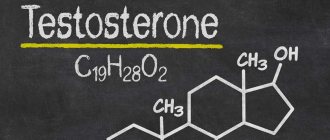
Testosterone is an androgen (a steroidal male sex hormone) that is produced in small quantities in a woman’s body. It takes part in the regulation of metabolism and affects reproductive function.
Physiological changes in testosterone concentration are observed during puberty, pregnancy and menopause. Pathological causes of hormonal imbalance are severe damage to organs and systems, diseases of the endocrine glands, and tumor formations.
One of the most common female hormonal problems is an increase in testosterone levels in the body. The official name of the disease is hyperandrogenism.
Testosterone hormone levels play an important role in a woman's health. Deviation from the norm of this indicator should be a signal for immediate action.
Optimal testosterone hormone levels
There are two types of this substance - free and common.
- Free testosterone hormone is the concentration of a substance not associated with sex hormones and proteins.
- Total testosterone is a total indicator of the concentration of a substance that is free and bound to hormones and proteins.
The normal level of total testosterone hormone is from 0.26 to 1.3 ng/ml. The concentration of the free component depends on the woman’s age:
- Before age 39, levels should be between 0.13 and 3.09 ng/L.
- In women over 40 to 59 years old - from 0.13 to 2.6 ng/l.
- In women over 60 years of age, from 0.13 to 1.8 ng/l.
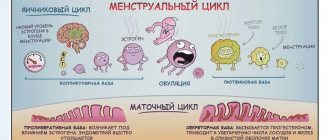
The production of the substance may vary depending on the time of day and the phase of the menstrual cycle.
What is estrogen?
Estrogen is a class of related hormones that includes estriol, estradiol, and estrone.
Estriol is produced from the placenta during pregnancy. It is the main sex hormone in pregnant women. It is formed from developing ovarian follicles.
Estradiol is responsible for female characteristics and sexual functioning. In addition, estradiol is important for bone health in women. Changes in estradiol levels contribute to the development of most gynecological problems, including endometriosis, fibroids and even female cancers.
Estrone is widely distributed throughout the body. This is the main estrogen present after menopause.
Decreased testosterone levels
Low concentrations of the hormone can cause low sexual desire, decreased muscle tissue and increased fat, dry skin, as well as apathy and depression.
If the production of testosterone and estrogen decreases at the same time, the production of lubricant during sexual intercourse stops, and therefore the woman experiences severe pain.
The main reason for low concentrations of the hormone testosterone in women is menstrual irregularities. This can lead to serious consequences, so this condition should not be ignored.
Insufficient testosterone production can also be caused by diseases of the endocrine system, insufficient physical activity, weak immunity, and vitamin deficiency.
To prevent hormonal disorders, experts recommend leading a full sex life, eating a balanced diet, and avoiding stress and emotional stress. In case of low testosterone levels, you should definitely consult a doctor who will prescribe corrective treatment.
The role of testosterone in a woman's body
Many people believe that testosterone is produced only in the male body. In fact, this is not at all the case and the hormone is found in a certain amount normally in the body of women, performing a number of functions. Why is it needed and what is the role of testosterone in a woman’s body?
Among the main functions and hormones in the female body are:
- Helps maintain normal muscle development
- helps strengthen bone tissue
- helps strengthen teeth
- supports sexual activity and is aimed at reproduction
- takes part in the development of hair follicles, controls the growth of body hair
- controls the functioning of the nervous system
- regulates the production of adipose tissue and maintains its level in the body
- participates in the metabolic process
- regulates water-salt metabolism
- prevents the development of diseases of the circulatory system
- Helps the body resist stress.
High concentration of testosterone
Excessive production of the hormone testosterone in women can be triggered by poor nutrition, too intense activity of the adrenal glands, or a hereditary predisposition.
The following symptoms may indicate an increased level of the hormone: male-pattern hair growth, changes in figure and voice, dry skin, hair loss, irritability, aggressiveness.
In the future, high levels of the hormone can cause the development of hyperandrogenism, menstrual irregularities, ovarian tumor formation and infertility. Therefore, if you have symptoms that indicate excessive testosterone production, you should definitely consult a doctor. Diagnostics and surgical treatment will help stabilize hormonal levels and protect against possible complications.
To the beginning of the section:: » Female hormones
Hyperandrogenism is a condition associated with excessive secretion of androgens and/or their enhanced effects on the body, which in women most often manifests itself as virilization (the appearance of male characteristics).
