, etc.) | 250 ml | 300 |
| Vanilla ice cream | 250 g | 208 |
| Vanilla milk | 250 ml | 283 |
| Yogurt (whole milk) | 250 g | 275 |
| Yogurt with low-fat milk additives | 250 g | 452 |
| Cheeses/ Dutch | 30 g | 195 |
| Cheddar | 30 g | 211 |
| Homemade, creamy | 30 g | 211 |
| Homemade, low-fat | 30 g | 138 |
| Cream cheese | 30 g | 23 |
| Parmesan | 1 spoon | 69 |
| Swiss | 30 g | 259 |
| Fish, seafood Shellfish (meat only) | 100 g | 88 |
| Oysters | 5–8 on average | 94 |
| Salmon, canned with bones | 100 g | 198 |
| Sardines, canned with bones | 100 g | 449 |
| Fruits Dried figs | 5 medium size | 126 |
| Orange | 1 medium size | 66 |
| Dried prunes | 10 large | 51 |
| Nuts, seeds Almonds or hazelnuts | 12–15 | 38 |
| Sesame* | 30 g | 38 |
| Sunflower seeds | 30 g | 34 |
| Vegetables/Tofu | 100 g | 128 |
| Gorbanzo beans | ½ cup | 80 |
| Spotted beans | ½ cup | 135 |
| Red beans, kidney-shaped | ½ cup | 110 |
| Broccoli, boiled | ⅔cup | 88 |
| Beetroot, boiled* | ½ cup | 61 |
| Cabbage (Brassica oleracia), boiled* | ½ cup | 152 |
| Fennel, raw | 100 g | 100 |
| Cabbage, boiled | ½ cup | 134 |
| Romaine lettuce | 3½ cups | 68 |
| Mustard greens, boiled* | ½ cup | 145 |
| Rutabaga, boiled | ½ cup | 59 |
| Seaweed, agar, raw | 100 g | 567 |
| Seaweed, kelp, raw | 100 g | 1,093 |
| Pumpkin | ½ medium size | 61 |
*Foods rich in oxalic acid, which slows down absorption.
Phytic acid is another plant component that binds calcium. The high phytin content of wheat bran explains its adverse effects on calcium absorption. Interestingly, adding calcium to wheat dough reduces phytin degradation by 50% during fermentation and baking. Wheat bran interferes with calcium absorption to such an extent that it has been used therapeutically for hypercalciuria.
Dark green, leafy vegetables often have relatively high calcium content. But the absorption of calcium from most vegetables is prevented by oxalic acid. Spinach, beet tops, and rhubarb are rich in it. Foods low in oxalic acid (cabbage, broccoli, turnips) are good sources of calcium. For example, calcium absorption from cabbage is almost as high as from milk.
Sodium increases urinary calcium excretion, so dietary salt intake should be reduced.
Want more new information on nutrition issues? Subscribe to the informational and practical magazine “Practical Dietetics”!
SUBSCRIBE
Caffeine and other drugs. Caffeine, found in coffee, tea, chocolate, cola and many over-the-counter medications, increases the amount of calcium lost in urine and feces. However, reasonable coffee consumption is a minor risk factor for osteoporosis. Up to 2 cups of black coffee per day may result in small urinary calcium excretion (up to 110 mg). This amount is easily covered by milk, which can be used with coffee, or calcium supplements.
The nicotine in tobacco reduces the body's ability to use calcium. Additionally, women who smoke tend to have lower estrogen levels and lower bone mass. It has been suggested that in smokers, estrogens are broken down more quickly in the liver and, as a result, fail to stimulate the secretion of enough calcitonin to prevent bone destruction.
Excessive alcohol consumption is a risk factor for osteoporosis and osteoporotic bone fractures. The development of osteoporosis is associated both with general metabolic disorders (malnutrition, liver cirrhosis, gastropathy, endocrine disorders) and with the direct effect of alcohol on bone tissue (a decrease in the volume of trabecular bone mass). Alcohol has been shown to have a direct toxic effect on osteocytes. Excessive alcohol consumption can also cause bone loss by impairing the absorption of calcium and vitamin D. Moderate alcohol consumption does not have a negative effect on bones.
Interaction between the absorption of calcium and other nutrients
Squirrels. The protein content in the diet of patients with osteoporosis should be at a physiological level, since protein deficiency leads to a negative nitrogen balance and a decrease in reparative processes. It is recommended to include in the diet specialized food products consisting of proteins with high biological value and digestibility (dry protein composite mixtures [GOST R 53861-2010]).
