Sexuality and attractiveness are factors that are very important for representatives of the fair half of humanity. Many people spend a huge amount of time and money to achieve such results and obtain such qualities. However, few people know that the male hormone testosterone is responsible for this.
This is a useful element for the female body, which is necessary not only for beauty, but also for health. If its indicators decrease, this automatically affects health and beauty. This is why it is so important to know how to increase testosterone in women.
Brief information about testosterone
The hormone is very important for the female body. Among the benefits that it provides a woman are the following:
- Provides a positive psychological attitude;
- Has a positive effect on normal sexual desire;
- Makes it possible to maintain youth and feminine attractiveness for many years;
- Forms building muscle tissue;
- Helps burn fat;
- Effectively controls the functioning of the sebaceous glands;
- Positively affects the functioning of the bone marrow;
- Completely normalizes natural lipid metabolism;
- Promotes protein production;
- Reduces the amount of glucose in the overall blood composition.
An insufficient amount of this hormone is quite dangerous for any woman.
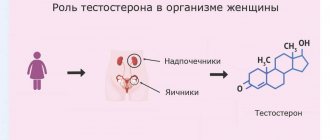
That’s why it’s so important to know how to properly increase testosterone. The production of this element occurs in the adrenal glands and ovaries. As for the main building material necessary for the formation of testosterone, this is ordinary cholesterol.
The highest levels of important testosterone are observed in young girls who have just passed natural puberty and during pregnancy in adult women. Then, with age, its amount begins to gradually decrease. The minimum amount of the hormone is observed during female menopause.
The role of androgens in women: what do we know?
Until recently, androgens in women were considered only as a cause of various metabolic and functional disorders, but their role in the female body is still not fully understood. Using polycystic ovary syndrome (PCOS) as an example, it is well known that elevated androgen levels often correlate with anovulation, infertility, as well as disorders of fat and carbohydrate metabolism [1]. At the same time, antiandrogen therapy did not solve these problems [2–4]. Androgens are perceived by most clinicians as “male” sex hormones, but is this true? In the last decade, androgen deficiency conditions in women, which can lead to a deterioration in the quality of life and sexual disorders, have been actively studied [5–7]. The influence of androgens on libido and a sense of well-being in women has now been proven [7–10], but their role in the genesis of metabolic disorders is still not fully known. The issues of the influence of androgens on bone, muscle tissue and hematopoiesis in the female body also remain unresolved.
Production and transport of androgens in the female body
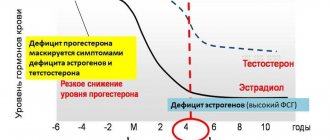
The pituitary gland regulates androgen secretion in women through the production of luteinizing hormone (LH) and adrenocorticotropic hormone (ACTH). The main androgens in serum in women with a normal menstrual cycle are testosterone and dihydrotestosterone. Dehydroepiandrosterone sulfate (DHEA-S), dehydroepiandrosterone (DHEA) and androstenedione are considered prohormones because only conversion to testosterone reveals their full androgenic properties. DHEA is produced mainly in the zona reticularis of the adrenal glands, as well as in the theca cells of the ovaries [11]. Testosterone is synthesized as follows: 25% is synthesized in the ovaries, 25% in the adrenal glands, the remaining 50% is produced as a result of peripheral conversion mainly in adipose tissue from androgen precursors, which are produced by both glands [12]. Healthy women of reproductive age produce 300 mcg of testosterone daily, which is approximately 5% of the daily production in men [13]. Unlike the fairly dramatic decline in estrogen production associated with menopause, levels of androgen precursors and testosterone decline gradually with age. A decrease in DHEA-S levels occurs as a result of decreased adrenal function. Concentrations of DHEA-S, which is not bound to any protein and does not change during the menstrual cycle, are approximately 50% in women aged 40–50 years compared to the concentrations observed in 20-year-old women [14–16]. Similar dynamics were also noted in the secretion of testosterone [17].
It is known that androgens are precursors of estrogens, which are formed from testosterone by aromatization in granulosa and theca cells of the ovaries, as well as in peripheral tissues.
In plasma, testosterone is predominantly in a bound state, with 66% bound to sex hormone binding globulin (SHBG), 33% bound to albumin, and only 1% in an unbound state [17]. Some diseases (thyrotoxicosis, liver cirrhosis), as well as taking estrogens as part of combined oral contraception (COCs) and hormone replacement therapy (HRT) can lead to a significant increase in SHBG and a decrease in the free fraction of testosterone [18]. Consequently, pathology of the pituitary gland, ovaries, adrenal glands, as well as diseases accompanied by a deficiency of adipose tissue or an increase in SHBG, can lead to the development of androgen deficiency conditions in women.
The final metabolites of testosterone are 5-alpha-dehydrotestosterone and estradiol, the amount of which is several times less than testosterone, from which we can conclude that the concentration of androgens in women is several times higher than the concentration of estrogens. Thus, studying the role of androgens, as well as replacement therapy for androgen deficiency conditions in women, including those receiving HRT with estrogens and progestins with insufficient effect, has a convincing biological basis.
The effect of androgens on fat and carbohydrate metabolism
One of the discussed side effects of testosterone is the negative effect on lipid metabolism, which consists of a decrease in high-density lipoproteins (HDL). Many studies have noted that higher levels of total testosterone and free androgen index were directly proportional to total cholesterol, low-density lipoprotein (LDL) and triglycerides, on the one hand, and lower levels of HDL, on the other [19–21]. This relationship was most clearly observed in women with PCOS [22]. Studies with oral methyltestosterone also showed a significant reduction in HDL cholesterol with normal or reduced LDL levels [23]. For many years, this fact was the main argument of opponents of the use of androgens in women.
At the same time, when using parenteral forms of testosterone (implants, intramuscular injections and transdermal drugs), no decrease in HDL was observed [24], and in women receiving estrogen replacement therapy, when adding testosterone undecanoate daily and even when supraphysiological concentrations of testosterone were achieved, significant reduction in total cholesterol and low-density lipoproteins [25].