In women, circulating androgens (a group of steroid hormones) are normally synthesized in the ovaries and adrenal glands, as well as through the peripheral conversion of androstenedione (AND) and dehydroepiandrosterone (DHEA) to testosterone (T). The following androgens and their precursors are of great importance in clinical practice: testosterone, dihydrotestosterone (DHT), 17-OH-progesterone (17-hydroxyprogesterone), AED, dehydroepiandrosterone sulfate (DHEAS).
Among all endocrine diseases in gynecological practice, the most common pathology of the thyroid gland and hyperandrogenism, which we are considering. To understand this problem, it is necessary to turn to the scheme of androgen synthesis, simplifying it as much as possible. The whole process is mainly controlled by pituitary hormones - ACTH (adenocorticotropic hormone) and LH (luteinizing hormone). The synthesis of all steroid hormones begins with the conversion of cholesterol to pregnenolone. It is important to understand the following: this stage occurs in all steroid-producing tissues! The remaining processes also occur to one degree or another in all organs related to steroidogenesis, but as a result, different organs produce both the same and different steroid hormones.
In addition, it must be added that the production of steroid hormones can occur not only in endocrine organs, but also in the periphery. In particular, for women, subcutaneous fatty tissue plays an important role in the production of steroids.
Most physicians do not consider it necessary to evaluate androgen levels in postmenopausal women with clinical symptoms or biochemically confirmed increases due to the lack of correlation between their levels and various changes in the body that occur during the natural aging process. However, in the presence of clinical symptoms of hyperandrogenism, it is imperative to carry out a differential diagnosis of diseases that lead to excess androgens in postmenopausal women. Consideration of possible key points in the history, physical examination, and appropriate laboratory and radiological evaluation will lead to the selection of optimal treatment strategies for the identified causes of hyperandrogenism.
Leading signs and symptoms
Most postmenopausal women with hyperandrogenism complain of hirsutism in the face and/or trunk along with hair loss on the scalp. A detailed history is critical in distinguishing progressive hirsutism from true virilization. Hirsutism is the excess growth of terminal hair, especially on the chin, upper lip and linea alba [13]. The degree of hirsutism can be assessed using the Ferriman-Gallwey scale (1961), which describes the degree of hair growth in points from 0 to 4 in 9 areas of the body. It is believed that in premenopausal women the degree of hirsutism is 8 points or more, although in postmenopausal women the total score is not as reliable. Virilization is a combination of severe hirsutism together with male pattern baldness, anabolic (android) type of obesity, decreased voice and clitoromegaly (≥1.5×2.5 cm) [13]. The presence of virilization symptoms suggests severe hyperandrogenism and the need for examination to detect a tumor (see table).
Polycystic ovary syndrome (PCOS)
PCOS (Stein-Leventhal syndrome) is a combination of oligomenorrhea or amenorrhea and bilateral multiple ovarian cysts. The most common causes of this disease are menstrual irregularities, infertility, hirsutism and obesity [4]. The diagnosis is made in the presence of hyperandrogenism and chronic anovulation [2]. With PCOS, there is an increased risk of developing insulin resistance and hyperinsulinemia; diabetes mellitus is observed in 20% of patients.
Women with PCOS are a special group because the disease persists until the menopausal transition. Because ovarian T synthesis decreases gradually during natural menopause [6], symptoms of hyperandrogenism associated with PCOS may persist. A carefully collected medical history confirming the regularity of the menstrual cycle, the time of onset of symptoms of hyperandrogenism and weight gain will help determine an accurate diagnosis [20]. Since women with PCOS have an increased risk of developing metabolic syndrome, type 2 diabetes mellitus and hyperplastic processes in the endometrium [15], methods for their early detection and appropriate treatment should be determined.
Hyperandrogenism caused by obesity
It is known that not all overweight women have PCOS. In clinical practice, the combination of obesity and symptoms similar to PCOS is widespread, but they are very different from the symptoms of classic PCOS, and therefore a separate group of women with “obesity-induced hyperandrogenism” has been identified [19]. These women usually experience timely menarche and a regular menstrual cycle, but a history of weight gain is often associated with pregnancy without a decline to baseline over subsequent years. Against this background, the menstrual cycle in women becomes irregular with the onset of premenopause. Later, signs of hyperandrogenism develop, often with the formation of cysts in the ovaries, but without the increased LH/FSH ratio characteristic of PCOS. It is believed that excess aromatase and 5α-reductase in adipose tissue causes a local increase in the levels of estrogens and androgens, leading to irregular cycles, hirsutism and acne, respectively. A similar scenario is observed when a critical threshold body weight is reached during the postmenopausal period. In this case, the history and timing of weight gain will also help distinguish between hyperandrogenism due to PCOS or caused by obesity. The stigmas associated with PCOS help make the diagnosis, but they do not go away with weight loss. As clinical practice shows, the phenotypic symptoms of hyperandrogenism caused by obesity disappear with a decrease in body weight.
