Perinatal encephalopathy - symptoms and treatment
Treatment of perinatal pathology in the acute period is carried out in the neonatology department.
Surgical treatment may be necessary if the child has a large hematoma inside the cranial cavity; its removal is only possible through surgery.
With increasing closed hydrocephalus, in order to avoid brain atrophy from compression by fluid, children undergo shunt surgery - a shunt is installed (a plastic tube through which excess fluid from the cranial cavity is drained into the abdominal cavity and absorbed there).
However, most parents are faced with the need to treat a child with perinatal encephalopathy during the recovery period of up to 1 year due to impaired muscle tone, developmental delay, hypertensive-hydrocephalic syndrome and other manifestations.
The greatest effect can be achieved using an integrated treatment approach.
Therapeutic medical massage is carried out to normalize muscle tone and improve trophic processes in tissues. Should only be performed by a massage therapist with medical training and experience working with children with perinatal brain damage. The procedure is carried out in children's clinics and rehabilitation centers [3][5][7].
Exercise therapy - physical therapy. May include various types:
- Vojta therapy;
- Bobat-terpiya;
- therapeutic exercises on balls;
- mechanotherapy (exercise exercises on a simulator);
- classes with an instructor in the swimming pool.
Exercise therapy should only be carried out by an instructor with special education [6][7][18].
Microcurrent reflexology (MCRT) is a new medical technology approved by the Ministry of Health of the Russian Federation and recommended for the treatment of children with organic brain damage, delayed motor, speech, mental development and cerebral palsy. Treatment is carried out on an outpatient basis in rehabilitation centers in various regions of the Russian Federation.
The therapeutic effect is physiological and painless, using currents in the microampere range on neuroreflex zones in various areas of the skin. Microcurrents are 10 times less than with standard physiotherapy. Treatment is carried out according to an individual scheme, taking into account all the manifestations of perinatal encephalopathy present in the child.
During the treatment process, normal reflex activity of the brain is restored, muscle tone is normalized: spastic (tense) muscles are relaxed, hypotonic (weak) muscles are stimulated. MPRT stabilizes cerebral vascular tone, which allows compensation of intracranial pressure.
The method promotes the acquisition of new motor skills by restoring the activity of neurons in various areas of the cerebral cortex and cerebellum affected by hypoxia and hydrocephalus [4][6][9][14][15].
Medicines of the nootropic group (Cortexin, Pantogam, Ceraxon, etc.) should be prescribed only by a pediatric neurologist strictly according to indications, i.e., if the child has delayed psychomotor development and there are no contraindications [11][16].
diuretics only when intracranial pressure increases (expansion of the cerebrospinal fluid spaces on the NSG, the presence of clinical manifestations of hypertensive-hydrocephalic syndrome).
Pediatric neurologists, as a rule, prescribe the diuretic drug Diacarb, which also has an additional effect on the choroid plexuses of the brain - it reduces excess formation of cerebrospinal fluid and reduces intracranial pressure. However, it should be used only in combination with Asparkam, since the use of this diuretic leads to the loss of such a trace element as potassium, Asparkam replenishes its loss [16]. In mild cases in infancy, it is advisable to use gentle herbal preparations: fennel tea, dill water, approved for use in childhood.
Hypoxic-ischemic encephalopathy (HIE)
What is perinatal encephalopathy?
Perinatal lesions of the nervous system in newborns are a number of conditions and diseases of the brain, spinal cord and peripheral nerves, combined into a common group according to the time of exposure to damaging factors. The perinatal period includes the antenatal, intranatal and early neonatal periods. The antenatal period begins with the 22nd week of intrauterine development and ends with the beginning of labor. The intrapartum period includes the act of childbirth from the onset of labor to the birth of the child. The neonatal period is divided into early neonatal (corresponding to the first week of a child’s life) and late neonatal (from the 8th to the 28th day of life inclusive) periods.
Why does perinatal encephalopathy occur?
Perinatal encephalopathy is a common complication of the pathology of pregnancy and childbirth. This condition is caused, as a rule, by oxygen starvation of the central nervous system, which occurs with various deviations in the course of the perinatal period, which is difficult for the child. Perinatal brain damage accounts for more than 60% of all pathology of the nervous system in early childhood, and is directly involved in the development of diseases such as cerebral palsy, epilepsy, and minimal brain dysfunction. Depending on the degree of damage to the central nervous system, as well as the availability of correct and timely treatment, different outcomes of perinatal encephalopathy are possible - a third of patients, as a rule, require minimal rehabilitation support for recovery, a third require serious, including drug treatment, and a third, even with competent and long-term medical care, will still have certain health problems in the future.
Among the causes of perinatal brain damage, the leading place is occupied by intrauterine and intrapartum (during childbirth) hypoxia of the fetus, the second most important place belongs to the factor of mechanical trauma to the child during childbirth - usually in combination with one or another severity preceding intrauterine hypoxia. The structure of etiopathogenetic factors of perinatal pathology also includes infectious (including viral) and toxic-metabolic variants of damage to the nervous system. Thus, among the factors causing perinatal damage to the central nervous system, the following are distinguished:
- Intrauterine hypoxia (oxygen starvation) of the fetus
- Fetal hypoxia during labor
- Mechanical trauma during childbirth
- Infectious (viral) factors
- Toxic factors
- Hereditary factors
- Combination of these factors
Among the risk factors for perinatal encephalopathy in children, a large share belongs to maternal morbidity, which, according to numerous studies, not only violates preconception health, that is, the health responsible for the birth of healthy offspring, the parent couple, but also has a direct negative impact on the fetus.
