Compound
| Nasal drops | 10 ml |
| active substance: | |
| naphazoline nitrate | 0.005 g |
| 0.01 g | |
| excipients: ethylenediamine; boric acid; methyl parahydroxybenzoate; water |
| Nasal spray | 10 ml |
| active substance: | |
| naphazoline nitrate | 0.01 g |
| excipients: ethylenediamine - qs (about 0.4 mg); boric acid - 0.17 g; methyl parahydroxybenzoate - 0.01 g; water - up to 10 ml |
Sanorin 0.05% 10 ml drops called.
APPROVED by the Order of the Chairman of the Committee for Control of Medical and Pharmaceutical Activities of the Ministry of Health and Social Development of the Republic of Kazakhstan dated "____"________201__ No. _______ Instructions for the medical use of the drug SANORIN Trade name Sanorin International nonproprietary name Naphazolin Dosage form Nasal drops 0.1%, 0.05% Composition 1 ml of solution contains the active substance - naphazoline nitrate 0.001 g, 0.0005 g excipients: boric acid, ethylenediamine, methylparaben, purified water. Description Colorless, transparent, odorless solution. Pharmacotherapeutic group: Nasal preparations. Anticongestants and other nasal preparations for topical use. Sympathomimetics. Naphazoline. ATC code R01AA08 Pharmacological properties Pharmacokinetics When applied topically, the vasoconstrictor effect occurs within 5-10 minutes and lasts from 2 to 6 hours. Absorbed into the systemic circulation and may have systemic effects. Pharmacodynamics Sanorin contains naphazoline, which is a systemic sympathomimetic acting on alpha-adrenergic receptors. It has a pronounced and long-lasting vasoconstrictive effect on the vessels of the mucous membranes. When administered intranasally, naphazoline constricts blood vessels, reduces exudation, thus facilitating nasal breathing and reducing swelling of the mucous membrane during inflammation of the upper respiratory tract. Indications for use - acute rhinitis - sinusitis - inflammation of the Eustachian tubes - inflammation of the middle ear - to relieve swelling of the nasal mucosa during diagnostic and therapeutic interventions Method of administration and dosage 0.1% solution Adults and adolescents over 15 years old - 1-3 drops of solution in each nasal passage, 2-3 times a day, with an interval of at least 4 hours. Use for 5-6 days. Solution 0.05% Children from 3 to 6 years old - 1-2 drops of 0.05% solution Children from 6 to 15 years old - 2 drops of 0.05% solution 2-3 times a day, with an interval of at least 4 hours. Use no more than 3 days. In case of bleeding from the front of the nose, you can insert a tampon soaked in a 0.05% Sanorin solution. For diagnostic purposes before nasal endoscopy (for example, for the diagnosis and treatment of nasal polyps), instill 3 - 4 drops of the drug into each nostril after cleaning the nose or insert a tampon soaked in a solution of Sanorin 0.05% for 1 - 2 minutes. The drug is instilled into each nostril, the head should be tilted back. When instilling into the left nostril, it is advisable to slightly turn your head to the left; when instilling into the right nostril, turn your head to the right. Side effects When used in recommended doses, the drug is usually well tolerated. Rarely: - nausea, headache, nervousness, tremor - tachycardia, increased blood pressure, palpitations - reactive hyperemia, increased sweating - burning sensation or dryness in the nose - swelling of the nasal mucosa (when used for more than 7 days) - atrophic rhinitis Very rare : - intense nasal congestion, after the effect of the drug wears off. Too frequent use can lead to dependence, accompanied by intense swelling of the mucous membrane, occurring within a relatively short time after use. Long-term use of the drug can lead to disruption of the epithelium of the mucous membrane, inhibition of ciliary activity and lead to irreversible damage to the mucous membrane and the development of dry rhinitis. Contraindications - hypersensitivity to the active substances or to the auxiliary components of the drug - dry rhinitis - children under 3 years of age for a 0.05% solution - children under 15 years of age for a 0.1% solution - diabetes mellitus - severe atherosclerosis - thyrotoxicosis Drug interactions Drug should not be used simultaneously with monoamine oxidase inhibitors, tricyclic antidepressants and maprotiline, as heart rhythm disturbances and increased blood pressure may occur. Special instructions The drug should be prescribed with great caution in diseases of the cardiovascular system (hypertension, coronary heart disease), pheochromocytoma or with potentially hypertensive drugs. With long-term use, the severity of the vasoconstrictor effect gradually decreases (the phenomenon of tachyphylaxis), and therefore it is recommended to use the drug for no more than 5 days. Caution should be exercised during general anesthesia with anesthetics that increase the sensitivity of the myocardium to sympathomimetics (for example, halothane) in