Bell R. et al. examined 587 women aged 18 to 75 years who did not present any complaints. There was no statistically significant relationship between the concentrations of endogenous testosterone, its adrenal precursors and HDL levels, while SHBG levels were inversely related to LDL and triglyceride levels [26].
A population-based study in Sweden found that women with low androgen levels had higher cardiovascular morbidity, including those on HRT, even if they controlled lipid levels. However, logistic regression analysis showed that the concentration of total testosterone was directly proportional to HDL and LDL in all women, while the level of androstenedione was positively associated with HDL and negatively with triglycerides [27].
Interestingly, levels of DHEA-S, total and free testosterone, and the free androgen index are inversely correlated not only with body mass index, but also with waist-to-hip ratio in both men and women [28, 29], however in the female population this pattern was less pronounced [28].
For many years, an association between hyperandrogenism and insulin resistance has been found in women with PCOS [1], but research data have shown that therapy with flutamide and gonadotropin-releasing hormone agonists did not improve insulin sensitivity in these patients [5–7]. Conflicting data that were obtained in women without PCOS in some studies did not confirm the relationship of testosterone with insulin resistance [30, 31]. Removal of an androgen-producing tumor in a patient with severe hyperandrogenism after 9 months led to a marked deterioration in peripheral insulin sensitivity [32].
Androgens and cardiovascular morbidity in women
Most often, researchers associate the effect of androgens on cardiovascular risk with the clinical model of hyperandrogenism in PCOS. In women with PCOS, there was an increase in the level of endothelin-1, a marker of vasopathy, free testosterone, and insulin. Administration of metformin, which increases the sensitivity of peripheral tissues to insulin, for 6 months contributed to a significant decrease in endothelin-1 levels, a decrease in hyperandrogenism and hyperinsulinemia, and an improvement in glucose utilization [33]. A meta-analysis of randomized clinical trials also showed that metformin therapy in patients with PCOS resulted in a decrease in androgen levels [34], suggesting a primary role of hyperinsulinemia in increasing androgen secretion in women.
The thickness of the intima-media of the carotid arteries, determined using ultrasonography, is one of the most popular markers used by researchers to determine the severity of atherosclerosis [35]. A large number of publications focused on measuring intima-media thickness and determining androgen levels once again confirms this. Bernini et al. 44 patients with physiological menopause were examined. The levels of total and free testosterone, androstenedione were studied, and the thickness of the intima-media of the carotid arteries was measured. An inverse correlation was noted between androgen levels and intima-media thickness, a sign that most reflects atherosclerotic changes in blood vessels: in women with the smallest intima-media thickness, androgen levels were in the upper third of the normal range, and in women with the greatest, in the lower quarter. Based on the study, the authors concluded that androgens may have a beneficial effect on the carotid artery wall in postmenopausal women [36]. Other authors came to a similar conclusion in their studies [37–39].
Hak et al. studied the relationship between the levels of total and bioavailable testosterone and the intima-media thickness of the abdominal aorta in men and women. While in men there was a clear inverse correlation between the levels of total and free testosterone, in women the levels of these androgens were positively correlated with aortic atherosclerosis, but this correlation became statistically insignificant after taking into account other cardiovascular risk factors [40].
An important factor in the development of serious cardiovascular complications is vasospasm. Worboys S. et al. investigated the effects of parenteral testosterone therapy in women receiving HRT with estrogens and progestins. We examined 33 postmenopausal women receiving HRT with testosterone implants (50 mg) for more than 6 months. The control group consisted of 15 women who did not receive any therapy. Ultrasound was used to study brachial artery diameter, reactive hyperemia (endothelium-dependent vasodilation) and the effect of nitroglycerin (endothelium-independent vasodilation). In the study group, there was an increase in testosterone levels, which was associated with a 42% increase in endothelium-dependent vasodilation. No changes were noted in the control group. Similar data were obtained regarding endothelium-independent vasodilation. The authors concluded that parenteral testosterone therapy in postmenopausal women receiving long-term HRT improves both endothelium-dependent and endothelium-independent vasodilation of the brachial artery [42].
The effect of androgens on the musculoskeletal system in women
A number of studies have shown a positive effect of endogenous androgens on bone mineral density (BMD) in postmenopausal women. E. C. Tok et al. examined 178 postmenopausal women who had never received HRT [43]. Androgen levels (DHEAS, androstenedione and free testosterone) and their correlation with BMD measured by dual-energy x-ray absorptiometry were examined. It was noted that DHEAS and free testosterone levels were positively associated with lumbar spine and femoral neck BMD. At the same time, data analysis using linear regression showed a different effect of androgens on bone tissue. Thus, free testosterone was independently associated with lumbar spine mineral density (trabecular bone tissue), while DHEAS was independently associated with femoral neck mineral density (cortical bone tissue). According to the authors, different androgens have different effects on different types of bone tissue. S. R. Davis et al. in their study showed that among two groups of postmenopausal women receiving HRT with estrogens and estrogens in combination with testosterone, BMD was significantly higher in group 2 [44].
Women with androgen deficiency associated with HIV infection are more likely than the general population to develop osteoporosis and increase the risk of fractures. In a study by S. Dolan et al. it was noted that the risk of osteopenia and osteoporosis in such patients was associated with low levels of free testosterone [45].
The influence of androgens on hematopoiesis
The effects of testosterone on erythropoietin were noted back in the 60s of the 20th century [46]. L. Ferrucci at al. in a study of 905 patients over 65 years of age (exclusion criteria were cancer, chronic renal failure, and taking medications that affect hemoglobin concentration), it was found that the hemoglobin level correlated with the level of free testosterone in both men and women; in addition, it was noted that that with low testosterone levels, the three-year risk of developing anemia was higher than with normal levels (4.1 times in women and 7.8 times in men) [47]. Another study in women with anemia associated with HIV infection showed a similar pattern [48]. In women with PCOS receiving antiandrogen therapy, a clear positive association was also found between free testosterone concentrations and hemoglobin and hematocrit levels [49].