With an adequate amount of protein in the diet, about 15% of calcium is absorbed, with a low protein content - about 5%. Excessive dietary protein (especially purified amino acids) increases urinary calcium loss and causes a negative calcium balance; surprisingly, it does not result in a compensatory increase in the efficiency of intestinal calcium absorption. A pure vegetarian diet fails due to insufficient amounts of calcium and other nutrients. Adding dairy products to a vegetarian diet improves bone health in postmenopausal women.
If you have excess fat, you need to increase the calcium content in your diet. In the presence of fat malabsorption (steatorrhea), calcium precipitates with fatty acids, forming insoluble soaps in the intestinal lumen. A lack of fat in the diet can lead to a deficiency of fat-soluble vitamins, including vitamin D, which is necessary for the absorption of calcium.
Long-term, continuous dietary intake of large amounts of phosphorus leads to hyperparathyroidism and secondary bone resorption.
Since both calcium and iron are commonly recommended for women, the interactions between these supplements are interesting. In one study, calcium carbonate and hydroxyapatite supplementation reduced iron absorption by approximately 50% in postmenopausal women. In another study, milk calcium inhibited iron absorption by 30%. When taken additionally with food, calcium inhibits the absorption of iron from its preparations (ferrous sulfate), dietary non-heme and heme iron. But if calcium carbonate was taken without food, even in high doses it did not inhibit the absorption of iron from ferrous sulfate. Thus, the use of dietary calcium supplements significantly affects iron absorption. Calcium likely affects intracellular iron transfer by the enterocyte.
Essential calcium salts
In terms of content and completeness of absorption, the best sources of calcium are milk and dairy products. 100 mg of calcium is contained in 85–90 g of milk and fermented milk drinks (kefir, yogurt, etc.), 70–80 g of cottage cheese, 10–15 g of hard cheese, 20–25 g of processed cheese, 110–115 g of sour cream or cream, 70–75 g milk or cream ice cream. Thus, if the daily diet includes 0.5 liters of milk and fermented milk drinks, 50 g of cottage cheese and 10 g of hard cheese, then this provides more than half of the recommended calcium intake, and in an easily digestible form.
The calcium content in the green mass of plants is significantly lower than the content in dairy products. Therefore, dairy products are the main ones. But the daily amount of calcium is difficult to cover with food alone. In this regard, calcium salts are used for medicinal purposes. The calcium content in its various salts is presented in Table 2. According to some data, it is best to add calcium carbonate to food in small doses, according to others, it is preferable to take it in the evening or at night. Persons with achlorhydria are prescribed calcium citrate, which is better absorbed when gastric secretory activity is low.
It has been shown that additional use of calcium supplements in the diet leads to a decrease in bone loss in older women. The greatest effect was observed in those who consumed little calcium in their diet.
Calcium supplementation to women early after the onset of menopause slightly reduced bone loss at the radius and femoral neck, but not at the spine. An analysis of prospective studies of postmenopausal women found that adequate vitamin D intake reduced the risk of hip fractures associated with osteoporosis, and milk consumption and a high calcium diet did not affect the incidence of hip fractures. The authors emphasize the need for supplemental vitamin D or increased consumption of fatty fish.
Table 2. Calcium content (Ca) in its various salts (Smolyansky B.L., Liflyandsky V.G., 2004)
| Calcium salts | Content of the Ca element, in mg per 1000 mg of Ca salt | Calcium salts | Content of the Ca element, in mg per 1000 mg of Ca salt |
| Carbonate | 400 | Lactate | 130 |
| Chloride | 270 | Phosphate dibasic anhydride | 290 |
| Citrate | 200 | Phosphate dibasic dihydride | 230 |
| Gluconate | 90 | Tribasic phosphate | 400 |
| Glycerophosphate | 190 |
You can't do without vitamin D
Vitamin D deficiency in the diet or disorders of its metabolism are of great importance in the pathogenesis of many forms of osteoporosis, but especially senile osteoporosis. Vitamin D is necessary for the absorption of calcium in the intestines, as well as for its absorption by cells, including bone cells.
In medical practice, active metabolites of vitamin D3 (calcitriol, alpha-calcidol) are more often used. It is these metabolites that are widely used in the treatment of osteoporosis.
The best sources of vitamin D in the diet are fatty fish, liver, fish roe, milk fats, and eggs. Vitamin D deficiency is easily prevented by eating these foods and/or taking small doses of vitamin D supplements.
Both in the treatment of osteoporosis and for preventive purposes, all women after menopause and people of both sexes after 65 years are prescribed calcium supplements in combination with vitamin D. These recommendations are especially important for people who consume little or no dairy products due to personal tastes, diseases (lactase deficiency, food allergies, etc.), strictly vegetarian diet. These drugs are often considered as dietary supplements - nutraceuticals.
