The causative agent of leishmaniasis is parasitic protozoa of the genus Leishmania, which has more than 20 species. It has been established that more than 90 species of mosquitoes can carry Leishmania parasites. There are 3 main forms of the disease:
- Visceral leishmaniasis (VL), also known as kala-azar, is fatal in 95% of cases if left untreated. It is characterized by irregular bouts of fever, weight loss, enlarged spleen and liver, and anemia. Most cases occur in Brazil, East Africa and India. It is estimated that between 50,000 and 90,000 new cases of VL occur annually worldwide, but only 25–45% of these are notified to WHO. This form of leishmaniasis remains one of the parasitic infections with the highest epidemic potential and mortality. In 2018, more than 95% of new cases notified to WHO were reported in 10 countries: Brazil, China, Ethiopia, India, Iraq, Kenya, Nepal, Somalia, South Sudan and Sudan.
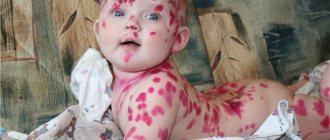
- Cutaneous leishmaniasis (CL) is the most common form of leishmaniasis and is accompanied by skin lesions, mainly ulcers, on exposed areas of the body. Skin lesions can leave permanent scars and lead to disability or stigmatization. About 95% of cases of CL are observed in the countries of the Americas, the Mediterranean basin, the Middle East and Central Asia. In 2021, more than 85% of new cases of CL were reported in 10 countries: Afghanistan, Algeria, Bolivia, Brazil, Colombia, Iran (Islamic Republic of), Iraq, Pakistan, and the Syrian Arab Republic and Tunisia. There are an estimated 600,000 to 1 million new cases of the disease each year worldwide.
- Mucocutaneous leishmaniasis leads to partial or complete destruction of the mucous membranes of the nose, mouth and larynx. More than 90% of cases of mucocutaneous leishmaniasis occur in Bolivia (Plurinational State of), Brazil, Ethiopia and Peru.
Transmission mechanism
Leishmania parasites are transmitted by the bites of infected female mosquitoes that feed on blood to lay eggs. The epidemiological characteristics of leishmaniasis may vary depending on the species of parasites and mosquitoes, the ecology of the areas where transmission occurs, the population's current or past exposure to the pathogen, and behavioral factors. It has been established that the natural reservoirs of Leishmania parasites are about 70 animal species, including humans.
Features of the epidemiology of the disease in different WHO regions
African region
Visceral, cutaneous and mucocutaneous forms of leishmaniasis are highly endemic in Algeria and East African countries. In East Africa, outbreaks of visceral leishmaniasis are common.
Americas region
The epidemiology of cutaneous leishmaniasis in the Americas is highly complex and highly heterogeneous with regard to transmission cycles, reservoirs, mosquito vector species, clinical manifestations and treatment response; in addition, different Leishmania species may circulate in the same geographic area. In 2021, more than 97% of VL cases in this region were reported in Brazil.
Eastern Mediterranean Region
This region accounts for 70% of all cases of cutaneous leishmaniasis in the world. Visceral leishmaniasis is highly endemic in Iraq, Somalia and Sudan.
European region
This region is endemic for cutaneous and visceral leishmaniasis. In 2018, more than 200 cases were reported in the region, mainly imported from Africa and the Americas region.
Southeast Asia region
The most common form of the disease in this region is visceral leishmaniasis, but the region is also endemic for cutaneous leishmaniasis. It is the only region with a regional initiative to eliminate visceral leishmaniasis as a public health problem by 2021. The region reported fewer than 5,000 cases in 2021, an all-time low. The region is confidently moving towards achieving its goal, and countries in the region intend to receive confirmation from the WHO of eliminating the disease by 2023.
Synonyms: acute necrotizing cutaneous leishmaniasis, wet cutaneous leishmaniasis, rural cutaneous leishmaniasis, furunculoid leishmaniasis. When an infected mosquito bites, Leishmania promastigotes are introduced into human skin, which after 24 hours transform into Leishmania amastigotes. The incubation period for zoonotic cutaneous leishmaniasis ranges from several days to 2 months.