Reasons for the development of androgen deficiency conditions in women
Androgen deficiency in women is characterized by decreased libido, decreased feelings of well-being, depression, decreased muscle mass, and prolonged unreasonable fatigue in combination with low levels of total and free testosterone with normal estrogen levels [50]. Among the causes of androgen deficiency are ovarian, endocrine, chronic diseases and medications [18, 50] (.).
The laboratory criterion for androgen deficiency in women is the concentration of total testosterone in the lower quartile or below the lower limit of the normal range [50].
Effects of androgen replacement therapy
Testosterone therapy in women was first used in 1936 to relieve vasomotor symptoms [51]. Currently, testosterone is used as off-label therapy in many countries for various diseases and conditions in women. A new era began in 2006, when the use of a patch containing 300 μg of testosterone was officially approved by the European Medicines Agency for the treatment of sexual dysfunction in women after oophorectomy [52]. Testosterone can be used either as an addition to traditional HRT [27, 53] or as monotherapy [54]. Randomized, placebo-controlled trials showed that transdermal testosterone monotherapy at a physiological dose of 300 mcg twice a week for 18 months in women with androgen deficiency caused by both hypopituitarism and HIV infection led to significant increases in BMD, muscle mass and strength, and also improved indexes of depression and sexual function in such patients. At the same time, fat mass indicators did not change, and side effects were minimal [55–57]. It was also noted that transdermal testosterone therapy in women with androgen deficiency caused by HIV-associated weight loss syndrome did not impair insulin sensitivity, total adipose tissue mass, regional distribution of subcutaneous fat, and did not affect markers of inflammation and thrombolysis [58]. ]. In addition, testosterone gel applied to the anterior abdominal wall led to a decrease in abdominal subcutaneous fat and a decrease in total body weight in postmenopausal women [59]. Topical application of androgen cream was effective against atrophic vaginitis and dyspareunia in postmenopausal patients [60, 61].
Combining testosterone with traditional HRT
One of the most commonly used estrogen-androgen drugs in women in the United States is Estratest, which contains conjugated equine estrogens and methyltestosterone. As shown by the WHI data, conjugated estrogens are not the drug of choice for HRT due to the relative increase in the risk of breast cancer and cardiovascular complications in older women. Therefore, the optimal drug for estrogen-progestin replacement therapy should meet safety criteria for the mammary glands and endometrium, not have a negative effect on lipid and carbohydrate metabolism, not increase the risk of cardiovascular complications and have a positive effect on bone metabolism.
Of the drugs containing native sex hormones, the drug of choice is Femoston, used for hormone replacement therapy in peri- and postmenopause and the only one on the modern market available in three dosages: 1/5, 1/10 and 2/10. Femoston is a combination drug that contains 17-beta-estradiol - a natural estrogen - and dydrogesterone - a pure analogue of natural progesterone that does not lose its activity when administered orally.
The use of dydrogesterone in combination with 17-beta-estradiol enhances the protective effect of estrogens on bone tissue. If estrogens act to reduce bone resorption, in vitro studies suggest that dydrogesterone may promote bone formation [62]. In addition, dydrogesterone does not have hormonal side effects and does not have a negative effect on the blood coagulation system, carbohydrate and lipid metabolism [63]. The results of clinical studies of Femoston have shown its high effectiveness for the treatment of menopausal disorders in perimenopausal women, safety and good tolerability, acceptability and ease of use. The drug helps reduce the atherogenic potential of the blood, and therefore can have a real preventive effect on the incidence of cardiovascular diseases. The combination of 17-beta-estradiol with dydrogesterone has a better effect on the lipid profile than some other HRT regimens. In a double-blind study, a comparative study of the effect of two options for HRT was carried out: Femoston 1/5 and conjugated equine estrogens orally (0.625 mg) + norgestrel (0.15 mg). Both options had an equally positive effect on LDL levels (decrease by 7% over 6 months), but in terms of their effect on HDL levels, Femoston 1/5 was significantly more effective (increase by 8.6% and decrease by 3.5%, respectively; p < 0.001 ) [64]. All this significantly reduces the risk of cardiovascular diseases in postmenopausal women. Thus, the combined use of Femoston with androgens may have potential benefits in women with psycho-emotional and sexual disorders caused by androgen deficiency and not relieved by traditional HRT.
Conclusion
Currently, in world practice there is extensive experience in the use of estrogen and estrogen-progestogen replacement therapy, however, more and more data have accumulated that in a number of patients, without correction of age-related androgen deficiency, it is not possible to improve the quality of life. Androgen replacement therapy, due to the lack of knowledge about the role of androgens, as well as due to the large number of prejudices about androgens as “male” sex hormones, is still not widely used. Currently, there are no algorithms for the use of androgens in women, the dosages at which the greatest effectiveness would be achieved with the least side effects are unknown, and the safety issues of long-term use of androgens have not been sufficiently studied.
Literature
- Azziz R., Nestler JE, Dewailly D. Androgen excess disorders in women. Second Edition. Humana Press, 2007.
- Diamanti-Kandarakis E., Mitrakou A., Hennes MM, Platanissiotis D., Kaklas N., Spina G., Georgiadou E., Hoffmann RG, Kissebah AH, Raptis S. Insulin sensitivity and antiandrogenic therapy in women with polycystic ovary syndrome / / Metabolism, 1995, vol. 44, p. 525–531.
- Dunaif A., Green G., Futtermeit W., Dobrjansky A. Suppression of hyperandrogenism does not improve of peripheral or hepatic insulin resistance in the polycystic ovary syndrome // J Clin Endocrinol Metab, 1990, vol. 70, p. 699–704.
- Lasco A., Cucinotta D., Gigante A., Denucco G., Pedulla M., trifiletti A., Fristina N. No changes in peripheral insulin resistance in polycystic ovary syndrome after long-term reduction of endogenous androgens with leuprolide // Eur J Endocrinol, 1995, vol. 133, p. 718–722.
- Sherwin BB Use of combined estrogen-androgen preparations in the postmenopause: evidence from clinical studies // Int J Fertil Womens Med, 1998, vol. 43, p. 98–103.
- Guay AT Decreased testosterone in regularly menstruating women with decreased libido: a clinical observation // J Sex Marital Ther, 2001, vol. 27, p. 513–519.