Table 3. Sample menu rich in calcium content (Bergman D., 1999)
| Products | Approximate calcium content, mg |
| Breakfast | |
| Orange (1 medium size) | 65 |
| Oatmeal (instant) | 170 |
| ½ cup skim milk | 75 |
| Beverages | |
| Lunch | |
| Turkey Sandwich | 260 |
| Swiss cheese (30 g) with whole grain bread | 50 |
| Apple | 10 |
| 1 cup skim milk | 300 |
| Snack | |
| 200 g fruit yoghurt (low-fat) with low-fat solids | 450 |
| Dinner | |
| Mushroom soup with chicken | 30 |
| Green salad with vinegar | 10 |
| Flounder fillet (100 g) | 25 |
| Broccoli(½ cup) | 90 |
| Boiled potatoes | 20 |
| Pear compote | 20 |
Other vitamins for osteoporosis
In recent years, soy products have been widely used in the treatment and prevention of osteoporosis. It is known that soy proteins contain isoflavones, which have estrogen-like effects. A number of studies have shown that the inclusion of soy products in postmenopausal women leads to a reduction in the incidence of bone fractures.
Experiments on animals revealed a negative effect on bone tissue from a deficiency of a number of vitamins (C, group B) and microelements (phosphorus, magnesium, zinc, copper, manganese, boron, silicon, strontium, fluorine). But a direct connection between the development of osteoporosis and a deficiency of these elements has not been found.
It has been established that vitamin K affects osteocalcin, which, being a modulator of osteoblasts, is involved in protein synthesis in bones. Low vitamin K intake is associated with low bone mineral density and an increased risk of hip fractures in women, but not in men. Therefore, taking vitamin K for osteoporosis may be important in cases of severe deficiency in the diet.
Prevent disease
Prevention of the development of osteoporosis should be carried out before the full bone mass is formed, and treatment - from the moment bone loss begins to be detected.
Early prevention should be carried out by adequate calcium supplementation, exercise and prevention of risk factors (smoking, excessive alcohol consumption, etc.). This is especially important in adolescence, when the bone gains mass. Individuals who have developed osteoporosis usually must rely on pharmacological interventions to maintain or improve bone health. Treatment of osteoporosis currently includes taking calcium supplements with vitamin D, estrogen, and calcitonin. Treatment in any case is carried out against the background of diet therapy.
Thus, all patients with osteoporosis who are not burdened with diseases requiring special dietary therapy should receive a rational, balanced diet with a physiological protein content in the diet, but with an increased content of calcium and vitamin D, including through special nutritional supplements and medications .
D. Bergman (1999) proposed a version of such a diet that supplies more than 1000 mg of calcium per day, and also meets the needs for other important minerals (Table 3).
Table 4 provides information on the effect of certain medications on calcium metabolism in the body.
Methods for artificial regulation of calcium metabolism in skin cells
The presented data substantiated the feasibility of including compounds in cosmetic creams that regulate calcium metabolism in skin cells as an additional means for preventing skin aging. The best effect among various chelating compounds capable of forming highly stable compounds with calcium was found when using bisphosphonates in the form of sodium-potassium salt5,6.
This compound is a synthetic analogue of inorganic bisphosphate, the advantages of which, compared to the natural regulator of cellular calcium metabolism, are its resistance to spontaneous and enzymatic hydrolysis. There are no enzymes in the human body that destroy this bisphosphonate, which makes it possible to obtain a regulatory effect on calcium metabolism when using small doses of the drug. When studying the pharmacokinetics of the drug administered through the skin, it was shown that it is absorbed through intact skin much better than from the gastrointestinal tract (3-5% compared to 0.5-1.0%, respectively). In addition, it was revealed that the drug enters the blood and is excreted in the urine within 36-48 hours. Maintaining the therapeutic concentration of the drug for 24-36 hours with transdermal administration provides a prolonged therapeutic effect, in contrast to the oral route of administration, in which a peak concentration in the blood is created within 3-5 hours, and by 10-12 hours after After a single dose, the drug is practically absent in the blood (urine)7.
We confirmed the calcium-regulating effect of bisphosphonate on the skin in an auto-experiment on skin biopsies of 2 women (40 and 60 years old), in whom a cream containing 0.5% of the drug was applied to the skin of the thigh on one side - once a day for 10 days. As a control, the skin of the thigh of the other leg was examined. The result shows that there are calcium deposits in the skin without the use of cream. In one case - in all cells in the form of single deposits in the apical region of the cells of the germ zone (40 years), which is equal to 1 point, and in the other (60 years) - 80% of the cells have deposits of calcium salts in the form of single deposits in the apical part of the germ zone zones and 40% of cells - in the form of circular deposits, which is equal to 2.4 points. In the skin of the thigh of both women after applying the cream, no deposits of calcium salts were found in the cells of the growth zone (Fig. 6a and 6b). The absence of deposits of calcium salts after treatment in the cells of the epidermal growth zones confirms the positive effect of the drug as a preventive agent for calcification of cells of the germ layer of the skin. When biopsy sections were stained with xydiphon (1% cobalt sulfate in an alkaline medium), it was shown that the drug accumulates precisely in the cells of the germinal (basal) layer of the epidermis, which most often undergo calcification.