Clinical manifestations on the skin are divided into 3 stages: tubercle, ulceration and scarring. After the incubation period, a limited, acutely inflammatory, painful red infiltrate (leishmanioma), 2–4 mm in size, appears at the site of the bite, which then transforms into a tubercle. At first it is flat, then it takes on a cone-shaped shape, similar to the initial stage of a boil, differing from it in less density and pain. The tubercle grows quickly, enlarges along the periphery and by the 2nd–3rd day reaches 8–10 and even 15–20 mm in diameter. As the infiltrate grows, the surrounding swelling of the skin also increases.
Then the tubercle ulcerates, necrosis forms in the center, and after the necrotic tissue is rejected, a small ulcer with a diameter of 2–4 mm with abrupt edges and a necrotic bottom is formed. Around the necrotic ulcer there is a fairly wide zone of infiltration with inflammatory edema of the surrounding skin. Leishmanioma resembles a boil, however, unlike the latter, there is no pain and necrotic core with leishmanioma. Necrotization occurs quickly (on days 3–7). Necrosis expands along the periphery both due to the disintegration of the surrounding infiltrate and due to new necrotic foci around the infiltrate.
Single ulcers are larger, and multiple leishmaniomas are small. The discharge from ulcers is serous-purulent and profuse. Sometimes this discharge dries out and the ulcer becomes covered with a loose brownish-purulent crust, which is easily torn off. Subsequently, the bottom of the ulcers begins to gradually clear of necrotic masses, red papillary granular growths resembling caviar appear. The shape of the ulcers is round, oval or irregular. The edges are smooth, undermined or scalloped, as if eaten away. In some cases, the edges hang over the bottom of the ulcer, forming pockets. The infiltrate around the ulcer is powerful; as it moves away from the ulcer, it becomes more gentle and gradually levels out with the surface of the skin. Ingoda infiltrate is involved in the ulcerative process in the form of a rim, slightly rising around the ulcer. Gradually, the bottom of the ulcer is completely cleared of necrotic deposits, the granulations quickly grow, the papillae become tall, they become covered with a whitish coating, deep grooves form between them, from which serous-purulent fluid is released when pressed. The papillae soon dry out and begin to be painlessly rejected, leaving a rough surface (Dobrotvorskaya’s symptom). The duration of the ulcerative stage without treatment is 2–4 months. After the papillae are rejected, the ulcer begins to scar (scarring stage). In the center of the ulcer, islands of epithelialization appear, which quickly increase, merge and cover the entire bottom of the ulcer. After the beginning of epithelization of the ulcer, further decay and necrotization of the infiltrate may continue. In this case, the bottom of the ulcer is covered with fresh epithelium, and its edges continue to ulcerate, hanging over the formed scar. The scarring stage of zoonotic leishmaniasis lasts 15–30 days. The period from the appearance of zoonotic leishmanioma to complete scarring is 3–6 months. Sometimes leishmaniomas are complicated by tubercles of contamination or leishmania lymphangitis.
Tubercles of contamination appear around the ulcer on the undissolved infiltrate. This occurs either as a result of repeated mosquito bites, or as a result of migration of amastigotes through the intercellular spaces from the main focus. Clinically, they are single or numerous rounded tubercles of dark red color, 2–5 mm in size. They may be isolated or merge with each other and with the underlying leishmanioma. Leishmania is found in the tubercles of infestation, but they appear in smaller quantities than in the main leishmanioma. With the zoonotic type of cutaneous leishmaniasis, tubercles of contamination are observed in 75% of patients
Other complications of the ulcerative stage of leishmanioma include lymphangitis and lymphadenitis. They arise in the first half of the ulcerative stage as a result of the migration of Leishmania through the lymphatic vessels up to the lymph nodes. Leishmania lymphangitis are dense, painless nodes ranging in size from a small pea to a hazelnut. The skin over them is initially slightly stretched, not fused with the nodes, of normal color, then the nodes begin to enlarge and become fused with the skin, which above them acquires a blue-red color and ulcerates. Other nodes do not open and remain in the same state for a long time, even after the underlying leishmanioma has healed.
A secondary infection may join the existing specific leishmaniasis lymphangitis. In such cases, swelling in some nodes increases, limited redness occurs that does not extend beyond the limits of lymphangitis, the temperature rises locally and body temperature also rises, and pain increases.