Differential diagnosis of PCOS and obesity-induced hyperandrogenism in postmenopausal women is quite difficult. While T levels fall sharply with surgical menopause, natural menopause results in a more gradual decline in T levels with age (H. Judd et al., 1974). S. Winters et al. [26] proved that T levels are always higher in women with PCOS compared to age-matched control women. However, a limitation of this study is that the oldest age group included only women aged 47 to 57 years and was not stratified by status regarding age at menopause. There were also no data examining detailed changes in androgen levels in women with PCOS after menopause compared with those in obese women with the same body mass index (BMI) who did not have PCOS.
Cushing's syndrome
Cushing's syndrome is a condition characterized by excess production of glucocorticoids by the adrenal glands. Most patients experience an increase in body weight with fat deposition on the face (moon face), neck, and torso. Characteristic clinical manifestations are hirsutism, menstrual dysfunction, infertility, limb muscle atrophy, osteoporosis, decreased immunoresistance, impaired glucose tolerance, depression and psychosis. The following variants of the syndrome exist:
A. ACTH-dependent pituitary syndrome - most often a tumor lesion of the pituitary gland - and ectopic - secretion of ACTH (or corticoliberin) by a tumor of any location.
B. ACTH-independent adrenal syndrome - cancer, adenoma or hyperplasia of the adrenal cortex - and exogenous - self-medication with glucocorticoid drugs or forced use of these drugs for another pathology.
Hyperthecosis (Frenkel syndrome)
Ovarian hyperthecosis is a bilateral proliferation of the ovarian stroma due to proliferation and luteinization. Clinically, it is manifested by high virilization of the female body due to the production of DHEA, androstenedione and T by ovarian stromal cells. The content of estrone (E1) in the plasma is significantly increased. The ratio of LH and FSH is normal or reduced. Clomiphene test is negative.
Back in 1942, J. Gains noted the connection between the clinical manifestations of virilization and the presence of islands of luteinized cells in the ovarian stroma. Subsequently, this phenomenon began to be called stromal hyperthecosis. According to E.G. Ivanov, hyperthecosis is the presence of a group of luteinized cells that are located in patches or diffusely in the stroma, occupying the chyme area. Unlike PCOS, in hyperthecosis cystic atretic follicles are present in small numbers or absent, and the tunica albuginea is most often not thickened.
Hyperthecosis is a severe form of PCOS that leads to excessive production of androgens by ovarian stromal cells (S. Braithwaite et al., 1978). This disease can occur in both pre- and postmenopausal women. Although the underlying etiology of the disease is unclear, the cause of ovarian hyperthecosis is believed to be associated with elevated levels of gonadotropins, mainly LH [12]. The ovaries may be normal size for premenopause but not for postmenopause; therefore, when interpreting ultrasound results, it is necessary to take into account the woman’s age and menopausal period [3]. With hyperthecosis, along with hypersecretion of androgens, the production of estrogen in the periphery is also increased, and these women are often at risk for developing endometrial hyperplasia and ovarian carcinoma [12]. A history of PCOS symptoms may help in making a differential diagnosis. Women with hyperthecosis are at risk of developing metabolic complications - hyperlipidemia and type 2 diabetes mellitus [12, 16], although there have been no large studies examining their incidence, clinical manifestations and long-term consequences in such postmenopausal patients.
Thus, hyperthecosis is a non-tumor pathology of the ovaries, in which proliferating islands of luteinized cells appear in their stroma. With hyperthecosis, the production of T, ANT and DHEA increases to a greater extent than with PCOS. LH and FSH levels may be normal or decreased, and the degree of insulin resistance and hyperinsulinemia exceeds that in PCOS.
Tumors of the ovaries or adrenal glands
Women with ovarian or adrenal tumors have rapidly progressive signs of virilization due to high androgen levels.
Androblastoma is a unilateral ovarian tumor that occurs at any age. It has a two-phase effect on a woman’s body: defeminization and then masculinization. Hypomenorrhea is characteristic, then amenorrhea, severe hirsutism, clitoral hypertrophy, hypoplasia of the uterus and mammary glands; in menopause - baldness, change in voice timbre.