patients with bronchial asthma. Long-term use and overdose should be avoided. Long-term use of drugs intended to relieve swelling of the mucous membrane can lead to swelling and subsequent atrophy of the nasal mucosa. This medicine contains methylparaben, which may cause allergic reactions (possibly delayed). Pregnancy and lactation There is insufficient information about the ability of naphazoline to cross the placenta and be excreted in breast milk. Therefore, it is necessary to consider the potential risks and benefits of treatment before administering the drug to pregnant or lactating women, prescribing the drug only in cases of urgent need. Features of the effect of the drug on the ability to drive a vehicle or potentially dangerous mechanisms. The drug does not affect the ability to drive vehicles or drive potentially dangerous machinery. Overdose Symptoms: Accidental consumption of the drug may lead to systemic adverse reactions such as nervousness, increased sweating, headache, tremor, tachycardia, palpitations or hypertension. Possible symptoms of overdose include nausea, cyanosis, fever, convulsions, cardiac arrest, pulmonary edema and respiratory or psychiatric problems. In addition, depression of the central nervous system may be noticed, accompanied by drowsiness, decreased body temperature, bradycardia, sweating, shock-like hypotension, apnea, or coma. Treatment: symptomatic. The risk of overdose is greater in children because they are more susceptible to adverse effects than adults. Release form and packaging 10 ml in a brown glass bottle, equipped with a dropper with a polyethylene cap with first opening control. Each bottle, equipped with a label, along with instructions for medical use in the state and Russian languages, is placed in a cardboard box. Storage conditions Store in a place protected from light, at a temperature from 10˚С to +25˚С. Protect from freezing. Keep out of the reach of children! Shelf life 3 years for 0.05% drops. 4 years for 0.1% drops. Do not use after the expiration date. Conditions for dispensing from pharmacies Without a prescription, Czech Republic "Teva Czech Enterprises s.r.o.", Czech Republic Name and country of the owner of the registration certificate "Teva Pharmaceutical Industries Ltd", Israel "Teva Pharmaceutical Enterprises Ltd", Israel Address of the organization receiving the territory of the Republic of Kazakhstan, claims from consumers regarding the quality of products (products): Ratiopharm Kazakhstan LLP 050000 Republic of Kazakhstan Almaty, Al-Farabi Avenue 19, Nurly Tau Business Center 1 B, office 603 Telephone, fax; 311-07-34 E-mail
Directions for use and doses
Intranasally.
Adults and children over 15 years of age: 1–3 drops or 1–3 doses of a 0.1% solution of Sanorin (drops or spray) in each nasal passage 3–4 times a day.
Children from 2 to 15 years old: 1-2 drops of a 0.05% solution of the drug Sanorin in each nasal passage 2-3 times a day with an interval of at least 4 hours.
Use short-term, no more than 1 week in adults and no more than 3 days in children. If nasal breathing becomes easier, the use of Sanorin can be stopped earlier. Repeated use is possible after a few days.
In case of nosebleeds, you can place a cotton swab moistened with a 0.05% solution of the drug into the nasal passage.
During rhinoscopy to prolong surface anesthesia: 2–4 drops of a 0.1% solution with 1 ml of anesthetic.
The drug Sanorin, drops, is instilled into each nasal passage with the head tilted back slightly. When instilling into the left nasal passage, you should tilt your head to the right, and when instilling into the right nasal passage, tilt your head to the left.
When using the drug Sanorin, spray for the first time, it is recommended to press the dosing device several times until a compact cloud of aerosol appears. Before direct use, remove the protective cap, keep the bottle in an upright position, insert the end part of the dosing device into the nasal passage, and then quickly and sharply press the applicator. Immediately after injection, it is recommended to inhale lightly through your nose. After using the drug, the applicator must be closed with a protective cap.
Sanorin spray naz 0.05% 10ml for children
Compound
Active substance: naphazoline nitrate 0.005 mg. Excipients: boric acid - 0.17 g, methyl parahydroxybenzoate - 0.01 g, ethylenediamine qs (about 0.0004 g), purified water - up to 10 ml.
Pharmacokinetics
There are no data on the distribution, metabolism and elimination of naphazoline in humans.
Indications for use
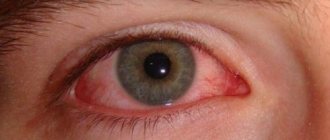
- Acute rhinitis of various etiologies;
- otitis media - as an additional means to reduce swelling of the nasopharyngeal mucosa;
- sinusitis;
- eustachitis;
- laryngitis;
- to reduce swelling of the mucous membranes of the nose, nasopharynx and paranasal sinuses during diagnostic and therapeutic procedures;
- if necessary to stop nosebleeds.