When studying the structure of the general pathology of pregnant women over the past five years, a significant increase in their morbidity is noted, primarily due to an increase in the frequency of diabetes mellitus and thyroid diseases. During pregnancy, these diseases lead to placental insufficiency and impaired absorption and assimilation of nutrients through the placenta, as well as a deficiency in the transport of oxygen and carbon dioxide, which is manifested by fetal growth retardation syndrome, intrauterine hypotrophy, immaturity of the lungs and surfactant. It has been established that a decrease in uteroplacental blood flow serves as an objective indicator of hypoxic brain damage.
How does perinatal encephalopathy manifest?
Clinically, perinatal encephalopathy is manifested by several symptom complexes, the most common of which are: “delayed motor and mental development”, “hypertensive-hydrocephalic syndrome”, “muscular dystonia syndrome”, “convulsive syndrome”, “brain stem lesion syndrome”.
What is psychomotor development delay and how to treat it?
The most difficult period in a child's life is the period of infancy. The rate of growth and development of babies is simply rapid. At the same time, a child is born with far from complete development of all organs and tissues, which after birth are gradually improved and approach the organs of an adult in their structure and functions, therefore a detailed study of the main aspects of the development of a child in the first year of life is an urgent need for pediatric doctors of all specialties. Tracing the development of a baby from the neonatal period to 1 year, experts focus on the child’s anthropometric data, his psychomotor development (motor activity), speech development, and the formation of skills and abilities.
Based on the characteristics of the psychomotor status of a healthy newborn child, carefully monitoring the decay of innate reflexes and the acquisition of motor and social skills over time, one can assess how correctly and harmoniously the baby’s development proceeds in the first year of life.
Delayed psychomotor development and the formation of motor skills is observed in various hereditary diseases, in children with pathologies of the central nervous system, leading to the formation of persistent motor disorders, including cerebral palsy. In this case, a delay is understood as a lag in development of 2 or more months. Particular attention should be paid to children with regression of previously acquired motor and psycho-speech skills, which may indicate degenerative diseases of the nervous system.
A mild (tempo) form of developmental delay is often found in premature infants, with chronic non-neurological diseases, rickets. For moderate to severe delays, special examination and treatment are recommended.
Non-drug correction of psychomotor delays in children of the first year of life includes:
Physical rehabilitation: a variety of therapeutic massage, therapeutic exercises, “position” treatment (styling, splints, “collars”, etc.), Vojta therapy; exercises in water and hydromassage; dry immersion (imitation of weightlessness); physiotherapy (alternating magnetic field, sinusoidal modulated currents, electrophoresis, paraffin therapy, laser therapy, light and color therapy)
Psychological and pedagogical correction: correctional (conductive) pedagogy; psychotherapeutic correction in the mother-child dyad (skin-to-skin contact, kangaroo care); music therapy, aesthetic therapy; tactile-kinesthetic stimulation. For premature babies, a combination of two or three “soft” methods of physical influence with psychoemotional and psychosensory correction is especially recommended, which helps simulate the effect of the so-called “sensory rooms” used in the rehabilitation of older patients.
Drug therapy , which is used by neurologists to correct motor and mental development in the first year of life, is mainly represented by peptide drugs that trigger endogenous compensation mechanisms and return the child to normal development.
What is muscular dystonia syndrome and how is it treated?
Tonic disturbances in an infant can be local (associated with one muscle group) or widespread (associated with several muscle groups). Based on the nature of changes in muscle tone, they are divided into hypotonia (decreased tone), hypertonicity (increased tone) and dystonia (incorrect combination of increased and decreased tone).
The typical and most common local tone disorder can be considered infantile torticollis.
Torticollis, as a rule, is caused by mild underdevelopment or traumatic damage to the sternocleidomastoid muscle and roots emerging from the C5-C6 segments of the spinal cord or accessory nerve; by the age of 6-9 months, torticollis usually disappears. However, mild symptoms of torticollis can also be detected at an older age. Resistance is felt when turning in the direction opposite to the affected muscle.
As treatment, your child may be prescribed applications, electrophoresis or traumeel injections, Vibrukol rectal suppositories, and placing the head in the correct position using a steering wheel pillow.
Muscular hypotonia ("floppy child" syndrome) is manifested mainly by a decrease in resistance to passive movements and an increase in their volume (the appearance of looseness in the joints). In severe cases, hypotension affects the child's posture: the extreme expression of diffuse muscular hypotonia is the “frog posture.” “Floppy baby” syndrome can occur in a wide range of diseases: severe non-neurological diseases, rickets, severe perinatal hypoxia (oxygen deficiency) and birth trauma, leading to the formation of cerebral palsy, metabolic disorders, neuromuscular diseases, developmental abnormalities and degenerative diseases of the nervous system, chromosomal syndromes, some forms of endocrine pathology.
Diffuse muscle hypotonia often occurs with hereditary pathology. In this case, insufficient weight and height gain, epileptic seizures, and episodes of acute cerebrovascular accident may occur. A pronounced decrease in muscle tone is often found in premature infants, and by 3-6 months they may transform hypotension into spastic syndrome.
In case of severe persistent muscle hypotonia, especially in combination with delayed psychomotor development, it is necessary to conduct regular rehabilitation courses (massage, exercise therapy, physiotherapy, hydro and balneotherapy, acupuncture, speech therapy classes, drug treatment).
Muscle hypertonicity can manifest itself from minor (not impeding movement and development) to severe up to spastic syndrome (an extreme form of hypertonicity). An increase in muscle tone can be combined with activation of tonic reflexes and a delay in the extinction of unconditioned reflexes.
Tonic reflexes in combination with increased muscle tone have a pathological effect on the child’s posture. This picture is often observed in premature and immature children, as well as in the formation of cerebral palsy. In the latter case, unconditioned reflexes may even intensify.
Dystonia and hyperkinesis (irregular, unnecessary, pretentious and excessive movements) - these clinical manifestations often appear delayed, after 3-6 months of life, but can also be observed from birth.