The histological picture of ovarian tumor tissue in patients with hyperandrogenism is represented by tumor Leydig cells, Sertoli cells and ovarian steroid cells (L. Morgan, 1990). The number of cases of these tumors is 10% among all ovarian tumors. The literature describes less than 150 cases of tumors consisting only of Leydig tumor cells [11]. This type of tumor produces T and/or other androgen fractions with or without estrogens.
Adrenal tumors secrete androgen prohormones: DHEA, DHEAS and, less commonly, T, glucocorticoids and/or estrogens [14, 23]. True Cushing's disease should be differentiated from non-pituitary Cushing's syndrome, in which excess endogenous cortisol is a manifestation of the so-called ectopic or paraneoplastic ACTH syndrome (ACTH-dependent glucocorticoid excess), adenoma or carcinoma of the adrenal cortex, or exogenous intake of glucocorticoids (ACTH-independent glucocorticoid excess) . Hormonal examinations in patients with Cushing's syndrome reveal very high levels of cortisol (hypercortisolism), high levels of T, but, as a rule, slightly reduced levels of DHEAS [28]. In contrast, high levels of DHEAS are observed in adrenal carcinoma, although the tumor grows slowly and is diagnosed when the size is greater than 8-10 cm. However, cases of adrenal tumors that secrete not only DHEAS, but also T [5] have been described.
Congenital adrenal hyperplasia
Congenital adrenal hyperplasia (CAH) is an inherited genetic disorder associated with inherited deficiency of 11β- or 21-hydroxylase.
Depending on the ethnicity of the patient, 2 forms of CAH are distinguished: congenital adrenal hyperplasia, classical (rare) or non-classical (more often), can cause hyperandrogenism in postmenopause [22]. Family history of early pubarche, short stature and ethnicity (increased risk in Eskimos, Ashkenazis, Spaniards, Italians) suggest CAH, confirmation of which requires laboratory and instrumental assessment. At the same time, the synthesis of corticosteroids increases, which leads to stimulation of the release of ACTH by the pituitary gland, and this in turn leads to increased secretion of androgens by the adrenal cortex, followed by its hyperplasia. Along with virilization, patients develop weakness, muscle pain, decreased blood pressure, and skin pigmentation. The level of 17-OX in urine is reduced, but as the total level of 17-KS increases, the level of DHEA increases. Radiologically, the shadow of the adrenal glands is more intense with hyperplasia than with a tumor.
Treatment [19] with aromatase inhibitors for breast cancer may provoke hyperandrogenism in postmenopausal women with previously unknown CAH.
Iatrogenic hyperandrogenism
The use of medications and/or nutritional supplements, as well as T gel used by a partner, can lead to hirsutism in postmenopausal women [13]. To treat menopausal symptoms, some women use a combination of estrogens with T or DHEA, which in pharmacological doses causes hirsutism and other symptoms of hyperandrogenism. Careful evaluation of your partner's medications, supplements, and hormone therapy will help identify these reversible causes.
Laboratory research
A medical history and physical examination are carried out before laboratory and instrumental examinations. The most important laboratory tests to detect hyperandrogenism are the determination of testosterone and DHEAS levels [13]. The definition of DHEA is not very informative, since this hormone has an unstable rhythm during the day and changes under stress. Most commercial tests have been developed for the determination of total T in men because the sensitivity threshold for T levels in women is very low [17]; however, T levels significantly above the normal range can be used to diagnose hyperandrogenism and monitor its dynamics during treatment [17, 22]. Considering that the secretion of androgens in the ovaries and adrenal glands decreases with age [6], one should be critical of the reliability of assessing androgen levels in hyperandrogenism in old age. Relatively recently, test systems have been developed to determine T levels using mass spectrometry, which are more sensitive to low T levels than the radioimmunoassay method. With the advent of this method, it became possible to determine normal or elevated levels of androgens throughout a woman’s life [18]. There is evidence to suggest that hyperprolactinemia may be a cause of hirsutism; in this regard, it is necessary to determine and monitor prolactin levels in women of different age groups [13]. The main diagnostic test for Cushing's syndrome is to determine the level of free cortisol in 24-hour urine or saliva after a test with 1 mg of dexamethasone [14, 23]. Patients with adrenal adenoma in most cases have normal levels of free cortisol. In this regard, the dexamethasone test is an ideal test for identifying patients with subclinical forms of Cushing's syndrome [28]. After some doubt and debate in the press about whether there are threshold levels of T and DHEAS that define the presence of ovarian or adrenal tumors, it has been determined that elevated levels of T >200 ng/dL (6.94 nmol/L) or DHEAS > 800 ng/ml (2171 nmol/l) may suggest the presence of ovarian or adrenal tumors. However, the results of a study of 478 women (pre- and postmenopausal) with signs and symptoms of hyperandrogenism are contradictory. In 11 (2-3%) cases, a high T level >250 ng/dL (8.68 nmol/L) was detected, and only 1 of 11 had an ovarian tumor. Of the 10 women with DHEAS levels >600 ng/ml (1628 nmol/l), 9 had an adrenal tumor [24]. In another study of 60 women diagnosed with an androgen-producing ovarian tumor, most had T levels ranging from 100 to 200 ng/dL (3.47 to 6.94 nmol/L) [1]. The results of this study indicate that androgen-producing ovarian tumors also occur at T levels ≤200 ng/dL. In order to carry out differential diagnosis between tumors of the ovaries and adrenal glands, some researchers suggest conducting 2-5-day tests with minimal doses of dexamethasone [9]. Reduction of initially elevated T, AED, or DHEAS levels allows identification of the ovarian origin of hyperandrogenism. Research in postmenopausal women with hyperandrogenism is very limited, and therefore this problem requires further study.