Fig. 6. Skin of a 40-year-old woman before (a) and after (b) application of REFARM cream (Cossa stain for calcium, magnification 40x).
Thus, with age, the skin loses its ability to regenerate and, as a result, elasticity, not only as a result of changes in its organic components, but also, obviously, due to the increasing accumulation of insoluble calcium salts in the cells of the germ layer.
Important and necessary calcium: the normal content of the element in the body
Calcium is a common macronutrient in the human body[1]. The mineral is involved in many processes: from the formation of the skeleton to the transmission of nerve signals. At the same time, according to research, most of the population of our country consumes insufficient amounts of calcium[2]. In the article we will talk about what level of calcium should be normal, what threatens a lack of macronutrients and how to prevent the development of deficiency conditions.
What should your calcium level be?
Calcium is found in all cells of the human body. The main part of the mineral is concentrated in bone tissue: 99% (which is about 1.2–1.4 kg) falls on the structures of bones and teeth and about 1% circulates in the blood [3]. It is impossible to detect calcium deviations from the norm without special laboratory tests. In private diagnostic centers and public health facilities, calcium levels in urine, blood, hair and nails are checked.
To assess calcium metabolism, the level of calcium in the blood is usually determined. Research on other biomaterials is carried out less frequently. The level of total calcium in the blood varies depending on the age of the person (see table).
Table. Total calcium in the blood: reference values[4].
| Age | Calcium norm, mmol/l |
| Up to 10 days | 1,90–2,60 |
| Up to two years | 2,25–2,75 |
| Up to 12 years | 2,20–2,70 |
| Under 18 years old | 2,10–2,55 |
| Up to 60 years old | 2,15–2,50 |
| Up to 90 years old | 2,20–2,55 |
| Over 90 years | 2,05–2,40 |
Why does the body need calcium?
- Calcium is necessary for the formation of bones and tooth enamel and for their further maintenance throughout life[5].
- Maintains heart rhythm[6].
- Promotes normal transmission of nerve impulses.
- Takes part in blood clotting processes.
- Regulates the activity of enzymes and endocrine glands.
- Participates in the processes of muscle contraction and relaxation.
- Reduces vascular permeability.
- Participates in the regulation of the acid-base state of the body [7].
How much calcium do you need per day?
The norm of the mineral depends primarily on the age of the person. According to the methodological recommendations of the Federal Center for Hygiene and Epidemiology of Rospotrebnadzor, the daily calcium requirement for newborns (up to three months) is 400 mg, children from four to six months need 500 mg of the mineral, up to one year - 600 mg, from one to three years - 800 mg, from three to seven years - 900 mg, for children 7-11 years old 1100 mg of calcium is recommended, from 11 to 18 years old 1200 mg of the macroelement is recommended[8].
The calcium intake rate for an adult 18-59 years old is approximately 1000 mg per day, after 60 years - 1200 mg daily [9]. In women during pregnancy and lactation, the need for calcium increases. The mineral is necessary for the intrauterine development of the fetus and for the mineralization of the skeleton and growth of the child during breastfeeding - while a woman experiences a decrease in bone mass, which is restored after stopping breastfeeding[10]. In the second half of pregnancy, the daily requirement for the mineral is 1300 mg, and during breastfeeding (up to a year) - 1400 mg[11].
Important! Research shows that the level of calcium also depends on the level of vitamin D in the body. Calciferol promotes the absorption of calcium in the intestines and maintains the necessary level of the mineral in the blood[12]. Therefore, it is recommended to take calcium together with vitamin D. According to the clinical recommendations of the Ministry of Health of the Russian Federation, the daily requirement of vitamin D for people 18-50 years old is 600-800 IU, for older people - 800-1000 IU. During pregnancy, 800–1200 IU of calciferol daily is indicated[13].
What are the dangers of deviations from the norm?