In the ulcerative stage of leishmaniasis, the bacterial flora has a great influence on both the clinical manifestations and the duration of the leishmaniasis process.
Histology
The tubercle is an infectious granuloma consisting of epithelioid cells, granular leukocytes, lymphoid and histiocytic elements. In the center, as a rule, there is a vessel with the phenomena of fibrinoid swelling and fibrinoid necrosis of the wall. Other vessels are thrombosed. The perivascular zone is saturated with protein fluid and leukocytes. Among epithelioid cells one can often see giant cells similar to Pirogov-Langhans cells. Other nodules are formed by an accumulation of histiocytes, granular leukocytes and lymphoid cells. They always contain many intracellularly located Leishmania.
Post-kala azar cutaneous leishmaniasis (PCCL)
Post-kala azar cutaneous leishmaniasis (PCCL) is usually a consequence of visceral leishmaniasis and manifests as a macular, papular or nodular rash, most often on the face, shoulders, trunk and other parts of the body. This clinical form of the disease is characteristic mainly of East Africa and the Indian subcontinent, where it is reported in 5-10% of patients with visceral (kala-azar) leishmaniasis. Typically, skin rashes appear between 6 months and one or more years after signs of visceral leishmaniasis have disappeared, but it can occur earlier. People with PCCL are considered a potential source of infection.
Leishmaniasis
In accordance with the clinical features, etiology and epidemiology, leishmaniasis is divided into the following types. Visceral leishmaniasis (kala-azar) 1. Zoonotic: Mediterranean-Central Asian (children's kala-azar), East African (dum-dum fever), mucocutaneous leishmaniasis (New World leishmaniasis, nasopharyngeal leishmaniasis). 2. Anthroponotic (Indian kala-azar).
Cutaneous leishmaniasis 1. Zoonotic (rural type of Borovsky's disease, Pendinsky ulcer). 2. Anthroponotic (urban type of Borovsky's disease, Ashgabat ulcer, Baghdad boil). 3. Cutaneous and mucocutaneous leishmaniasis of the New World (espundia, Breda disease). 4. Ethiopian cutaneous leishmaniasis.
Visceral Mediterranean-Asian leishmaniasis. Incubation period. Varies from 20 days to 3-5 months, in rare cases up to 1 year or more. In young children and rarely in adults, long before the general manifestations of the disease, a primary affect occurs in the form of a papule.
Initial period of the disease. Characterized by the gradual development of weakness, loss of appetite, adynamia, pallor of the skin, and a slight enlargement of the spleen. Body temperature rises slightly.
High period. It usually begins with a rise in body temperature to 39-40 °C. The fever takes on a wave-like or irregular pattern and lasts from several days to several months with alternating episodes of high fever and remissions. In some cases, body temperature during the first 2-3 months can be low-grade or even normal.
When examining patients, polylymphadenopathy (peripheral, peribronchial, mesenteric and other lymph nodes), enlargement and hardening of the liver and even to a greater extent of the spleen, painless on palpation, are determined. In cases of development of bronhadenitis, a cough is possible, and pneumonia of a secondary bacterial nature is not uncommon.
As the disease progresses, the condition of patients progressively worsens. Weight loss (even cachexia) and hypersplenism develop. Bone marrow lesions lead to progressive anemia, granulocytopenia and agranulocytosis, sometimes with necrosis of the oral mucosa. Manifestations of hemorrhagic syndrome often occur: hemorrhages in the skin and mucous membranes, bleeding from the nose, and gastrointestinal tract. Fibrous changes in the liver lead to portal hypertension with edema and ascites, which is facilitated by progressive hypoalbuminemia.
Due to hypersplenism and the high position of the diaphragm, the heart shifts somewhat to the right, its sounds become muffled, tachycardia and arterial hypotension develop. These changes, along with anemia and intoxication, lead to the appearance and worsening of signs of heart failure. Possible diarrhea, menstrual irregularities, impotence.
Terminal period. Cachexia, a drop in muscle tone, thinning of the skin, the development of protein-free edema, and severe anemia are observed.