- Braunstein GD, Sundwall DA, Kate M., Shifren JL, Buster JE, Simon J. A, Bachman G., Aguirre OA, Lucas JD, Rodenberg C., Buch A., Watts NB Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: randomized placebo-controlled trial // Arch Intern Med, 2005, vol. 165, p. 1582–1589.
- Buster JE, Kingsberg SA, Aguirre O., Brown C., Breaux JG, Buch A., Rodenberg CA, Wekselman K., Casson P. Testosterone patch for low sexual desire in surgically menopausal women: A randomized trial // Obstet Gynecol, 2005, vol. 105, p. 944–952.
- Davis SR, Bouchard C., Kroll R., Moufarege A., Von Schoultz B. The effect of a testosterone transdermal system on hypoactive sexual desire disorder in postmenopausal women not receiving systemic estrogen therapy, the aphrodite study. 82nd Annual Meeting of the Endocrine Society; Boston USA, 2006.
- Nathorst-Boos J., Floter A., Jarcander-Rollf M. Treatment with percutaneous testosterone gel in postmenopausal women with decreased libido-effects on sexuality and psychological well-being // Maturitas, 2006, vol. 53, p. 11–18.
- Burger HG Androgen production in women // Fertil Steril, 2002, vol. 77 (Suppl 4), p. 3–5.
- Balthazart J. Steroid control and sexual differentiation of brain aromatase // J Steroid Biochem Mol Biol, 1997, vol. 61, p. 323–339.
- Southren AL, Gordon GG, Tochimoto S. Further study of factors affecting the metabolic clearance rate of testosterone in man // J Clin Endocrinol Metab, 1968, vol. 28, p. 1105–1112.
- Guay A., Munarriz R., Jacobson J., Talakoub L., Traish A., Quirk F., Goldstein I., Spark R. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: Part A. Serum androgen levels in women aged 20–49 years with no complaints of sexual dysfunction // J Impot Res, 2004, vol. 16, p. 112–120.
- Labrie F., Belanger A., Cusan L., Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging // J Clin Endocrinol Metab, 1997, vol. 82, p. 2396–2402.
- Orentreich N., Brind JL, Riser RL, Vogelraan JH Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations through out adulthood // J Clin Endocrinol Metab, 1984, vol. 59, p. 551–555.
- Pancer C., Guay A. Testosterone replacement therapy in naturally and surgically menopausal women // J Sex Med, 2009, vol. 6, p. 8–18.
- Riverra-Woll LM, Papalia M., Davis SR, Burger HG Androgen insufficiency in women: diagnostic and therapeutic implications // Human Reproduction Update, 2004, vol. 10, no. 5, p. 421–432.
- Mudali S., Dobs AS, Ding J., Cauley JA, Szklo M., Golden SH Endogenous postmenopausal hormones and serum lipids: the Atherosclerosis Risk in Communities Study // J Clin Endocrinol Metab, 2005, vol. 90, p. 1202–1209.
- Lambrinoudaki I., Chrisotdoulakos G., Rizos D., Economou E., Argeitis J., Vlachou S., Creatsa M., Kouskouni E., Botsis D. Endogenous sex hormones and risk factors for atherosclerosis in healthy Greek postmenopausal women // Eur J Endocrinol, 2006, vol. 154, p. 907–916.
- Debing E., Peeters E., Duquet W., Poppe K. Velkieners B., Brande P. Van de. Endogenous sex hormone levels in postmenopausal women undergoing carotid artery endarterectomy // Eur J Endocrinol, 2007, vol. 156, p. 687–693.
- Vrionidou A., Papatheodorou A., Tavridou A., Terzi Th., Loi V., Vatalas I.-A., Batakis N., Phenekos C., Dyonissou-Asteriou A. Association of hyperandrogenemic and metabolic phenotype with carotid intima- media thickness in young women with polycystic ovary syndrome // Obst gyn Surv, 2006, vol. 61, No. 2, r. 104–106.
- Hickok L.R., Toomey C., Speroff L. A comparison of esterified estrogens with and without methyltestosterone: effects on endometrial histology and serum lipoproteins in postmenopausal women // Obstet Gynecol, 1993, vol. 82, p. 919–924.
- Shifren JL, Davis S., Moreau M., Waldbaum A., Bouchard C., DeRogatis L., Derzhko C., Baernson P., Kakos N., O'Neill S., Levine S., Wekselman K., Buch A., Rodenberg C., Kroll L. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM 1 study // Menopause, 2006, vol. 13, p. 770–779.
- Floter A., Nathorst-Boos J., Carlstrom K., von Schoulz B. Serum lipids in oophorectomized women during estrogen and testosterone replacement therapy // Matutritas, 2004, vol. 47, no. 2, p. 123–129.
- Bell RG, Davison SL, Papalia M.-A., McKenzie D., Davis S. Endogenous androgen levels and cardiovascular risk profile in women across the adult life span // Menopause, 2007, vol. 14, no. 4, p. 630–638.
- Khatibi A., Agardh C.-D., Shakir YA, Nerbrand C., Nyberg P., Lidfeldt J., Samsioe G. Could androgen protect middle aged women from cardiovascular events. A population-based study of Swedish women. The Women's Healh in the Lund Area (WHILA) study // Climacteric, 2007, vol. 10, no. 5, p. 386–392.
- Manolakou P., Angelopoulou R., Bakoyiannis C., Bastounis E. The effects of endogenous and exogenous androgens on cardiovascular disease risk factors and progression // Reprod Biol Endocr, 2009, vol. 7, p. 44.
- Bernini GP, Moretti A., Sgro M., Argenio GF, Barlascini CO, Cristofani R., Salvetti A. Influence of endogenous androgens on carotid wall in postmenopausal women // Menopause, 2001, vol. 8, p. 43–50.
- Evans DJ, Hoffman RG, Kalkhoff RK, Kissebach AH Relationship of androgenic activity to body fat topography, fat cell morphology, and metabolic aberrations in premenopausal women // J Clin Endocr Metab, 1983, vol. 57, p. 304–310.