Deviations in calcium levels from the norm, as a rule, do not make themselves felt immediately. At an early stage, calcium deficiency may be asymptomatic. In addition, the first pronounced signs of this condition cannot be called specific: increased fatigue, muscle weakness, and sleep disturbances. All this looks more like banal fatigue, and therefore almost no one pays attention to such signals, and the disease progresses. Over time, the deficiency negatively affects a person’s appearance: nails become brittle, hair becomes brittle, the skin takes on an unhealthy color and becomes dry, problems with teeth may appear, and posture is disturbed[14,15].
In more advanced cases, numbness of the fingers and convulsive syndrome occur - all this is caused by a disorder in the transmission of nerve impulses and intracellular signaling in muscle tissue [16,17]. A lack of macronutrients can cause a decrease in cognitive abilities [18]. Also, a lack of calcium leads to the destruction of bone tissue: the consequence of a long-term deficiency is almost always a decrease in bone mineral density, which further increases the risk of developing calcium deficiency diseases - osteoporosis, spondylosis, osteochondrosis, osteoarthrosis [19,20].
An excess of calcium is no less harmful than its deficiency. When the level of the mineral increases in the body, memory loss, depression, drowsiness, muscle and joint pain, itching, loss of appetite, and nausea may occur. Hypercalcemia can provoke kidney damage, seizures, arrhythmia, peptic ulcers and cholelithiasis and other disorders[21].
Excess calcium is still less common than deficiency[22]. As mentioned above, there is a widespread lack of calcium intake in our country. An unbalanced diet and insufficient consumption of foods high in macronutrients lead to calcium deficiency.
Important! The most physiological, but at the same time difficult way to maintain normal calcium levels is a balanced diet. The macroelement is found in dairy products, sesame, poppy seeds, soybeans, nuts (walnuts, hazelnuts, almonds), greens and vegetables, and so on [23].
According to the clinical recommendations of the Ministry of Health of the Russian Federation, if there is insufficient consumption of the mineral in food, it is recommended to take special calcium supplements to maintain the daily norm[24]. Let's look at several drugs that are on the market:
- “Calcium-D3 Nycomed Forte”[25] (number in GRLS - P N013355/01)[26]. This is a drug for the treatment and prevention of calcium and vitamin D3 deficiency, as well as osteoporosis and its complications. The active ingredients are calcium carbonate and colecalciferol. The product is available in the form of chewable tablets with lemon flavor. The average retail price of package No. 60 is 576 rubles, No. 120 is 767 rubles[27].
- "Calcium 600"[28]. This is a dietary supplement from Solgar® with calcium and vitamin D3. The additive is used as an additional source of nutrients. The manufacturer notes that the product is primarily recommended for people over 30 years of age. The price of a package (60 pieces) of “Calcium 600” from Solgar® is 904 rubles[29].
- “Calcemin® Advance”[30] (number in GRLS - P N015747/01)[31]. Suitable for the prevention and treatment of deficiency of calcium, vitamin D and some other nutrients, and can be used as part of complex therapy for osteoporosis. In addition to calcium and vitamin D, Calcemin® Advance contains other active ingredients: magnesium, zinc, copper, manganese, boron. The average retail price of Kalcemin® Advance package No. 30 is 522 rubles, No. 60 is 762 rubles and No. 120 is 1060 rubles[32].
Even a slight deviation of the level of calcium in the blood from the norm can negatively affect a person’s health. Therefore, it is important to maintain normal levels of the mineral in the body. With modern pharmaceutical drugs this is quite easy and convenient.
"Complivit® Calcium D3 Forte" is a preparation of calcium and vitamin D3 in the form of chewable tablets
It is used for the prevention and treatment of calcium and vitamin D3 deficiency, as well as in the complex treatment of osteoporosis and its complications.
The product regulates the exchange of calcium and phosphorus in the body (in bones, teeth, nails, hair, muscles). Reduces resorption (absorption) and increases bone density, enhances the absorption of calcium in the intestines and the reabsorption of phosphates in the kidneys, promotes the mineralization of bone and dental tissue. One tablet of Complivit® Calcium D3 Forte contains 500 mg of calcium and 400 IU of vitamin D3.
To compensate for the deficiency of calcium and vitamin D3, adults and children over 12 years old are recommended to take two tablets per day, children 3–12 years old - one tablet per day or as prescribed by a specialist.
The average retail price of Complivit® Calcium D3 Forte No. 30 is 260 rubles, No. 60 is 407 rubles, package No. 100 will cost an average of 537 rubles, package No. 120 - 576 rubles[33].
Complivit® Calcium D3 Forte should not be taken by children under three years of age, people with severe renal failure, high concentrations of calcium and vitamin D in the body, and in some other cases. More information about contraindications can be found in the instructions.
* The registration certificate number of the drug in the GRLS is LSR-008769/09 dated November 2, 2009[34].