Hyperkinesis, as a rule, characterizes damage to the subcortical nuclei of the brain, which are often combined with other neurological symptoms (for example, hearing loss). Both hypertonicity and dystonia, forming long-term incorrect postural attitudes in a child, over time lead to deformations of the baby’s skeleton. Therefore, if there are motor disorders, in addition to a neurologist, such a child must be observed by an orthopedist.
From the moment of diagnosis, it is recommended to begin a course of rehabilitation treatment. Restorative therapy (in the absence of contraindications from the cardiovascular, respiratory systems, absence of epileptic paroxysms, etc.) includes massage, physical therapy, orthopedic styling, physiotherapeutic treatment, hydro and balneotherapy, acupuncture, speech therapy classes, classes in sensory rooms.
Early development methods and drug therapy are chosen individually, depending on the severity of the syndrome and its combination with developmental delay, hyperkinetic and dystonic syndromes.
Drug therapy prescribed to correct muscle tone in children in the first year of life is divided into drugs that reduce muscle tone (antispastic drugs) and drugs that correct muscle hypotension.
What is hypertensive-hydrocephalic syndrome and how to treat it? Normally, the increase in head circumference at 1 year of life is 11-12 cm:
- During the first trimester of life, head circumference increases by 4 cm (1.5 cm/month)
- During the second trimester of life - by 3 cm (1 cm/month)
- During the second half of life, head circumference increases by 3-4 cm (0.5 cm/month)
A pathological increase in head circumference as a symptom of hydrocephalus develops as a result of blockage of the cerebrospinal fluid ducts at various levels and when the relationship between the processes of production and absorption of cerebrospinal fluid is disrupted.
Hydrocephalus:
Congenital hydrocephalus is a disease that develops in the fetus during pregnancy and the child is born sick. The main causes of congenital hydrocephalus are developmental defects, less often intrauterine infection, much less often the cause of hydrocephalus is hemorrhage into the ventricles of the brain in the fetus.
Acquired hydrocephalus - the disease develops after the birth of a child, sometimes in the earliest stages of life. The causes of acquired hydrocephalus are intraventricular hemorrhages, infections affecting the central nervous system - meningitis, encephalitis, traumatic brain injury, brain tumors.
Clinical symptoms of intracranial hypertension:
- Changes in the child's behavior: restlessness, frequent and monotonous crying, throwing back the head, frequent regurgitation
- Delayed mental, motor and psycho-speech development
- Opening of the sagittal suture more than 0.5 cm, bulging, tension of the large fontanelle
- Changes in the shape of the skull with a high forehead (tower skull) or with a sharply protruding occipital protuberance in combination with excessive growth of head circumference, predominance of head circumference over chest circumference
- Graefe's sign, “stagnant” changes in the fundus
- Increased muscle tone, mainly in the hands and feet
- Tremor (shaking) of the hands with a tendency to open them
In addition to hydrocephalus, children with the following conditions may have a “hydrocephalic shape” skull with a large head circumference:
- Rickets
- Constitutionally large-headed children
- Infants with syndromic conditions and hereditary diseases
To adequately assess the situation, it is always necessary to compare the head circumference with the chest circumference, assess the size of the head of the child’s parents, and focus on the results of additional research methods (fundus examination, NSG, CT, MRI).
Drug therapy for the correction of hydrocephalic syndrome in the first year of life is mainly represented by drugs with diuretic and vascular effects.
Hydrocephalus is a disease that is treated not by a neurologist, but by a neurosurgeon. Without consulting a neurosurgeon, a neurologist cannot make a decision on the treatment of hydrocephalus. If for some reason surgical treatment of hydrocephalus is not indicated for a child, a neurologist and a neurosurgeon observe such a patient together.
Insufficient growth of head circumference is observed in progressive hereditary degenerative diseases, in severe organic lesions (secondary microcephaly - when the brain does not grow and, as a result, skull growth slows down), in craniostenosis (pathology of the skull bones, requiring surgical treatment by a neurosurgeon).
It should be noted that the rate of overgrowth of the large fontanel in each child is strictly individual, and is more likely related to the characteristics of mineral metabolism than to the pathology of the nervous system. The preservation of an open large fontanel by the end of the second half of the baby’s life in the absence of neurological complaints is not a cause for great concern, just as the accelerated closure of a large fontanel is in no case a reason to stop the planned prevention of rickets in an infant.
What is seizure syndrome and how to treat it? In the practice of a pediatric neurologist, there are many conditions that occur paroxysmally, i.e. arising suddenly, short-lived and abruptly ending. An example of paroxysms are epileptic seizures, usually accompanied by loss of consciousness. However, not all paroxysms occur with impaired consciousness; some of them are characterized by very unusual symptoms, which can cause diagnostic errors.
It is particularly difficult to identify and differentiate various paroxysmal conditions in infancy. To choose the right treatment tactics, it is necessary to distinguish between paroxysmal states of epileptic and non-epileptic nature; however, only an experienced neurologist-epileptologist can clearly distinguish between the cause of paroxysms and their prognosis.
Affective-respiratory paroxysm is a short-term cessation of breathing at the height of crying with pale or blue discoloration of the skin. It is necessary to differentiate affective-respiratory paroxysms from apnea syndrome (cessation of breathing), which often occurs in premature and immature infants, as well as in children with pathologies of the cardiovascular and respiratory systems, and the brain stem. For clear differentiation, a polysomnography study is carried out, which allows, using simultaneous recording of an EEG, electrocardiogram and spirogram (breathing graph), to determine what is the root cause of breathing problems in the baby. By themselves, affective-respiratory paroxysms are not epileptic, but the frequency of their further transformation into epilepsy is quite high.