The disease can manifest itself in acute, subacute and chronic forms. • Acute form. Occasionally found in young children. It develops rapidly and without treatment quickly ends in death. • Subacute form. Seen more often. Severe clinical manifestations are characteristic, lasting 5-6 months. • Chronic form. It develops most often, often occurring subclinically and latently.
With visceral anthroponotic leishmaniasis (Indian kala-azar), in 10% of patients, several months (up to 1 year) after therapeutic remission, so-called leishmanoids appear on the skin. They are small nodules, papillomas, erythematous spots or areas of skin with reduced pigmentation, which contain Leishmania for a long time (years and decades).
Cutaneous zoonotic leishmaniasis (Pendinsky ulcer, Borovsky disease). Found in tropical and subtropical countries. The incubation period varies from 1 week to 1.5 months, on average 10-20 days. At the site of the entrance gate, primary leishmanioma appears, initially representing a smooth pink papule with a diameter of 2-3 mm. The size of the tubercle quickly increases, and it sometimes resembles a boil, but is painless or slightly painful on palpation. After 1-2 weeks, necrosis begins in the center of the leishmanioma, resembling the head of an abscess, and then a painful ulcer up to 1-1.5 cm in diameter is formed, with undermined edges, a thick rim of infiltrate and abundant serous-purulent or sanguineous exudate; Small secondary tubercles often form around it, the so-called “tubercles of seeding”, which also ulcerate and, when fused, form ulcerative fields. This is how sequential leishmanioma is formed. Leishmaniomas are most often localized on exposed parts of the body, their number varies from a few to dozens. The formation of ulcers in many cases accompanies the development of painless lymphangitis and lymphadenitis. After 2-6 months, epithelization of the ulcers and their scarring begin. The total duration of the disease does not exceed 6-7 months.
Diffuse infiltrating leishmaniasis . It is characterized by pronounced infiltration and thickening of the skin with a large area of distribution. Gradually the infiltrate resolves without a trace. Minor ulcerations are observed only in exceptional cases; they heal with the formation of barely noticeable scars. This variant of cutaneous leishmaniasis is very rare in older people.
Tuberculoid cutaneous leishmaniasis . Sometimes observed in children and young people. It is characterized by the formation of small tubercles around scars or on them. The latter can increase and merge with each other. As the disease progresses, they occasionally ulcerate; subsequently the ulcers heal with scarring.
Cutaneous antroponotic leishmaniasis . It is characterized by a long incubation period of several months or even years and two main features: slow development and less severe skin lesions.
Complications and prognosis Advanced leishmaniasis can be complicated by pneumonia, purulent-necrotic processes, nephritis, agranulocytosis, and hemorrhagic diathesis. The prognosis of severe and complicated forms of visceral leishmaniasis with untimely treatment is often unfavorable. In mild forms, spontaneous recovery is possible. In cases of cutaneous leishmaniasis, the prognosis for life is favorable, but cosmetic defects are possible.
Main risk factors
Socio-economic conditions
Poverty is a risk factor for leishmaniasis. Poor housing conditions and unsanitary conditions (eg, lack of waste disposal systems, open sewers) can increase the number of breeding sites for mosquitoes, as well as their proximity to humans. Mosquitoes are attracted to crowded living conditions that are favorable for feeding on human blood. Behavioral factors such as sleeping outdoors or on the ground may also be associated with an increased risk of infection.
Poor nutrition
Protein-energy malnutrition and dietary deficiencies of iron, vitamin A and zinc increase the risk of developing clinical disease in the event of infection.
Population movement
Epidemics of the two most common forms of leishmaniasis are often associated with migration and the movement of non-immune people into areas where the infection is circulating. Professional activities and large-scale deforestation remain important morbidity factors.
Environmental changes
Urbanization and increased intensity of economic activity in forest areas may be factors for the incidence of leishmaniasis.
Changing of the climate
The epidemiology of leishmaniasis depends on a number of climatic factors:
- changes in temperature, precipitation and humidity can have a significant impact on the distribution area, survival and population sizes of vectors and reservoirs of infection;
- small fluctuations in temperature can have a profound effect on the developmental cycle of Leishmania promastigotes in mosquitoes, which may create conditions for transmission of the protozoa in areas not previously endemic for the disease;
- drought, famine and floods can trigger mass displacement and migration of populations into areas where leishmaniasis circulates, and poor nutrition can have a negative impact on immunity.