** All remedies given in the article have contraindications. Before using them, you should consult a doctor.
*** The material is not a public offer. Information on the cost of calcium supplements is presented for informational purposes only.
All information related to health and medicine is presented for informational purposes only and is not a reason for self-diagnosis or self-medication.
Study of the clinical effectiveness of cosmetic creams containing xidifon
The use of creams containing bisphosphonate has a therapeutic and preventive effect, preventing calcium deposits in skin cells, which, in combination with the preventive effect of the nourishing base of the cream, suggests an increase in the anti-aging effect of cosmetics. In this regard, this part of the work is devoted to an experimental and clinical study of the biological activity of creams produced by the REPHARM Institute of Pharmaceutical Reagents. In the experiment, the following was carried out:
- Histological examination of skin biopsies of white rats after 30 applications.
- Studying the effect of creams on the state of the protective function of the skin of laboratory animals using the method of determining the time of alkali neutralization according to the Burghart method.
- Clinical assessment of the state of functional indicators of the skin under the influence of the presented creams included:
- Study of the moisture-holding capacity of the skin;
- Study of the state of lipid metabolism of the skin;
- Study of skin elasticity;
- Study of skin profile (width and depth of wrinkles).
The following results were obtained:
- A histological study of animal skin preparations treated with REPHARM creams revealed their effect on water-electrolyte and protein metabolism in the skin, manifested in an increase in the hydration properties of the skin.
- A study of the effect of creams on the state of the protective function of the skin showed that in group 1 of animals, i.e. in rats that received the cream, restoration of the initial time of neutralization of alkali by the skin (6 minutes) occurred after 2 days. After 3 days, this indicator decreased and amounted to 4 minutes, i.e. a prolonging effect of the drug was noted. In group 2 of animals, i.e. in rats on whose skin the cream was not applied, restoration of the initial time of alkali neutralization occurred only on the 7th day after cessation of exposure to alkali, i.e. almost 2.5 times slower than in rats of group 2. Thus, the results obtained indicated a pronounced positive effect of the cream on the condition of one of the main functions of the skin - protective.
- The clinical trials involved 20 volunteers - clinically healthy women without a significant allergic history, aged 35 to 50 years. 11 patients were diagnosed with combination facial skin, 9 with dry skin complicated by hyperkeratosis. Clinically, this manifested itself in dry skin and the presence of a network of fine wrinkles. The cream was applied to previously cleansed skin of the face and body every day, 2 times a day for 3 weeks.
The study of the effect of creams on the moisture-holding capacity of the skin was carried out using the Moisture Meter device, Russia. 20 minutes after the first application of the cream, the moisture content in the skin increased by an average of 67.3% compared to the initial level, after 2 hours this figure was 51.5%, after 4 hours - 36.6%, after a course of use by 15 .6%.
The data obtained indicated a positive effect of the cream on water-electrolyte metabolism in the skin and a pronounced hydration effect of the drug.
When studying the effect of creams on the elastic properties of the skin, it was found that the stretching indicator as a result of a course of cream use increased by an average of 10.9% compared to the initial one.
When studying the skin profile (width and depth of wrinkles) using the Tester T-2000 device, it was found that as a result of 3 weeks of use of the cream, there was a decrease in the Rt tooth by 5.09%, Rmax by 6.7%, Rz-d by 6.3% compared to the initial level, i.e. a positive effect of the cream on indicators characterizing the skin profile was stated.
Based on the analysis of the formulation and the results of the research, we can conclude that REFARM cosmetic creams, which include a calcium metabolism regulator, are effective cosmetics, the use of which has a pronounced hydrating effect, improves the elasticity and tone of the skin, smoothes out a network of fine wrinkles, improves the protective function of the skin.
These cosmetics can be recommended for use in broad cosmetology practice to care for the body's skin as an effective means of preventing and correcting skin aging processes.
Application
The main use of calcium metal is as a reducing agent in the production of metals, especially nickel, copper and stainless steel. Calcium and its hydride are also used to produce difficult-to-reduce metals such as chromium, thorium and uranium. Alloys of calcium and lead are used in some types of batteries and in the production of bearings. Calcium granules are also used to remove traces of air from vacuum devices. Pure metallic calcium is widely used in metallothermy for the production of rare earth elements.
Calcium is widely used in metallurgy for the deoxidation of steel, along with aluminum or in combination with it. Extra-furnace processing with calcium-containing wires occupies a leading position due to the multifactorial influence of calcium on the physico-chemical state of the melt, the macro- and microstructure of the metal, the quality and properties of metal products and is an integral part of steel production technology. In modern metallurgy, injection wire is used to introduce calcium into the melt, which is calcium (sometimes silicocalcium or aluminocalcium) in the form of powder or pressed metal in a steel sheath. Along with deoxidation (removal of oxygen dissolved in steel), the use of calcium makes it possible to obtain non-metallic inclusions that are favorable in nature, composition and shape, and are not destroyed during further technological operations.