- Peiris AN, Mueller RA, Struve MF, Smith GA, Kissebah AH Relationship of androgenic activity to splanchnic insulin metabolism and peripheral glucose utilization in premenopausal women // J Clin Endocr Metab, 1987, vol. 64, 162–169.
- Volpi E., Lieberman SA, Ferrer DM, Gilkison Ch. R., Rassmussen BB, Nagamani M., Urban RG The relationship between testosterone body composition, and insulin resistance. A lesson from a case of extreme hyperandrogenism // Diabetes Care, 2005, vol. 28, no. 2, p. 429–432.
- Diamanti-Kandarakis E., Spina G., Kouli Ch., Migdalis I. Increased endothelin levels in women with polycystic ovary syndrome and the beneficial effect of metformin therapy // Journ Clin Endocr Met, 2001, vol. 86., No. 10, p. 4666–4673.
- Barba M., Schunemann H., Sperati F., Akl E., Mussico F., Guyatt G., Muti P. The effects of metformin on endogenous androgens and SHBG in women: a systematic review and meta-analysis // Clin Endocr , 2009, vol. 70, no. 5, p. 661–670.
- Manolakou P., Angelopoulou R., Bakoyiannis Ch., Bastounis E. The effects of endogenous and exogenous androgens on cardiovascular disease risk factors and progression // Reprod Biol Endocrinol, 2009, vol. 7, p. 44–52.
- Bernini GP, Sgro M., Moretti A., Argenio GF, Barlascini CO, Cristofani R., Salvetti A. Endogenous androgens and carotid intimal-medial thickness in women // J Clin Endocrinol Metab, 1999, vol. 84, p. 2008–2012.
- Golden SH, Maguire A., Ding J., Crouse JR, Cauley JA, Zacur H., Szklo M. Endogenous postmenopausal hormones and carotid atherosclerosis: a case-control study of the Atherosclerosis Risk in Communities Cohort // Am J Epidemiol, 2002 , vol. 155, p. 437–445.
- Montalcini T., Gorgone G., Gazzaruso C., Sesti G., Perticone F., Pujia A. Role of endogenous androgens on carotid atherosclerosis in non-obese postmenopausal women // Nutr Metab Cardiovasc Dis., 2007, vol. 17, p. 705–711.
- Debing E., Peeters E., Duquet W., Poppe K., Velkieners B., Brande P. Van de. Endogenous sex hormone levels in postmenopausal women undergoing carotid artery endarterectomy // Eur J Endocrinol, 2007, vol. 156, p. 687–693.
- Hak AE, Witteman JCM, de Jong FH, Geerlings MI, Hofman A., Pols HA Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam Study // J Clin Endocrinol Metab, 2002, vol. 87, p. 3632–3639.
- Worboys S., Kostopoulos D., Teede H., McGrath B., Davis S. Evidence that parenteral testosterone therapy may improve endothelium-dependent and endothelium-independent vasodilation in postmenopausal women already receiving estrogen // Journ Clin Endocr Met, 2001, vol . 86, no. 1, p. 158–161.
- Tok EC, Ertunc D., Oz U., Camdeviren H., Ozdemir G., Dilek S. The effect of circulating androgens on bone mineral density in postmenopausal women // Maturitas, 2004, vol. 48, no. 3, p. 235–242.
- Davis SR, McCloud P., Strauss BJ, Burger H. Testosterone enhances estradiols effects on postmenopausal bone density and sexuality // Maturitas, 2008, vol. 61, p. 17–26.
- Dolan SE, Carpenter S., Grinspoon S. Effects of weight, body composition, and testosterone on bone mineral density in HIV-infected women // Journal of AIDS, 2007, vol. 45, no. 2, p. 161–167.
- Rishpon-Meyerstein N., Kilbridge T., Simone J., Fried W. The effect of testosterone on erythropoietin levels in anemic patients // Blood, 1968, vol. 31, no. 4, p. 453–460.
- Ferucci L., Maggio M., Bandinelly S., Basaria S., Lauretani F., Ble A., Valenti G., Ershler WB, Guralinik JM, Longo DL Low testosterone levels and the risk of anemia in older men and women / / Arch Intern Med, 2006, vol. 166, no. 13, p. 1380–1388.
- Behler CM, Shade SB, Gregory K., Abrams DI, Volberding PA Anemia and HIV in the antiretroviral era: potential significance of testosterone // Blood, 2004, p. 104, abstract 3722.
- Berria R., Gastaldelli A., Lucidi S., Belfort R., De Filippis E., Easton C., Britzki R., Cusi C., Jovanovic L., DeFronzo R. Reduction in hematocrit level after pioglitasone treatment is correlated with decreased plasma free testosterone level, not hemodilution, in women with polycystic ovary syndrome // Clin Pharm Ther, 2006, vol. 80, p. 105–114.
- Bachmann G. A., Bancroft J., Braunstein G., Burger H., Davis S., Dennerstein L., Goldstein I., Guay A., Leiblum S., Lobo R. et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification and assessment // Fertil Steril, 2002, vol. 77, p. 660–665.
- Bachmann GA Androgen cotherapy in menopause: evolving benefits and challenges // Am J Obstet Gynecol, 1999, vol. 180, p. 308–311.
- Radestad AF Testosterone treatment in women - an overview // Cur Wom Heal Rev, 2009, vol. 5, no. 1, p. 29–43.
- Flooter A., Nathorst-Boos J., Carlstrom K., Ohlsson C., Ringertz H., von Schoultz B. Effects of combined estrogen/testosterone therapy on bone and body composition in oophorectomized women // Gynec Endocr, 2005, vol. 20, no. 3, p. 155–160.
- Arlt W. Androgen therapy in women // Eur Journ Endocr, 2006, vol. 154, p. 1–11.
- Miller KK, Biller BMK, Beauregard C., Lipman JG, Jones J., Schoenfeld D., Sherman JC, Swearigen B., Loeffler J, Klibanski A. Effects of testosterone replacement in androgen-deficient women with hypopituitarism: a randomized, double -blind, placebo-controlled study // J Clin Endocrinol and Metabol, 2006, vol. 91, no. 5, p. 1683–1690.