Paroxysmal sleep disorders represent a fairly large group of conditions that need to be differentiated from both non-neurological diseases and epileptic paroxysms. The most typical representatives of paroxysmal sleep disorders are nightmares, during which the baby sharply and piercingly begins to scream and cry, without fully waking up and reacting little to the environment. The presence of frequently recurring nightmares may indicate problems in the child’s mental sphere.
Febrile seizures are the most common type of paroxysmal conditions in childhood. Febrile seizures (FS) are paroxysms of varying duration, occurring primarily in the form of convulsive seizures in infants and young children at a body temperature of at least 37.8-38.5°C. FS have an increased likelihood of transformation into epilepsy.
There are typical and atypical FS. The first ones have a short duration (up to 15 minutes), a generalized nature (all limbs are involved, consciousness is briefly lost); indicators of the child’s psychomotor development usually correspond to age; there are no typical changes on the EEG. In atypical FS, the duration of the attack is more than 15 minutes (up to several hours), there is a predominant involvement of the limbs on one side; sometimes after an attack Todd's paresis occurs - transient muscle weakness in the limbs affected by the spasm (in 0.4% of cases); epileptic focal changes are not uncommon on the EEG.
Neonatal seizures (NS) occur during the first 4 weeks of life of a full-term newborn (from the 1st to the 28th day) and somewhat later in premature infants. Neonatal seizures are quite difficult to distinguish by clinical manifestations from benign movements of the child - their presence is established using an EEG with video recording. Myoclonic seizures have the most severe prognosis and may indicate the onset of early severe epilepsy.
Epilepsy is a chronic brain disease characterized by repeated unprovoked seizures that cause impairment of motor, sensory, autonomic, mental or mental functions resulting from excessive neural discharges in the cerebral cortex. The prevalence of epilepsy among the child population is about 10 cases per 1000 children.
Epilepsy is a very unpleasant but very common neurological disease, especially in childhood. In addition to creating life-threatening conditions for the infant during prolonged and complicated convulsive seizures, epilepsy leads to long-term negative consequences, disrupting the cognitive, psycho-social and motor development of the child. Modern anticonvulsants provide control over most forms of epilepsy, but parents are afraid to give them to children because of the poor reputation of older anticonvulsants for fear of their side effects. All your concerns should be discussed with a specialist. Don't deprive your child of a healthy future because of your prejudices.
What are the symptoms of brain stem damage and how are they treated?
The brain stem, or brain stem, is a traditionally distinguished part of the brain, which is an extended formation that continues the spinal cord.
The brain stem contains vital centers responsible for breathing, swallowing, cardiac activity, coordinated eye movements, etc. In newborns with neurological problems, the activity of these centers may be insufficiently developed or damaged. Underestimation of symptoms of brain stem damage can lead to critical consequences.
Bulbar and pseudobulbar disorders in children with damage to the nervous system are noted from birth. They manifest themselves as sluggish sucking, then they begin to choke on saliva and food, choke, food pours out through the nose, the tone of the tongue changes, and speech formation is disrupted.
Oculomotor disorders are very typical for infants with central nervous system pathology. The range of possible disorders includes nystagmus (trembling of the eyeballs), various types of strabismus. It is extremely important to isolate patients from this group who are visually impaired, since oculomotor disorders may be the first symptoms of impaired visual function (for example, the so-called “floating nystagmus of the blind”). Patients require joint supervision by a neurologist and an ophthalmologist.
Respiratory and heart rhythm disturbances are also typical for patients with impaired function of stem structures. Infants born premature and low birth weight are especially susceptible to breathing problems (losses) or apnea and arrhythmias. In such a situation, the fundamental task is the differential diagnosis of neurological and non-neurological (cardiological, respiratory) causes of the identified disorders. Such patients require careful diagnosis and observation by a neurologist, pulmonologist, cardiologist and ENT doctor.
Be sure to ask specialists about the presence of brainstem disorders in your baby. Their consequences can be very serious (for example, respiratory disorders such as apnea cause sudden infant death syndrome, and swallowing disorders and chronic “choking” lead to severe aspiration pneumonia), but with proper and careful care (correct position of the child during sleep, feeding with a raised head end and a special diet) troubles can be avoided even without additional medications.
Do all developmental abnormalities in an infant require special treatment?
Variants of the norm in the first year of life are the following events:
- Physiological tremor - rhythmic twitching of the arms or chin during crying or feeding. Normally, it occurs in half of children under 3 months of age. Tremors are often observed in premature newborns up to 6 months of age.
- Physiological astasia-abasia - from 2 to 5 months, the child, when “suspended”, does not rest on the plane with his legs, presses them in, and accordingly there are no stepping movements (due to the fading of the support reflex)
- Yactation - rocking (self-soothing) movements of the body and/or head, which appear in a child mainly before bedtime, are characteristic of children raised without parental care
- The revival complex is a variety of chaotic movements that accompany emotional, usually positive, “outbursts.” Particularly common in children with immobilized limbs (for example, those receiving casting or orthopedic devices)
Thus, all children with perinatal encephalopathy require long-term dynamic observation by specialists and individually selected repeated courses of rehabilitation treatment, with an interval of no more than 3 months and with regular monitoring of its effectiveness, during the first year of life in outpatient rehabilitation centers, hospital departments and district clinic
However, in order for the rehabilitation process to be continuous, healthcare professionals are not enough - it is necessary to involve the child’s family in the rehabilitation process. Professionals interested in the results of their work will be happy to train mothers in the basics of exercise therapy, infant swimming, and methods of early child development. Contrary to popular belief, restorative treatment of children with perinatal pathology of the nervous system should begin immediately after diagnosis, and stop only after the child “catch up” with his peers in all parameters of physical, motor and psycho-speech development.