The 48Ca isotope is one of the effective and most commonly used materials for the production of superheavy elements and the discovery of new elements on the periodic table. This is because calcium-48 is a doubly magic nucleus, so its stability allows it to be sufficiently neutron-rich for a light nucleus; the synthesis of superheavy nuclei requires an excess of neutrons.
Literature
- VC. Bauman, “Biochemistry and physiology of vitamin D” Riga, Zinatye, 1989, 480 p.
- Fleisch, Bisphosphonates in bone disease, 1997, London, 184p.
- Feldman, D., and Mallon, P. J. and Gross, C. (1996). Vitamin D: metabolism and action.In Marcus, R., Feldman, D. and Kelsey, J. (eds) Osteoporosis, pp.205-35. (San Diego: Academic Press).
- Bushinsky, D. A. and Krieger, N. S. (1992). Integration of calcium metabolism in the adult. In Coe, FL and Favus, MJ (eds.) Disorders of Bone and Mineral Metabolism, pp.417-32. (New York: Raven Press).
- O.G.Arkhipova, E.A. Yuryeva, N.M. Dyatlova, “Prospects for the use of xydiphone”, J. Vses. chem. total them. DI. Mendeleeva, 1984, XXIX, 3, pp. 76-80.
- T.A. Matkovskaya, N.P. Tatarnikova, etc. Pat. RF RU 2124881 C1.
- E.A. Yuryeva, I.P. Dunaeva, G.I. Kulakova “The effectiveness of xydiphone depending on the method of its use”, In: New chelating agent xydiphone. Pharmacology, toxicology and therapy. Moscow, 1990, pp. 62-70.
(The article is abbreviated. The full text was published in the journal: Cosmetics and Medicine, 1999, No. 5/6, p. 71.)
The role of diet in preventing osteoporosis
Because osteoporosis usually progresses to a clinically advanced stage before its effects become apparent, preventing bone loss is the single best way to avoid the possibility of fractures and resulting disability. Although increasing calcium in the diet is one of the strategies most often proposed, many studies have failed to show a clear relationship between dietary absorption of this mineral and bone density. Consumption of calcium and vitamin D-rich foods is likely more important during the bone-growing years before peak mass is reached rather than later in adulthood.
Dairy products contain more than 70% of the calcium in the diet of the “average” resident of our country. The calcium found in these foods is quickly absorbed because these foods contain lactose and some are fortified with vitamin D.
Vegetarians who avoid animal foods can get calcium from soy milk, tofu (soy curd) processed with calcium sulfate, grains (especially unrefined or slightly refined grains), some green leafy vegetables (cabbage, turnips), beans and nuts. Some types of currently popular mineral drinks and tap water also contain calcium, the amount of calcium varies depending on the source.
Supplement Efficacy
Due to widespread and questionable advertising linking dietary calcium to osteoporosis, this mineral has been added to many products, including orange juice, soda, and bread products.
Calcium supplements have also become popular, but their value as a source of calcium remains controversial. Supplements vary in absorption capacity, and many people take them incorrectly. For example, until recently, calcium supplements came in the form of large, difficult-to-take pills. Supplement manufacturers have reduced the size of the pills to make them easier to swallow, but they may have compressed the pills so much that they do not dissolve in the stomach and pass intact through the gastrointestinal tract.
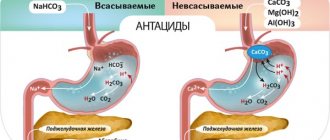
Calcium supplements vary in the percentage of the mineral in each dose and their effect on the body. Among the most commonly used compounds are calcium carbonate (Kalmagin, Calcium D3 Nycomed, etc.) and calcium citrate (Citrical, etc.). Calcium carbonate contains up to 40% pure calcium. It reacts with hydrochloric acid in the stomach to form calcium chloride, a highly soluble and available compound.
Undesirable effects of large amounts of calcium carbonate include flatulence, nausea and constipation. Excessive use of supplements may also cause excess hydrochloric acid due to stimulation of gastric secretion; The possibility of developing kidney stones has also been reported. Calcium carbonate may reduce the absorption of medications such as aspirin, tetracycline, atenolol, and ferrous sulfate if the supplement and these medications are taken together. Antacids containing both calcium carbonate and aluminum actually block calcium absorption.
Calcium citrate contains less calcium, approximately 24%. This compound does not require hydrochloric acid to dissolve, making it more suitable for those who are deficient in this component of gastric juice. This condition, known as achlorhydria, is quite common in old age.