Cutaneous leishmaniasis
rural (zoonotic) type is characterized by a relatively short incubation period (from 1-2 to 3-5 weeks) and a not very long course (3-6 months). Typically, conical tubercles with a wide base, red-bluish color with a brownish or yellowish tint, and doughy consistency appear on exposed areas of the skin. Subsequently, the tubercles increase in size and after 1-3 months. open with the formation of a round or irregularly shaped ulcer with an uneven bottom and abundant serous-purulent exudate, which shrinks into layered dense crusts. The edges of the ulcer seem to be corroded. A doughy infiltrate of pinkish-bluish color forms in the circle, behind which strands of inflamed lymphatic vessels and the so-called rosary of secondary leishmaniomas are palpated. In children, a more acute course is observed with a furuncle-like, fluctuating pustular formation of the lesion, quickly abscessing and necrotizing. Often in adults and children the process is complicated by a purulent infection with the development of phlegmon and erysipelas. The inflammatory process ends in 3-8 months. with the formation of a scar and stable immunity to this pathogen. The urban (anthroponotic) type is found in cities and large settlements. It is characterized by a lengthened incubation period (on average 5-8 months, and sometimes 1-2 years) and a slow course of the process, hence the name - yearling. The disease is transmitted from an infected person or carrier through a mosquito vector. Small bumps of pinkish or reddish-brown color with a yellowish tint appear on exposed areas of the skin. Elements of round shape, doughy consistency. The infiltrate is not clearly expressed and disintegrates late. The ulcers are superficial with uneven roller-like edges and a granulating bottom, covered with grayish-yellow serous-purulent discharge. A border of inflammatory infiltrate usually forms around the ulcers. As with the zoonotic form, nodular lymphangitis (“rosary beads”) can form along the periphery. They sometimes ulcerate, turning into small, secondary (daughter) leishmaniomas.
The anthroponotic form includes a rare clinical form of cutaneous leishmaniasis - lupoid, or tuberculoid cutaneous leishmaniasis (metaleishmaniasis). This form is difficult to distinguish from ordinary lupus due to the appearance of tubercles on scars formed after regression of leishmania or along the periphery. The tubercles are flat, barely rising above the skin level, brownish in color, soft in consistency, giving a distinct brownish color on diascopy (apple jelly symptom). The number of tubercles can gradually increase, persist for a long time, and be difficult to treat. Tuberculoid leishmaniasis is most often localized on the skin of the face and is observed in childhood and adolescence. The development of this form of leishmaniasis is associated with immunodeficiency due to the presence of a focus of chronic infection, hypothermia, injury, or possible natural superinfection.
The atypical form of the anthroponotic type includes mucocutaneous and diffuse cutaneous leishmaniasis . Characteristic of these varieties is the slow formation process. Ulcerations develop late or are absent. Healing occurs within 1-3 years or even longer. The primary elements of mucocutaneous leishmaniasis are similar to the usual type in the form of a tubercle followed by ulceration. Metastatic spread of the process to the mucous membrane of the mouth, nose and pharynx occurs at an early stage of the disease, but can sometimes occur several years later. Erosion and ulceration of the tubercles is accompanied by the destruction of soft tissues, cartilage of the oral cavity and nasopharynx. At the same time, swelling of the nasal mucosa and red border of the lips develops. A secondary infection often occurs. The process ends with pronounced mutations.
Diffuse cutaneous leishmaniasis manifests itself as widespread elements of multiple tubercles on the face and on exposed areas of the extremities. Merging, the rashes resemble lesions in leprosy. Characterized by the absence of ulcerations and lesions of the mucous membranes. The disease does not go away spontaneously and is prone to relapse after treatment.
Diagnosis and treatment
Diagnosis of visceral leishmaniasis is made on the basis of the clinical picture in combination with parasitological or serological studies (for example, rapid testing). For the diagnosis of cutaneous and mucocutaneous leishmaniasis, serological tests are not of great interest; in these cases, the diagnosis is made on the basis of the clinical picture and the results of parasitological examination.