O.V. Bykova
Doctor of Medical Sciences, Chief Researcher of the Scientific and Practical Center for Pediatric Psychoneurology
Department of Health of Moscow
Diagnostics
Perinatal ischemia syndrome due to cerebral hypoxia begins to be diagnosed by performing a visual examination of the child. It's the same with adults. Despite all the advances in medicine, a unique test that can accurately identify DIE has not yet been invented. All laboratory techniques are aimed at identifying how badly the brain is damaged and the current state of the whole organism.
What kind of research will be done depends on the symptoms and how they developed. To decipher the tests, there are special biomarkers that give a complete picture of the degree of HIE. The patient's blood is needed for the study.
Neuroimaging is carried out using:
- neurosonography and/or MRI, a tomograph showing internal brain damage and changes in it;
- Doppler ultrasound, recording the functioning of cerebral blood flow;
- electroneuromyograph to determine the sensitivity of fibers of the periphery of the nervous system.
Additional can be used:
- electroencephalograph to detect developmental delay at an early stage and whether there is epilepsy;
- video monitoring to study the motor activity of children.
If necessary, the victim is examined by an ophthalmologist to determine the condition of the optic nerves and fundus of the eye, as well as the presence of genetic diseases in this area.
New treatment for HIE
For HIE, the mononuclear fraction of umbilical cord blood containing stem cells is used. When it is administered intravenously to a child, restoration and regeneration of brain cells occurs, and the functioning of the immune system is regulated. If therapy is started at an early stage of the disease, then encephalopathy can be cured due to the powerful regenerative potential of umbilical cord blood and restoration of the population of nerve cells. The uniqueness of cell therapy for encephalopathy lies in the achievement of high treatment results in each case. Today, in Russia alone, more than 330 children suffering from cerebral palsy have already been saved with the help of harvested umbilical cord blood.
Treatment of hypoxic-ischemic encephalopathy with the mononuclear fraction of umbilical cord blood in the early stages is the most important factor in a favorable prognosis, reducing the risk of developing cerebral palsy, as well as the correct development of the child and improving his quality of life in subsequent years.
Thus, by deciding to preserve cord blood, parents give their baby wonderful “biological insurance” against cerebral palsy: if necessary, treatment can begin from the first day of the newborn’s life, using his own cord blood cells, right in the maternity hospital.
Parents - be vigilant: prepare your child's umbilical cord blood and thereby you will save him from many diseases.
Kinds
Doctors distinguish between congenital and acquired encephalopathy. The first occurs against the background of an abnormal course of pregnancy or childbirth and, often, develops while the fetus is in the womb. Its signs are detected immediately after birth or appear in the first weeks of life. Neonatologists and pediatricians are involved in the diagnosis and treatment of this condition.
Acquired encephalopathy occurs in adulthood. It is divided into several types depending on the cause of neuronal death:
- post-traumatic: occurs against the background of a traumatic brain injury; often develops within a few years after it and often leads to severe mental disorders;
- toxic: associated with acute or chronic poisoning of the body with alcohol, poisons, narcotic drugs, medicines, salts of heavy metals, etc.; Alcoholic encephalopathy is often distinguished separately within this type;
- metabolic: associated with metabolic disorders in the body; The following subtypes of pathology are distinguished: hepatic: occurs when the liver or biliary tract is damaged;
- uremic: associated with impaired renal function;
- diabetic: is one of the frequent complications of diabetes mellitus, occurs against the background of persistent microcirculation disorders and increased blood viscosity;
- anoxic: develops after clinical death and is associated with oxygen starvation of the brain with the subsequent development of a “metabolic storm”;
- Gaye-Wernicke syndrome: encephalopathy caused by vitamin B1 deficiency;
- pancreatic: is a complication of inflammation of the pancreas;
- hypoglycemic: occurs against the background of a sharp decrease in blood glucose;
- atherosclerotic: develops due to atherosclerosis and thickening of the walls of blood vessels;
Depending on the speed of development of the process, encephalopathy is divided into acute and chronic. The first can develop within a few days or hours, and more often occurs against the background of severe intoxication, trauma, or an infectious process. The chronic process can last for years or decades.
Premature birth is a leading cause of infant mortality and a significant factor in the loss of human potential of surviving children during later life. According to foreign authors, several million children with very low body weight (VLBW) are born every year around the world [1]. In the United States, 90% of the 65,000 newborn VLBW infants survive the neonatal period due to great advances in intensive care, but 5–10% of these children are later diagnosed with cerebral palsy [2].
The introduction of modern medical technologies in the last decade has been marked by a decrease in perinatal and infant mortality. At the same time, an increase in the survival rate of children with VLBW and extremely low body weight (ELBW) at birth entails an increase in morbidity and the formation of early disability [3]. Among the causes of childhood disability, pathology of the nervous system ranks first, and the contribution of perinatal lesions reaches 60–80% of all neurological diseases [4]. In Russia, annually no more than 2.5-5% of those examined as disabled since childhood are recognized as able to work, compared to 50% abroad [5].
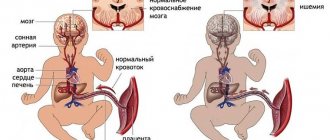
Among the factors that adversely affect the antenatal period, disruption of the uteroplacental circulation, which can be caused by both extragenital and somatic pathology of the mother, is of great importance [6]. Disturbances in the uteroplacental blood flow, in turn, lead to the development of hypoxia, which is the central link in the pathogenesis of antenatal damage to the fetus, and primarily to the central nervous system (CNS). The works of many authors have established a whole complex of physiological adaptive reactions of the fetus to unfavorable developmental conditions, in particular to hypoxia [7-10]. However, there is insufficient information on the biochemical status of the nervous tissue of the fetus and newborn in this pathology. Of great interest in this regard is the study of glucose metabolism, the characteristics of free radical oxidation and glutamate metabolism, the processes of necrosis and apoptosis.