Contraindications
- Hypersensitivity to the components of the drug;
- chronic rhinitis;
- atrophic rhinitis;
- angle-closure glaucoma;
- severe eye diseases;
- arterial hypertension;
- severe atherosclerosis;
- tachycardia;
- hyperthyroidism;
- diabetes;
- simultaneous use of monoamine oxidase inhibitors and a period of up to 14 days after the end of their use.
- Sanorin 0.05% solution is contraindicated in children under 2 years of age.
With caution Pregnancy, lactation, coronary heart disease (angina), prostatic hyperplasia, pheochromocytoma.
Directions for use and doses
Intranasally.
Adults and children over 15 years of age: 1-3 drops of 0.1% Sanorin solution in each nasal passage 3-4 times a day.
Children 2-15 years old: 1-2 drops of 0.05% Sanorin solution in each nasal passage 2-3 times a day with an interval of at least 4 hours.
Use short-term, no more than 1 week in adults and no more than 3 days in children.
If nasal breathing becomes easier, then the use of Sanorin can be stopped earlier. Repeated use of the drug is possible after a few days. In case of nosebleeds, you can place a cotton swab moistened with a 0.05% Sanorin solution into the nasal passage.
During rhinoscopy to prolong surface anesthesia: 2-4 drops of a 0.1% solution with 1 ml of anesthetic.
The drug is instilled into each nasal passage with the head tilted back slightly. When instilling into the left nasal passage, you should tilt your head to the right, and when instilling into the right nasal passage, tilt your head to the left.
Storage conditions
Store at a temperature of 10 to 25°C, protected from light. Do not freeze. Keep out of the reach of children.
Best before date
3 years. Do not use after expiration date.
special instructions
Caution should be exercised when performing general anesthesia with the use of anesthetics that increase the sensitivity of the myocardium to sympathomimetics (halothane). In rare cases, rebound hyperemia and swelling of the nasal mucosa develops. May have a resorptive effect. As a result of irritation of the sympathetic nervous system and the general effect on the body, nausea, tachycardia, headache, irritability, increased sweating, allergic reactions, rash, and increased blood pressure are extremely rare.
Description
Anticongestant - alpha-adrenergic agonist.
Use in children
Allowed from 2 years.
Pharmacodynamics
Naphazoline is an alpha2-adrenergic agonist with a direct stimulating effect on alpha-adrenergic receptors of the sympathetic nervous system.
When administered intranasally, it has a rapid, pronounced and long-lasting vasoconstrictor effect on the vessels of the mucous membrane of the nasal cavity, nasopharynx and paranasal sinuses - it reduces swelling and hyperemia, thereby improving the patency of the nasal passages and facilitating nasal breathing.
Along with this, the patency of the Eustachian tubes is restored.
The therapeutic effect usually occurs within 5 minutes after administration of the drug and lasts for 4-6 hours.
Side effects
Local reactions: irritation of the mucous membrane (depending on the place of application); when used for more than 1 week - swelling of the mucous membrane, atrophic rhinitis.
From the cardiovascular system: reactive hyperemia, tachycardia, increased blood pressure.
From the side of the central nervous system: headache.
From the digestive system: nausea.
Use during pregnancy and breastfeeding
The use of naphazoline during pregnancy and lactation (breastfeeding) is possible only according to strict indications in cases where the expected therapeutic effect for the mother exceeds the potential risk of side effects in the fetus or child.
Interaction
When using the drug simultaneously with MAO inhibitors or tricyclic antidepressants (and within 14 days after stopping their use), an increase in blood pressure is possible, which is due to the release of deposited catecholamines under the influence of naphazoline. Therefore, the use of the drug Sanorin simultaneously with MAO inhibitors and within 14 days after their discontinuation is contraindicated.
Naphazoline slows down the absorption of local anesthetics, which leads to an increase in the duration of their action.
Overdose
Symptoms
Nervousness, increased sweating, headache, tremor, tachycardia, palpitations, increased blood pressure, nausea, cyanosis, fever, convulsions, cardiac arrest, pulmonary edema, respiratory and mental disorders are also possible. In case of depression of the central nervous system, bradycardia, weakness, drowsiness, decreased body temperature, increased sweating, collapse, apnea and coma are observed.
Treatment
Discontinuation of the drug, symptomatic therapy.
Children are more vulnerable to the side effects of the drug, so the risk of overdose is higher in children than in adults.
Release form
Nasal spray [for children], 0.05%.
Impact on the ability to drive vehicles and operate machinery
The drug does not affect or slightly affects the ability to drive vehicles and other potentially hazardous activities that require increased concentration and speed of psychomotor reactions, taking into account the side effect profile.