- Dolan S., Wilkie S., Aliabadi N., Sullivan MP, Basgoz N., Davis B., Grispoon S. Effects of testosterone administration in human immunodeficiency virus-infected women with low weight. A randomized placebo-controlled study // Arch Intern Med, 2004, vol. 164, p. 897–904.
- Dolan S., Collins M., Lee H., Grispoon S. Effects of long-term testosterone administration in HIV-infected women: a randomized placebo-controlled trial // AIDS, 2009, vol. 23, p. 951–959.
- Herbst KL, Calof OM, Hsia SH, Sinha-Hikim I., Woodhouse LJ, Buchanan TA, Bhasin S. Effects of transdermal testosterone administration on insulin sensitivity, fat mass and distribution, and markers of inflammation and thrombolysis in human immunodeficiency virus-infected women with mild to moderate weight loss // Fertil Steril, 2006, vol. 85, no. 6, p. 1794–1802.
- Gruber DM, Sator MO, Kirhengast S., Joura EA, Huber GC Effect of percutaneous androgen replacement therapy on body composition and body weight in postmenopausal women // Maturitas, 1998, vol. 29, no. 3, p. 253–259.
- Witherby S. Efficacy and safety of topical testosterone for atrophic vaginitis in breast cancer patients on aromatase inhibitors: a pilot study // Breast Cancer Res Treat, 2007, 106: abstract 6086.
- Labrie F., Archer D., Bouchard C., Fortier M., Cusan L., Gomez GL, Girard G., Baron M., Ayotte N., Moreau M., Dube R., Cote I., Labrie C. , Lavole L., Berger L., Gillbert L., Martel C., Balser J. Intravaginal dehydroepiandrosterone (Prasterone), a physiological and highly efficient treatment of vaginal atrophy // Menopause, 2009, vol. 16, no. 5, p. 907–722.
- Verhaar HJ L, Damen CA, Duursma Scheven BAA A comparison of action of pro-gestins and estrogen on the growth and differentiation of normal adult human osteoblast-like cells in vitro // 11 Bone, 1994, v. 15, p. 307–311.
- Voetberg GA, Netelenbos JC, Kcnemans P. et al. Estrogen replacement therapy con-tinuosly combined with four different dosages of dydrogesteronc; effect on calcium and lipid metabolism // J Clin Endocrin Metab, 1994, v. 79, p. 1465–1469.
- Siddle N., Jesinger D., Whitehead M. // Br J Obst Gynaecol, 1990, vol. 97, p. 1093–1100.
S. Yu. Kalinchenko , Doctor of Medical Sciences, Professor S. S. Apetov , Candidate of Medical Sciences
RUDN University , Moscow
Contact information for authors for correspondence
Decrease in testosterone – what is dangerous?
The fact that a woman's testosterone levels are decreasing is indicated by certain changes in her appearance. They develop in the absence of proper therapy and quite slowly, so it is almost impossible to notice them. The main signs of hormone reduction include:
- The appearance of fatty deposits.
- Formation of sagging skin and muscles.
- Increased bone fragility.
- Decreased quality of skin, hair, and nail plates. The skin becomes dry and nails often break.
Frequent occurrence of depression and decreased mood. Instead of enjoying every day around the clock, a woman begins to fall into a depressed state and feels tired. At the same time, a decrease in testosterone negatively affects memory and concentration. Women begin to perceive information worse and worse and stop fully remembering it.
All these are quite unpleasant phenomena that will not please any woman. This is why it is so important to know how to effectively increase testosterone levels. This can be done in different ways - by eating special foods, using traditional methods and modern medicines.
Testosterone levels for women
The normal concentration may vary significantly between different clinical laboratories. This is due both to the characteristics of the equipment used and the determination method itself.
However, most laboratories indicate limits as a physiological norm from eleven to thirty-three nanomoles per liter for men and from zero point twenty-four to three point eight for women.
Testosterone Boosting Products
If a woman’s body does not have certain pathological conditions that arise due to a lack of testosterone, it can be increased naturally.
To increase testosterone naturally, the first thing you need to pay attention to is the quality of your daily diet.
In your standard diet, it is very important to include foods that contain substances that have a direct effect on the production of such important testosterone. Among the main such products are:
- All types of meat of special low-fat varieties;
- Eggs;
- All types of dairy products;
- Fish and all possible seafood;
- Nuts and different varieties of seeds;
- All types and categories of vegetables and fruits;
- Cereal porridge
As noted above, the hormone is secreted from the beneficial form of cholesterol. It is for this reason that the diet should include the maximum amount of animal products and fats and other special foods that affect the building block for testosterone - cholesterol.
Here are some of the most basic foods that increase total good cholesterol:
- You need to eat as much meat as possible. This is an ideal source of protein and cholesterol, which are important for the body. Protein effectively builds muscle fibers, and the more there are, the more testosterone is produced.
- Nuts are nature's healthy fat bombs. They provide the body with the cholesterol it needs. The best option is to use special Brazil nuts.
- Olives of all varieties and avocados are one of the main sources of healthy fat.
- Green broccoli is an excellent source of indole. This is a substance that helps reduce bad cholesterol and produce good cholesterol.
- Olive oil is a very good dressing for all fresh salads. It helps the body absorb good cholesterol more efficiently.
- Dressing in the form of balsamic vinegar - maintains insulin levels in the desired position.
Certain components may not be considered available, but you can still freely modify them, combine them with each other, and replace missing components with those that are available.
There is a very important chemical element in which a large amount of testosterone is present. It's zinc! You can get it in large quantities from fish and seafood.
If you eat a large amount of vegetables every day, you can saturate your body with useful macro- and microelements that can increase the sensitivity of receptor cells to the hormone. There is no particular difference between vegetables; you can eat a lot of everything, giving preference to those products that ripen not in the ground, but above its surface.
It is equally important to eat fruit. Preference should be given to such products as:
- All types of citrus fruits;
- Apricot;
- Persimmon;
- Melon;
- Grape;
- A pineapple;
- Pear.