In utero, the fetus is in a state of hypoxia, but this environment is physiological for it, moreover, in the early stages of the embryonic period it is necessary for normal cell differentiation. Fetal oxygenation depends on oxygen partial pressure gradients between maternal and placental blood, fetal blood and fetal tissue. It is known that in the first weeks after conception in the embryonic period, the level of partial pressure of oxygen (pO2) is extremely low and amounts to about 18-20 mm Hg. Presumably, this is necessary to protect the embryo, which is very sensitive to the damaging effects of reactive oxygen species [11]. Hypoxia in the embryonic period causes angiogenesis and is a prerequisite for maintaining pluripotency of stem cells [12]. It is noteworthy that in the first trimester of pregnancy, embryonic stem cells develop at a pO2 level of about 10-15 mm Hg, while in the endometrium, pO2 is about 25 mm Hg. Stem cells demonstrate more efficient growth and differentiation at low oxygen pressures of 10-15 mmHg. [13]. Prolonged hypoxia will stimulate angiogenesis through transcriptional and post-transcriptional regulation of growth factors: vascular endothelial growth factor, erythropoietin, placental growth factor and angiopoietin1 [14].
The main regulator of adaptive cell responses to hypoxia is hypoxia-inducible factor 1 (HIF-1), a heterodimeric transcription factor including subunits (HIF-1α and IGF-1β). IGF-1α stabilizes when the oxygen concentration is below a certain critical threshold, thus accumulating in a hypoxic environment. IGF-1β is present in the cell nucleus, and under hypoxic conditions it dimerizes with IGF-1α, improving oxygen delivery to the tissue [15, 16]. At the 14-16th week of pregnancy, pO2 rises to stable values of 45-50 mmHg. and remains so until the end of pregnancy. In late pregnancy, the rate of cell proliferation and differentiation decreases [17, 18]. Lipid peroxidation processes are present from the very beginning of pregnancy, which contributes to the normal development of the fetus. At the end of the first trimester, physiological oxidative stress causes regression of the villi that were formed throughout the surface of the chorionic sac to form the final discoid placenta [19]. The postnatal increase in oxygen concentration causes a surge in the formation of its reactive species, with the expression of antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase dynamically increasing during the last weeks of pregnancy. Similarly, the availability of the most important non-enzymatic antioxidants increases: glutathione, heme oxygenase, vitamins C and E, β-carotenes, etc. [20]. The premature infant is at greater risk of free radical damage [21]. The use of high concentrations of oxygen during neonatal resuscitation is thought to cause hyperoxemia. At the same time, a significant correlation was found between oxidized glutathione (GSSG), pO2 and the activity of enzymes in the glutathione redox cycle [22, 23]. Many diseases associated with prematurity, such as retinopathy, bronchopulmonary dysplasia, and intraventricular hemorrhage, are associated with free radical damage as a result of the immaturity of the antioxidant system of preterm infants [24].
Nervous tissue is most vulnerable when exposed to hypoxia. Hypoxia leads to disruption of the exchange of oxygen and carbon dioxide, which in turn causes metabolic disorders and hemodynamic disorders [25]. The following mechanisms underlying cerebral damage during hypoxia-ischemia are known: local disturbances in the metabolism of high-energy compounds, excessive lipid peroxidation and disturbance of Na+/K+-ATPase activity, extracellular accumulation of K+ and intracellular accumulation of Ca2+, intracellular acidosis, disturbance of neurotransmitter metabolism [26 ]. The main links of hypoxic-ischemic stress are presented by P. Marro [27].
- Lack of oxygen. A deficiency of oxygen as an electron acceptor in tissues leads to disruption of electron transport in the Krebs cycle and respiratory chain, replenishment of energy by increasing cerebral blood flow and anaerobic metabolism [28].
— Glutamate-calcium cascade. An increase in glutamate concentration activates N-methyl-D-aspartate (NMDA) receptors, which is accompanied by an increase in intracellular Ca2+ [29]. Disturbances in mitochondria and endoplasmic reticulum can lead to further accumulation of intracellular Ca2+. An increase in Ca2+ concentration inside the cell promotes the formation of free radicals, which in turn causes lipid peroxidation of the cellular and intracellular membranes. Along with this, the accumulation of intracellular Ca2+ is naturally accompanied by an increase in its concentration in the cell nucleus. Excess intranuclear Ca2+ is a factor in the activation of protoapoptotic genes, which trigger genetically programmed cell death - apoptosis [30].
— The role of free radicals. Hypoxia-ischemia causes inadequate saturation of mitochondrial cytochrome oxidase, disruption of electron transport in mitochondria, which leads to an increase in the concentration of superoxide anion and the entry of free radicals from mitochondria into the cytoplasm [31]. An increase in intracellular Ca2+ concentration activates NO synthetase, cyclooxygenase and lipoxygenase, which promotes the formation of free radicals. Their excess leads to additional release of excitatory amino acids and activation of NMDA receptors [32].
- Inflammatory factors. The effect of hypoxia-ischemia on microglia promotes the synthesis of cytokines, interleukin-1β (IL-1β), tumor necrosis factor α (TNFα) [33]. IL-1β activity is accompanied by the production of specific proteases and the development of apoptosis. Excessive production of TNFα has a direct toxic effect and causes vascular infiltration with the release of cytotoxic factors, reactive oxygen species and cytokines [34].