The choice of treatment for leishmaniasis depends on a number of factors, such as the clinical form, the presence of associated pathologies, the type of parasite and the geographical area. Leishmaniasis is treatable and can be cured completely, however, the effectiveness of drugs depends on the state of the patient’s immune system, and relapses are possible if the immune system is weakened. All patients with visceral leishmaniasis are advised to immediately receive a full course of treatment. Detailed information on the treatment of different forms of leishmaniasis depending on the geographical area is given in the WHO technical report series No. 949 on the control of leishmaniasis.
Publications in the media
Leishmaniasis is the general name for protozoal vector-borne infections caused by intracellular parasitic flagellated protozoa of the genus Leishmania.
Etiology • Various species of Leishmania • Vectors are female mosquitoes of the genus Phlebotomus and Lutzomyia. Pathomorphology • Severe lymphocytic infiltration in the affected organs and tissues in combination with necrotic and degenerative processes, accumulations of Leishmania, fibrosis • Ulcerations on the skin and nasopharyngeal mucosa.
Classification, epidemiology and clinical picture • Visceral leishmaniasis (Leishman-Donovan disease , internal leishmaniasis, cachectic fever, tropical splenomegaly) •• The causative agent is Leishmania donovani •• The main reservoirs in Eurasia and Latin America are rodents, foxes, jackals, porcupines and dogs. In Eastern India and Bangladesh, where the natural reservoir is humans, epidemics of leishmaniasis are recorded every 20 years •• Symptoms develop 3-12 months after infection. In the Asian region, in 75% of cases, symptoms appear in the first month after infection •• Clinical picture: abnormal fever, febrile attacks continue, gradually fading, for 2–8 weeks, then appear at irregular intervals; malabsorption and diarrhea; enlarged liver and spleen; lymphadenopathy; swelling; in persons with weak skin pigmentation, grayish spots are observed on the face and head; anemia and thrombocytopenia with subsequent hemorrhages, agranulocytosis, leukopenia; It is acute and severe with possible death.
• East African visceral leishmaniasis is a type of visceral leishmaniasis. The causative agent is Leishmania donovani subspecies archibaldi. Distributed throughout East Africa (from the Sahara in the North to the Equator). The disease is registered more often in men aged 10–25 years; It is characterized by skin lesions in the form of nodes, often ulcerating, with subsequent damage to internal organs.
• Indian visceral leishmaniasis (kala-azar, Assam fever, dum-dum fever ) is a type of visceral leishmaniasis. The causative agent is Leishmania donovani subspecies donovani. Distributed in Eastern India and Bangladesh; It is distinguished by dark skin coloring due to damage to the adrenal cortex.
• Visceral Mediterranean-Central Asian leishmaniasis (kala-azar Mediterranean childhood) is a type of visceral leishmaniasis. The causative agent is Leishmania donovani subspecies infantum. Distributed in Southern Europe, North Africa, Central Asia, the Middle East and North-West China. Observed mainly in children; characterized by an acute onset with high body temperature and enlarged lymph nodes.
• Cutaneous leishmaniasis of the New World (leishmaniasis mucocutaneous, leishmaniasis of the mucous membranes, American leishmaniasis) •• Pathogens - Leishmania braziliensis, Leishmania mexicana •• The disease is characteristic of the humid forests of Central and South America; reservoir - large forest rodents. Usually recorded among workers engaged in forestry and road work, among the population of forest villages •• Symptoms appear 1–4 weeks after the bite of the vector •• Clinical picture: characterized by painless, deforming lesions of the mouth and nose, metastasizing to neighboring areas with the appearance of mushroom-shaped and erosive ulcers on the tongue, buccal mucosa and nose; relapses are possible several years after the spontaneous disappearance of the primary lesions; destruction of the nasal septum, hard palate and destructive lesions of the pharynx are observed; The disease is accompanied by fever, weight loss and secondary bacterial infections.
• Brazilian mucocutaneous leishmaniasis (Espundia) is a New World type of cutaneous leishmaniasis. The causative agent is Leishmania braziliensis subspecies braziliensis. Distributed in the forests of South America east of the Andes, it occurs with extensive damage to the skin, subsequently the mucous membranes, usually the upper respiratory tract, sometimes with deep destruction of soft tissues and cartilage.