It is worth eating a variety of different cereals every day. These are products containing fiber. It, in turn, has a positive effect on blood circulation and significantly improves the functioning of the endocrine system.
What symptoms can you suspect?
Symptoms of male hormone deficiency in the female body are quite nonspecific, and are similar to the manifestations of many other pathological conditions that negatively affect the functioning of the endocrine system.
Among the manifestations are:
- Unprovoked mood swings or persistent decline. Sometimes there is no need to rush into starting treatment for depression. Sometimes extremely similar manifestations can be observed with a lack of testosterone.
- A persistent feeling of fatigue that does not go away even after nine to ten hours of sleep, which can also be a problem, since low testosterone concentrations lead to sleep disorders.
- A deficiency of male sex hormones also negatively affects sex life. Characterized by a sharp decrease in libido, vaginal dryness, and problems achieving orgasm.
- Hair may become brittle and fall out. This applies to both hair on the head and growing on other parts of the body.
- Problems concentrating. Tasks that previously did not cause any difficulties at all can, with low testosterone, require quite a lot of time and effort.
- Anxiety is also common. Moreover, the severity of this manifestation can vary from minor to full-fledged panic attacks.
Folk remedies to increase testosterone
To effectively increase the amount of testosterone, you can use special medicinal infusions, herbal natural decoctions and all possible useful tinctures. You can make such drinks from the following components:
- Dog-rose fruit;
- Rowan berries;
- Aloe pulp;
- Ginseng root;
- Damian;
- Wild yam.
Such products not only increase the total amount of testosterone, but at the same time improve the general condition of the body. If such funds are not enough, you can prepare special medicinal infusions and mixtures. Here are a few of the most effective ones.
pollen
To prepare a remedy in this category, you need to prepare the following components:
- 1⁄2 kg of regular pollen purchased at the pharmacy;
- Two cans of condensed milk.
The ingredients should be placed in a small container, mixed thoroughly and put in the refrigerator. The mixture is aged for two full weeks.
The product should be consumed first one teaspoon at a time, and over 4 days the amount should be increased to one tablespoon. The frequency of administration is once a day, and the product should be consumed strictly 20 minutes before meals.
Healthy salad
It is very useful to eat salad, which is prepared from such components as:
- Celery;
- Red and white cabbage;
- Apples.
A similar salad is dressed with ordinary vegetable oil, to which table wine and apple cider vinegar are added. This is a very healthy dish that will not only increase the amount of testosterone in the blood, but will improve your overall well-being.
Rowan and rosehip decoction
To prepare a medicinal infusion, you will need to take three small handfuls of the fruit mixture, put it in a thermos and add a liter of hot water. The resulting infusion should be drunk instead of tea, without adding sugar to the drink.
Healthy mixture of honey and nuts
You need to take some nuts, chop them thoroughly and mix with honey. It is advisable to consume the resulting mixture every day in a volume of 30 g three times a day.
Ginger tea
To prepare this medicinal drink, you need to take 20 grams of the root and pour 300 ml of boiling water over it. The tea needs to be steeped for 5 hours. When ready, the drink should be drunk four times a day, 100-120 ml.
Decoction of hop cones
To prepare the remedy, you need to take one tablespoon of the product and pour 300 ml of hot water into it. A small container with the prepared mixture is placed on low heat. The infusion needs to be boiled for about 10 minutes. The finished decoction must be cooled well and consumed 100 ml twice a day.
Drug treatment
If testosterone is significantly reduced, experts prescribe conservative treatment for women. The standard treatment regimen includes drugs that contain artificial or natural testosterone.
Modern medicines come in different forms. You can take regular tablets, give injections, or use special patches or gels. Among the most popular tablet drugs are:
- Andriol is a medicine that is made on the basis of special testosterone undecanoate;
- Methyltestosterone.
A very popular drug for injection is Testosterone Propionate. As for the transdermal method, we can mention the Androderm patch. It is glued directly to the surface of the skin - on the back, thigh or abdominal area. You can purchase Androgel gel and apply it to your stomach or shoulders two to three times a day.
Hormone replacement therapy is characterized by the presence of a large number of contraindications. For this reason, it should be carried out exclusively under the supervision of an experienced, qualified physician and strictly after a thorough examination. As soon as the specialist is convinced that there are no chronic pathologies, he will prescribe appropriate medications and monitor their use.
Additional Methods to Increase Testosterone
If testosterone deviations are minor, their levels can be completely normalized using natural methods. Here are a few recommendations that, if followed, will effectively increase the amount of testosterone in the body:
- You need to sleep at least 8 hours;
- It is important to avoid any stressful situations;
- You need to consume vitamin C every day, and in an amount of at least 1000 mg;
- You need to drink vitamins A, B and E on a regular basis;
- It is important to exercise regularly and do light strength training;
- It is necessary to have sex regularly;
- It is advisable to exclude grapefruits from the daily diet;
- Quitting alcoholic beverages is extremely important.
If the decrease in testosterone is insignificant, it is quite enough to switch to this regimen. This way you can significantly improve not only your health, but also your sexuality and attractiveness.
Sports and testosterone
Moderate physical activity also has a positive effect on testosterone production. This benefit is based on the fact that the growth of muscle tissue increases and the amount of excess fat decreases. There are several simple rules that must be followed when organizing training:
- It is worth choosing basic exercises that involve large muscles;
- High-intensity training increases testosterone levels;
- Each exercise should be performed for a maximum of two to three repetitions. There is no need to work to complete failure;
- Rest between sets should range from one to two minutes.
From time to time, you can supplement your workouts with high-intensity interval training. The essence of this technique is to alternate short, fairly intense and longer-term exercises. There are quite a lot of options for such training, you can easily choose the optimal scheme.
You need to know that in the process of playing sports you need to avoid such a phenomenon as overtraining, since you can achieve the exact opposite effect. If you exercise before the appearance of signs such as lack of physical strength, pain, lethargy and permanent fatigue, you can get such an unpleasant effect as a decrease in the amount of testosterone.
You must play sports only to the best of your ability and physical strength.