— The role of nitric oxide (NO). NO synthetase is found in endothelial cells, astrocytes, and neurons. There are 3 isoforms of NO synthetase: neuronal (regulates synaptogenesis and remodeling and depends on Ca2+); endothelial (regulates vascular tone, especially vasodilation, and depends on Ca2+); inducible (present in macrophages and astrocytes, induced by cytokines, independent of Ca2+) [35]. Activation of NMDA receptors causes the production of neuronal NO synthetase, which promotes the formation of nitric oxide (NO.) radical and damage to neuronal DNA [36].
- Apoptosis. The processes described above develop in the first minutes of acute hypoxia, after which the apoptosis mechanism is activated [37]. Hypoxia, through a number of pathogenesis links, promotes the accumulation of intracellular Ca2+, activation of endonucleases, and damage to gene expression. This leads to disinhibition of the phagocytic activity of glial cells and neurons, which phagocytose the damaged neuron, causing a decrease in its size and sequestration [38].
The most significant loss of nervous tissue cells develops 2-6-48 hours after birth, due to pathological oxidative stress. Under such conditions, in the first hours and days of life after birth, newborns who have suffered hypoxia develop a pronounced imbalance in the cerebral circulation regulation system, which aggravates the course of the ischemic process [39].
A feature of premature infants is the immaturity of the antioxidant system, since a physiological increase in antioxidant capacity occurs at the end of pregnancy, which is why they are more susceptible to oxidative stress, especially when their condition requires respiratory therapy [40]. In this regard, there is a need to study oxidative stress in these children, in particular by measuring lipid peroxidation products and components of the antioxidant system.
Antioxidants are known to have anti-inflammatory activity, and the glutathione system is considered a critical factor in the development of inflammation and immune responses [41, 42]. This is confirmed by changes in the levels of cytokines, acute phase proteins and glutathione during inflammation [43, 44]. The glutathione system includes its forms, a number of enzymes for its synthesis and catabolism, and transport mechanisms. All these components make an important contribution to changes in glutathione status [45].
When studying the classification of cerebral lesions in newborns, it should be noted that the most popular among neonatologists is the classification of hypoxic encephalopathy according to H. Sarnat and M. Sarnat [46]. It combines clinical signs of cerebral ischemia and electroencephalography (EEG) results. This classification evaluates the main indicators of a newborn: level of consciousness, neuromuscular status, reflexes, autonomic function, presence of seizures, EEG. Depending on the severity of cerebral dysfunction, stage I, II or III of encephalopathy is established. Canadian neonatologists modified the Sarnat classification, adding thermoregulation disorders and excluding EEG and some other indicators [47]. Neonatologists in Great Britain use the classification of hypoxic cerebral disorders by L. Dubowitz et al.[48]. In the International Classification of Diseases, 11th revision (ICD-11), hypoxic-ischemic brain lesions in newborns belong to the group of “neurological disorders characteristic of the perinatal and neonatal periods” [49]. The main difference from the classification of the 10th revision is that in ICD-11 this group is supplemented with diseases that were not previously identified separately. For example, perinatal arterial stroke and neonatal cerebral sinovenous thrombosis. At the same time, congenital hydrocephalus was excluded from the proposed classification. The diagnosis of newborn asphyxia was placed in the “group of other disorders arising in the perinatal period.” At the same time, newborn asphyxia with an Apgar score of 0-3 points and newborn asphyxia with an Apgar score of 4-6 points were separately identified. As for cerebral injuries of a hypoxic-hemorrhagic nature, they were classified into the group of “hemorrhagic and hematological disorders in the fetus and newborn.” At the same time, the classification of intraventricular hemorrhages has changed somewhat. In ICD-11, it is customary to distinguish 4 degrees of intraventricular hemorrhage, while in ICD-10 the 3rd and 4th degrees were combined.
Symptoms of severe central nervous system damage may not appear immediately after birth, but may occur several hours later. However, clinical symptoms do not always reflect the true severity of the disease. In this regard, intravital assessment of changes that occur in the cells of nervous tissue in the early neonatal period is of particular relevance. Ultrasound examination (ultrasound) allows us to identify structural changes in the brain of newborns due to perinatal hypoxic damage to the central nervous system. Analysis of ultrasound and pathomorphological data indicates that the nature of ischemic brain damage depends not only on the severity of perinatal hypoxia, but also on the maturity of the child [50]. In full-term newborns, cerebral ischemia is accompanied by the occurrence of selective neuronal necrosis, subcortical and multicystic encephalomalacia, and cerebral infarctions [51]. Severe perinatal hypoxia in premature infants 34–37 weeks of gestation, as a rule, leads to the development of periventricular leukomalacia. In connection with the nursing of extremely premature infants with ELBW, ultrasound has become more likely to detect such forms of ischemic damage as diffuse leukomalacia and periventricular hemorrhagic infarction. It should be noted that in the first 24-48 hours of the debut of neonatal arterial ischemic stroke, this method does not have sufficient sensitivity and specificity, since the focus of ischemic damage begins to appear only on the 2-3rd day from the onset of ischemia, which is associated with the course of pathohistological processes. The severity of the inflammatory reaction, the intensity of necrosis and apoptosis reach their peak 48–72 hours after circulatory disturbance [52]. This leads to a change in the echogenicity of the damaged brain parenchyma. Further assessment of the evolution of the ischemic focus is not inferior in information content to magnetic resonance imaging (MRI) [53, 54]. The use of Doppler ultrasound helps to increase the sensitivity of ultrasound in the early stages of cerebral damage. Duplex scanning allows you to objectively assess the hemodynamic characteristics of cerebral vessels. The value of Doppler ultrasound technique in the acute period of the disease is the identification of the vasodilation phase, the earliest sign of ischemic damage, which occurs within 30 minutes after the development of a vascular accident and persists for the first 5-6 days [55]. Vasodilation develops in response to the action of various metabolites and increases the supply of glucose and oxygen to ischemic tissue. Its characteristic features are an increase in blood flow velocity and a decrease in peripheral resistance indices in the damaged vascular system [56, 57]. MRI has become the most informative method for diagnosing perinatal brain damage. The presence of various pulse sequences provides high sensitivity and specificity, even in the early stages of the development of a vascular accident [58, 59]. MRI with diffusion-weighted images and the construction of maps of the measured diffusion coefficient (MCD) makes it possible to detect an ischemic lesion within 30 minutes from the moment of its occurrence. ICD serves as a quantitative characteristic of diffusion in tissue and reflects the presence of intracellular edema [60]. The presented diagnostic methods are necessary to identify, determine the localization, severity of brain damage and prognosis. Their disadvantages are the short diagnostic time interval and the limited possibility of repeated examination [61]. Over the past 20 years, the diagnostic value of biomarkers has been studied to predict the outcome of cerebral injury in newborns [62, 63]. Considering the pathophysiological changes that occur as a result of damage to brain tissue, neuroproteins, calcium-binding protein, vasoactive substances, markers of oxidative stress, inflammatory mediators, etc. have been studied in detail [64-67]. However, despite the promise of studying biomarkers, there is no data on their practical use in medicine.