• Leishmaniasis Uta (uta) is a New World variant of cutaneous leishmaniasis. The causative agent is Leishmania peruviana. Distributed in the highlands of South America. Proceeds with the formation of single ulcers, scarring within a year.
• Cutaneous diffuse leishmaniasis is a New World variant of cutaneous leishmaniasis. Pathogens: Leishmania mexicana subspecies amazoniensis, Leishmania mexicana subspecies pifanoi, Leishmania mexicana subspecies venezuelensis, Leishmania mexicana subspecies garnhami. Clinical manifestations do not differ from Asian and African types of cutaneous leishmaniasis. However, cases of spontaneous recovery are observed less frequently. The carriers are mosquitoes of the genus Lutzomyia. Exceptions are lesions caused by Leishmania mexicana subspecies mexicana (rubber canker), common in Mexico, Guatemala, Belize; detected in rubber tappers (chiclero) and lumberjacks; The carrier is the mosquito Lutzomyia olmeca. Characterized by the formation of painless, non-metastasizing chronic (several years) ulcers, usually localized on the neck and ears. As a rule, gross deformations of the auricles (ear chiclero) are observed.
• Cutaneous leishmaniasis of the Old World (Borovsky's disease) •• Causative agent - Leishmania tropica •• Endemic infection with the highest incidence in the autumn months •• Natural reservoir - small rodents (mice, rats, hyraxes), carriers - mosquitoes Phlebotomus papatasi (the main carrier), Phlebotomus duboscqi, Phlebotomus salehi, Phlebotomus longpipes and Phlebotomus pedifer •• Distributed in areas bordering deserts with low groundwater •• Clinical picture: the incubation period lasts from 2 weeks to 5 months; characterized by lesions on open areas of the body, prone to ulceration and centrifugal spread; the bottom of the ulcer is covered with granulation tissue, the edges are inflamed; After 3–12 months, spontaneous healing is observed with the formation of a rough, pigmented scar.
• Cutaneous anthroponotic leishmaniasis (urban cutaneous leishmaniasis, late ulcerating cutaneous leishmaniasis) is a type of cutaneous leishmaniasis of the Old World. The causative agent is Leishmania tropica subspecies minor. Distributed in cities of the Mediterranean, the Near and Middle East, and the western part of the Hindustan Peninsula; characterized by a long incubation period and prolonged necrosis of infiltrates.
• Zoonotic cutaneous leishmaniasis (cutaneous acute necrotizing leishmaniasis, rural cutaneous leishmaniasis) is a type of cutaneous leishmaniasis of the Old World. The causative agent is Leishmania tropica subspecies major. Distributed in oases of deserts and semi-deserts of the Middle East, Central Asia, India and Africa. It is characterized by a short incubation period and rapid necrosis of infiltrates.
• Recurrent (lupus) leishmaniasis is an Old World type of cutaneous leishmaniasis. The causative agent is Leishmania tropica subspecies tropica. It is characterized by partial healing of lesions, intensive formation of granulomas, frequent development of concomitant lesions that contribute to the formation of granulomatous tissue without signs of cure, sometimes for many years.
• Cutaneous lupoid leishmaniasis (cutaneous tuberculoid leishmaniasis, metaleishmaniasis, paraleishmaniasis) is a relapse of cutaneous leishmaniasis, characterized by the formation of dense confluent brownish-red tubercles around healed leishmaniamas. Laboratory tests • Detection of pathogens in Romanovsky-Giemsa-stained smears and in cultures grown after culture of aspiration material from lymph nodes, bone marrow, spleen, or biopsy material from lymph nodes. In macrophages, bean-shaped amastigotes with a dark round nucleus and short rod-shaped kinetoplasts (Leishman–Donovan bodies) are found • The direct agglutination reaction reveals IgM, characteristic of the acute phase • Skin tests with leishmanin (Montegoro test): with the cutaneous form of leishmaniasis, positive 6-8 weeks after recovery, but not with diffuse leishmaniasis. For visceral leishmaniasis, the results are negative. The test is used only for epidemiological studies • For species identification, monoclonal antibodies or the DNA hybridization method are used • ELISA is a highly sensitive and specific method in the diagnosis of visceral lesions.