Approaches to the treatment of postmenopausal hyperandrogenism
Treatment methods depend on the etiology of hyperandrogenism. Drug and/or surgical treatment of the primary disease (ovarian or adrenal tumors, hyperthecosis, iatrogenic causes, CAH or pituitary tumor) has a positive effect, manifested by a decrease in hyperandrogenism. GnRH analogues can be used for both diagnostic and therapeutic purposes. If androgen levels decrease in response to GnRH analogs, the tumor is considered gonadotropin-dependent; however, this has not been confirmed by controlled studies.
Treatment of hirsutism is discussed in the latest recommendations of the Endocrine Society and includes the use of drugs such as spironolactone or flutamide, which have an antiandrogenic effect [13]. Although cyproterone acetate, a potent antiandrogenic progestogen, is not used to treat hyperandrogenism in the United States, it is widely used in Europe [13]. Insulin sensitizers have been found to suppress androgens in premenopausal women with PCOS [10], although no data exist on their effects in postmenopausal women.
Cardiometabolic consequences of hyperandrogenism. Do I need to treat?
Data regarding the frequency and extent of androgen excess during menopause are limited. There is no available information about the long-term effects of excess androgen production at the organ and system level. However, high levels of androgens are known to disrupt blood lipids (increase LDL, decrease HDL, and increase triglycerides) [25]. Recently, there have been reports of an association between advanced glycation end products and T levels in postmenopausal women, regardless of the presence or absence of insulin resistance [7]. There is also evidence of an increase in hematocrit in women with tumors, followed by a fall after tumor removal [27]. A high T/E ratio is accompanied by worsening insulin resistance and can provoke arterial hypertension and fluid retention. Recent studies [8] have shown that high T levels in women correlate with an increased risk of breast cancer and heart disease. According to L. Shaw et al. [21], in a group of 390 postmenopausal women with clinical signs of ischemia, 104 women with a history of irregular menstrual cycles and hyperandrogenism, according to angiogram, were more likely to have coronary heart disease, as well as obesity, metabolic syndrome and diabetes mellitus. Postmenopausal women with symptoms of hyperandrogenism need to undergo a thorough examination and adequately selected treatment. A carefully collected anamnesis, physical examination, laboratory (tandem mass spectrometry) and radiological examination methods will allow us to establish a diagnosis, which will lead to the selection of appropriate medical or surgical treatment for postmenopausal women with hyperandrogenism.
Why do estrogen levels drop?
Why do estrogen levels drop?
There are many reasons why estrogen levels drop, including:
- hypogonadism;
- hypopituitarism;
- disruption of pregnancy (estriol);
- perimenopause and menopause (estradiol);
- polycystic ovary syndrome (PCOS);
- anorexia nervosa (eating disorder);
- extreme exercise or training;
- medications that block estrogen, such as clomiphene.
Additionally, women experience low estrogen levels immediately after childbirth, as well as during breastfeeding.
How do you know if your hormone levels are normal?
A gynecologist-endocrinologist can assess your health status and symptoms already during the physical examination. At this point, he determines whether further laboratory tests are needed to check hormone levels. These tests may be important if PCOS is suspected or if the patient has stopped menstruating due to excessive exercise or anorexia nervosa.
If tests show abnormal hormone levels, the doctor will definitely prescribe treatment.
If you find an error, please select a piece of text and press Ctrl+Enter