Treatment of hypoxic brain lesions is the subject of heated debate and extreme opinions, ranging from complete denial of the need for treatment with neurotropic drugs to aggressive polypharmacy [26]. According to modern views, hypoxic-ischemic encephalopathy occurs during asphyxia, usually in the structure of multiple organ disorders, therefore the main principle of therapy is to remove the child from asphyxia and maintain vital functions [68]. Specific treatment for hypoxic-ischemic encephalopathy is therapy for cerebral edema and neuroprotection, which primarily involves controlling the volume of cerebrospinal fluid (CSF), cerebral perfusion and the volume of brain matter. Cerebral perfusion depends on arterial inflow, venous outflow and the metabolic rate of nervous tissue. At this stage, the adequacy of artificial ventilation and the effectiveness of hypothermia are of great importance. It should be noted that hypothermia is carried out in newborns with a gestational age of at least 36 weeks if at least one of the signs set out in the special criteria is present [69]. Control of fluid volume in the CSF pathways is achieved by inhibiting the production of CSF and improving its outflow. Controlling brain volume involves enhancing active transport and stabilizing neuronal membranes. It should be noted that at the moment the use of drugs in neonatology is limited due to the high risk of side effects and lack of evidence base. However, the search for such drugs continues to this day. In particular, over the past two decades there have been significant changes in the understanding of the role of erythropoietin as a neuroprotector. Erythropoietin has been shown to be involved in neurogenesis and angiogenesis during embryonic development and is activated after brain injury [70], and also has a cytoprotective effect on endothelial, glial cells and neurons [71]. The neuroprotective role of erythropoietin was first identified in several in vitro
and
in vivo
[72]. At the same time, it was found that erythropoietin has anti-apoptotic [73], antioxidant [74] and anti-inflammatory effects [75]. In addition, it attenuates the effects of inflammation by reducing reactive astrocytosis and suppressing microglial activation, and reduces the number of immune cells at the site of inflammation [76]. The absence of endogenous erythropoietin is known to increase ischemic brain damage and worsen neuronal survival [77]. The established protective effects of erythropoietin during ischemia and reperfusion have prompted the use of recombinant erythropoietin in premature infants with cerebral ischemia, intraventricular hemorrhage, and periventricular leukomalacia [78]. Thus, one study assessed the effectiveness and safety of erythropoietin in neonatal hypoxic-ischemic encephalopathy. This study included 167 newborns with moderate to severe cerebral damage. All children were divided into two groups. Children of the first group received standard therapy for hypoxic-ischemic encephalopathy, and children of the second group received erythropoietin at a dosage of 300 and 500 U/kg to standard therapy. The drug was administered in the first 48 hours every other day for 2 weeks. It turned out that mortality and disability were 19.2% more common in the group of children who did not receive erythropoietin [79].
Thus, the problem of ischemic cerebral damage in premature newborns is very relevant. First of all, this is confirmed by the high incidence of this pathology and the high risk of death and disability in children. The question of complex diagnosis of cerebral disorders in premature infants remains open, because there is no single algorithm that combines data from the clinical picture, instrumental and laboratory research methods. Of great interest is the search for predictors of unfavorable outcome in order to optimize treatment approaches.
The authors declare
no conflict of interest.
The authors declare no conflicts of interest.
Information about authors
Anuriev A.M. - https://orcid.org/0000-0002-6724-5067; e-mail
Gorbachev V.I. — https :// orcid . org /0000-0001-6278-9332
How to quote:
Anuriev A.M., Gorbachev V.I. Hypoxic-ischemic brain lesions in premature newborns. Journal of Neurology and Psychiatry. S.S. Korsakov.
2019;119(8 issue 2):63-69. https://doi.org/10.17116/jnevro201911908263
Corresponding author:
— Anuriev Alexey Mikhailovich — e-mail
What treatment is prescribed for HIE?
The best treatment for HIE is prevention and early treatment of intrauterine hypoxia and newborn asphyxia. But, despite significant advances in the prevention of complications of childbirth, moderate and severe hypoxic-ischemic encephalopathy still occurs with a frequency of 1-2 per 1000 children born. Until recently, medicine could offer such children only supportive therapy for organ dysfunction.
Since 2010, induced hypothermia has become the standard treatment for HIE. This method consists in maintaining the baby’s body temperature at 33.5 °C for 72 hours, starting 6 hours after birth. Unfortunately, even after the use of induced hypothermia, a significant number of infants with HIE continue to have neurological deficits of varying severity.
Today, for the treatment of newborns with HIE, scientists are proposing a new method - regenerative therapy with umbilical cord blood stem cells.