In addition to the evidence regarding the potential of calcium supplements to cause gastrointestinal problems, there is other evidence that casts doubt on their value. Calcium supplements alone have little beneficial effect on postmenopausal bone mineral loss. They may slow compact bone loss but are not very effective in preventing trabecular bone loss.
Additionally, medications that also contain vitamin D may cause toxic accumulation of this fat-soluble vitamin if taken in excess amounts. Bone meal and dolomite contain calcium and are relatively inexpensive, but they also contain toxins such as lead and should be avoided.
Typical mistakes in the diet of patients with osteoporosis:
- insufficient calcium in the diet;
- excessive amounts of dietary fiber, phytic and oxalic acid, which impair the absorption of calcium;
- protein deficiency in the diet, leading to a negative nitrogen balance and a decrease in reparative processes;
- too much protein in the diet, which contributes to increased excretion of calcium in the urine (for every 50 g of protein in excess of the norm, 60 mg of calcium is lost in the urine);
- excess carbohydrates in the diet, which also leads to increased loss of calcium in the urine;
- excess phosphorus in the diet, which impairs calcium absorption;
- abuse of alcohol and drinks with a high caffeine content (coffee, strong tea, cola, chocolate), which increases the loss of calcium in urine and feces;
- too much sodium, leading to loss of calcium in the urine;
- excessive (less often - insufficient) energy value of the diet;
- deficiency of vitamin D in the diet and insufficient insolation necessary for endogenous synthesis of the vitamin;
- lack of fat in the diet, which leads to impaired absorption of all fat-soluble vitamins, including vitamin D;
- underestimation of the role of dietary supplements and preparations containing calcium and vitamin D for the prevention and treatment of osteoporosis.
Sources of calcium
Strong base
Women & calcium
- Calcium-rich foods have been clinically proven to help relieve symptoms associated with PMS.
- 5% bone mass is lost annually at the onset of menopause. At this time, the body rapidly reduces the production of estrogen, which promotes the accumulation of calcium in the bones.
- 14 million people in Russia suffer from osteoporosis, a disease in which bones become porous and brittle. Of these, 80% are women.
Calcium is the main component of the inorganic basis of the skeleton, and it is needed to keep bones and teeth strong and healthy. However, this macronutrient is also necessary for:
- regulation of the blood clotting process;
- ensuring muscle activity, including myocardial contractions;
- nervous system;
- important intracellular and intercellular processes;
- proper functioning of enzymes and hormones.
In order for all these mechanisms to work without failure, you should provide yourself with the necessary amount of calcium.
Source of calcium
White chalk
There is an opinion that the most reliable sources of calcium are chalk and eggshells. They do have this macronutrient, but mainly in the form of an insoluble salt - calcium carbonate, which is practically not absorbed.
The most reliable source of calcium is dairy products. In them, this element is in the form of lactate, which is easily absorbed and almost all of it goes to its intended purpose. One glass of milk covers almost a third of the daily calcium requirement, and 100 g of cheddar cheese satisfies it completely. If you want to reduce your fat intake, you can switch to skim milk - it contains no less calcium than regular milk.
By the way
If you do not tolerate lactose well, lean on sesame seeds rich in calcium: 100 g is the daily requirement of this useful element.
Poorly learned
60% Calcium from food is absorbed by the body of children and adolescents. And only 20% are adults over 21 years old.
In combination with certain substances, for example, oxalic and phytic acids and sugar, calcium is absorbed with great difficulty. Oxalic acid is found in calcium-rich chard, spinach and broccoli, and black tea that does not contain it, phytic acid is found in legumes, oatmeal and bran. So it turns out that cottage cheese with sugar, tea with cream or oatmeal with milk are tasty dishes, but from the point of view of calcium absorption they are rather stupid.
By the way
Caffeine interferes with calcium absorption. 2-3 cups of coffee a day won't hurt, but if that's not enough for you, drink at least half a glass of milk for every additional cup.
Keep balance
Vitamin D is necessary for the absorption of calcium. It helps transport this macronutrient into the blood. Most of vitamin D is formed in the skin under the influence of sunlight, a smaller part comes from food: fatty herring, mackerel, salmon from a can, egg yolk, butter. Don't give up these foods and get out in the sun as often as possible.
By the way
If there is a deficiency of magnesium and phosphorus, the calcium eaten will be useless. To prevent this from happening, eat more foods that contain these elements in the correct proportions. Ideal in this regard are cottage cheese, eggs, fresh herbs and some types of fish (for example, horse mackerel).
Author: Anna Fedorova Published: March 15, 2016