Physical activity
To activate blood circulation and increase testosterone levels, it is recommended to do:
- race walking;
- bodybuilding;
- athletics;
- swimming.
The loads should be intense and constant, at least 3 times every 7 days. Sets (2-3) must be alternated with a short rest (1-2 minutes). With such physical activity, excess fat will disappear and muscles will grow.
In order not to reduce testosterone, during sports there should not be:
- fatigue;
- loss of strength;
- lethargy;
- pain.
Useful vitamins and various sports supplements
Proper nutrition allows the body to effectively synthesize testosterone in the body. And the presence of modern technologies allows us to do this even more efficiently. In other words, proper nutrition must be supplemented with special high-quality supplements. Among the most effective and affordable of them are:
- D3. It is not a vitamin supplement, but a hormone that our body receives from regular sunlight. If a woman is not lucky enough to live in sunny, warm regions, if her work is carried out in an office, supplementing with this vitamin would be the best option.
- Omega-3. High-quality fish oil consumed every day promotes effective hormone production.
- Special whey protein. This is a supplement that is optimal not only for men, but also for women. With this supplement you can get the required amount of protein for health. This substance allows you to restore, protect and build the muscles necessary for the body.
- Caffeine. Numerous studies have shown that drinking 100 mg of regular caffeine before going to the gym helps increase the production of the necessary hormone.
Good nutrition and optimal physical activity should be accompanied by adequate sleep.
Scientists have proven that testosterone synthesis occurs exclusively when a person is sleeping. If you are chronically sleep deprived, if you sleep less than 5 hours a day for a long time, the body simply will not have time to produce the much-needed hormone. In addition to the production of testosterone, adequate sleep and rest also reduces the amount of cortisol, which tends to block testosterone.
To ensure yourself a good night's rest, you should protect yourself from your smartphone and from taking special invigorating drinks in the evening. All you need to do is take a warm, relaxing bath or shower.
Vitamins
Dietary supplements and vitamins that increase testosterone include:
- Tribulus based on saponins from Tribulus terrestris.
- Vitamin C , which:
- keeps androgen normal;
- strengthens bones;
- neutralizes cortisol;
- restores the body;
- regenerates tissue;
- eliminates bad cholesterol.
- Vitamin D. This component:
- normalizes the absorption of phosphorus and calcium;
- maintains order in the hormonal and immune systems;
- gives the body strength;
- maintains normal weight.
- Fish oil , which performs the following functions:
- improving the health of the body;
- activation of testosterone biosynthesis;
- strengthening stress resistance;
- normalization of mood and blood pressure.
- Vitamin E, it:
- restores reproduction;
- cleanses of toxins;
- participates in the production of testosterone;
- slows down aging;
- strengthens the heart.
- B vitamins, which are also involved in:
- supporting skin health;
- protein synthesis;
- normalization of digestion;
- regulation of the functioning of the nervous system;
- liver protection;
- construction for nucleic acids.
Effective stress management
As you know, frequently repeated stress causes the formation of large amounts of cortisol. In small dosages it is relatively harmless and necessary. It effectively triggers a vital reaction in a woman's body. Problems can begin when a stressful situation drags on for a little while.
A woman automatically begins to have health problems, so there can be no question of testosterone production. To effectively cope with stress, you need to regularly do meditation, simple breathing exercises and walks.
How to increase testosterone: key microelements
Several different nutrients are involved in testosterone synthesis. Among them are vitamin D3 and zinc, which are often supplied in insufficient quantities, leading to deficiency.
Zinc is an important mineral that occurs naturally in some foods. Zinc deficiency is accompanied by a wide range of symptoms, as it is involved in many important processes for the body 5,6.
Vitamin D and zinc are two key microelements necessary for the natural production of testosterone
Vitamin D is produced during exposure to the sun (in unprotected areas of the skin). In climates where there is little sun, the risk of developing a deficiency of this vitamin is very high.
In order to provide the body with vitamin D, there is no need to sunbathe for hours, but regular sun exposure is vital.
Stress is a natural testosterone killer. Under stress, the adrenal glands secrete the hormone cortisol, which suppresses the action of testosterone . In this case, even if your natural testosterone level is high, its effectiveness will be significantly reduced. Avoid stressful situations 7!
When stressed, the hormone cortisol is released, which suppresses the effect of testosterone.
We recommend : Vitamin and mineral deficiency: how to understand which vitamins are missing?
Maintaining Testosterone Levels
Increasing the amount of testosterone in the body with the help of medications or folk remedies is quite simple. It is much more difficult to maintain the result obtained. To maintain hormone levels at a high level for a long time, you should adhere to the following recommendations:
- It is important to completely give up bad habits - smoking and alcohol;
- It is necessary to lead an active lifestyle, play sports;
- It is required to constantly monitor your weight - it is strictly forbidden to allow weight gain;
- Medicines should be taken only on the recommendation of doctors;
- It is necessary to avoid any stressful situations and various nervous overloads;
- It is advisable to undergo a medical examination every year.
The female body is a rather complex system. For its ideal and full functioning, the balance of hormones is very important. You should lead a healthy lifestyle and eat right. This is the only way to avoid deviations in the amount of testosterone.
Conditions accompanied by similar clinical manifestations
Symptoms of low testosterone levels in women are quite nonspecific and may resemble the clinical manifestations of many other pathological conditions. It is not possible to independently understand the cause of the appearance of disturbing manifestations, since it may be necessary to consult more than one specialist to make an accurate diagnosis.
Among the diseases whose clinical manifestations may be similar to testosterone deficiency include a lack of thyroid hormones. Deterioration of hair and skin condition, mood changes and decreased ability to work do not allow clinical differentiation of these two diseases; additional laboratory tests are needed to indicate the cause of such symptoms.
Major depressive disorder, which is characterized by decreased mental capacity, depressed mood, sleep problems, decreased sex drive, loss, or, conversely, weight gain without obvious changes in eating habits.
However, in the case of such disorders, it is first necessary to exclude the possible somatic nature of such conditions - similar manifestations are characteristic of both the pathology of the thyroid gland and the lack of testosterone itself.