Differential diagnosis • Malaria • Brucellosis • Tuberculosis • Typhoid • Liver abscess • Syphilis • Leprosy • Yaws. TREATMENT Management tactics • Bed rest, oral hygiene, enhanced nutrition • Blood transfusions for anemia and antibacterial chemotherapy for bacterial complications (in addition to specific therapy) • Periodic monitoring of the functions of the cardiovascular system, liver, kidneys • To prevent relapses, examinations are carried out after 3 and 12 months.
Surgical treatment - splenectomy. Drug therapy • Drugs of choice •• Meglumine antimoniate (glucantim) - 20–60 mg/kg deep IM 1 time per day for 20–30 days •• If the disease relapses or treatment is insufficiently effective, a second course of injections should be given within 40–60 days. Additional administration of allopurinol 20–30 mg/kg/day in 3 doses orally is effective • Alternative drugs for relapses of the disease and resistance of the pathogen to the drugs of choice •• Amphotericin B - 0.5–1.0 mg/kg IV every other day •• In the absence of chemotherapy effect, additional human recombinant -IFN.
Complications • In the later stages of the disease, edema, cachexia, hyperpigmentation are possible • Bacterial superinfections and bleeding from the gastrointestinal tract can cause death in untreated patients with visceral leishmaniasis • 3–10% of treated patients develop skin changes in the form of depigmented spots and wart-like nodules on the face and extensor surface of the extremities • In patients with mucocutaneous leishmaniasis, metastatic destructive lesions of the nasopharyngeal mucosa are possible.
Course and prognosis • With early diagnosis and timely treatment, more than 90% of patients recover • Even with chemotherapy, mortality reaches 15–25% • Without treatment, 95% of adults and 85% of children die within 3–20 months. Prevention • Extermination of insect vectors, rodents and stray dogs in areas adjacent to populated areas • Persons visiting endemic foci are recommended to use personal protective equipment (repellents, mosquito nets, etc.) • Timely treatment of patients with leishmaniasis.
ICD-10 • B55 Leishmaniasis
Prevention and control
Prevention and control of leishmaniasis requires a multimodal approach because transmission occurs within a complex biological system involving the human or animal reservoir (hosts), the parasite and its vector (the mosquito). The main measures to prevent leishmaniasis include:
- Early diagnosis and prompt initiation of effective treatment help reduce the prevalence of the disease and prevent disability and death in patients. This provides an opportunity to reduce transmission and monitor the spread and burden of disease. There are now highly effective and safe drugs for the treatment of leishmaniasis, especially its visceral form, although their use can be difficult. WHO's efforts to harmonize prices and the WHO-brokered free drug program have greatly increased access to medicines.
- Vector control helps reduce disease or interrupt transmission by reducing mosquito populations. Insecticide spraying, insecticide-treated netting, environmental engineering measures and personal protective equipment are used to control vectors.
- Effective surveillance is important because it allows for rapid monitoring and intervention during epidemics and in situations where there are high case fatality rates among patients under treatment.
- Control of animal populations as reservoirs of infection requires a complex set of measures and therefore must be carried out taking into account local conditions.
- Social mobilization and strengthening partnerships: Community mobilization and health education and effective behavior change interventions must always be locally tailored. Working in partnership and collaboration with various stakeholders and programs to control other vector-borne diseases is critical.
WHO activities
WHO's work on leishmaniasis control is carried out in the following areas:
- technical and financial assistance to national leishmaniasis control programs to update guidance documents and develop disease control plans that include sustainable and effective surveillance systems and epidemic preparedness and response systems;
- monitoring epidemiological indicators and assessing the effectiveness of disease control activities, which contributes to increasing awareness and advocacy on the global burden of leishmaniasis, as well as ensuring equitable access to health care;
- developing evidence-based strategies and standards for the prevention and control of leishmaniasis, and monitoring their implementation;
- strengthening cooperation and coordination among partners and stakeholders;
- promoting research and use of effective treatments for leishmaniasis, including safe, effective and affordable drugs, diagnostics and vaccines;
- providing support to national disease control programs to ensure patient access to quality-assured medicines.