Instructions for use
In the article you can find useful information regarding pharmacology, method of administration, possible analogues, conditions of administration for damaged liver, indications, contraindications, and much more. All this data is necessary for safe and effective use of the drug.
Pharmacological properties
An anthraquinone derivative inhibits the synthesis of interleukin-1 , which plays a major role in the pathogenesis of cartilage destruction and inflammation in arthrosis. It inhibits the effects of other inflammatory cytokines.
Several times slows down the production of metalloproteins, which are directly related to cartilage damage. It stimulates the synthesis of glycosaminoglycans and haluronic acid. As a result of taking the medicine Diacerein, the narrowing of the gap between the joints slows down.
Mechanism of action of Diacerein
The drug is absorbed quite well from the gastrointestinal tract . The bioavailability of the drug increases as a result of taking the drug by as much as 25%. The maximum concentration of the active substance in the blood is observed after 2.4 hours.
With prolonged use of the medicine, the concentration increases. This effect is associated with the accumulation of the drug. The metabolite can penetrate barriers. The medicine is excreted from the body primarily by the kidneys. The half-life is 4 hours.
Video: “The role of interleukin in the pathogenesis of osteoarthritis”
Composition and release form
The drug is available in the form of capsules intended for oral administration..
The capsule contains the active substance - diacerein - no more than 50 mg . In addition, additional substances are also present, for example, croscarmellose sodium, lactose monohydrate, hypromellose, colloidal silicon dioxide, magnesium stearate.
The capsule contains methyl parahydroxybenzoate, titanium dioxide, propyl parahydroxybenzoate, sodium lauryl sulfate, azorubine dye, patent blue dye, gelatin, water.
Diacerein is available in capsules of 50 mg of active substance
Black ink consists of ethanol, isopropanol, shellac, black iron oxide, isopropanol, butanol, concentrated ammonia solution, macrogol, black iron oxide. White ink consists of shellac, ethanol, titanium dioxide, isopropanol, polysorbate-80, and concentrated ammonia solution.
Indications for use
The medicine is used to treat diseases of the musculoskeletal system, in particular joints: primary and secondary forms of osteoarthritis.
Method of use of the medicine
It is important to understand that if for some reason it suddenly hurts you to take the capsule in its entirety, you can open it and take only the contents by dissolving the powder in a glass of water.
According to the instructions, the drug must be taken orally after meals, washed down with plenty of water .
Take the drug one capsule twice a day (in the morning and evening) .
As a result of the first few weeks of taking the medication, a person may experience increased intestinal transit. If this happens, the dosage is reduced to one capsule per day. The medicine should be taken preferably in the evening with food .
Reception is carried out for at least 4 weeks , after which the dosage is again increased to two capsules per day. The general course of treatment is about 4 months.
If necessary, the doctor may decide to repeat the course of treatment and adjust the dosage.
Interaction with other drugs
Before starting treatment, you should inform your doctor about all medications you are taking, including those taken without a prescription.
Antacids reduce drug absorption.
In the case of simultaneous use of the drug with other drugs that affect the intestinal microflora (including antibiotics), the frequency of occurrence of adverse intestinal symptoms increases several times.
Medicines containing magnesium hydroxide/aluminum hydroxide reduce the bioavailability of the active component of the drug Diacerein.
It is not recommended to take Diacerein and laxatives .
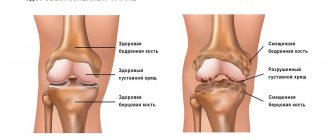
The use of diacerein for osteoarthritis of the joints of the hands
Osteoarthritis (OA) is one of the most common diseases of the musculoskeletal system, especially in older age groups. In old age, along with lesions of the knee and hip joints, OA of the distal and proximal interphalangeal joints of the hands occupies a significant place. The prevalence of this type of OA in European countries increases from 10% in patients aged 40–49 years to 92% in patients over 70 years of age.
The study of the pathogenesis of OA contributed to the development and implementation of drugs classified as symptomatic slow-acting drugs, which have not only a symptomatic effect, but may also have a structure-modifying effect [1]. These drugs include diacerein, which has an inhibitory effect on the production and activity of interleukin-1 [2–7]. Clinical studies have demonstrated the effectiveness of diacerein compared to placebo and non-steroidal anti-inflammatory drugs (NSAIDs) in terms of the effect on pain and functional state of the hip and knee joints [8, 9], while the therapeutic value of diacerein in patients with OA of the joints of the hands has been practically not studied. [10].
The purpose of this study was to evaluate the clinical efficacy and safety of diacerein in patients with OA of the hand joints.
Materials and research methods
A comparative controlled randomized study included 60 women who met the clinical diagnostic criteria for OA of the joints of the hands [11]. The study was conducted in accordance with the core principles of Good Clinical Practice (GCP) and the Declaration of Helsinki. All patients expressed voluntary informed consent in writing, and a positive decision was made by the local ethics committee. Using the adaptive randomization method, patients were divided into two groups: 30 patients (group 1) took Arthrodarin (diacerein) at a dose of 50 mg 2 times a day for 4 months and diclofenac sodium 100 mg/day, the remaining 30 patients (group 2) group) - diclofenac sodium 100 mg/day. The groups of patients before the start of the study were comparable in terms of main clinical and demographic indicators (p > 0.05) (Table 1). The average age of patients in group 1 was 57.5 ± 5.3 years, in group 2 - 58.9 ± 7.4 years. The duration of the articular syndrome of less than 5 years was noted in 46.7% of patients of the 1st group and 53.3% of patients of the 2nd group, from 6 to 10 years - in 36.7% and 23.3%, more than 10 years - in 16.6% and 23.3% of patients, respectively. In 83.3% of patients in group 1 and in 66.7% of patients in group 2, Heberden and/or Bouchard nodes were detected. In 18 patients (60%) of the 1st group and 20 (66.6%) patients of the 2nd group, radiographic stage II of the disease was observed, in 12 patients (40%) of the 1st group and 10 (33.3%) patients Group 1 - I x-ray stage. Inclusion criteria were: a reliable diagnosis of OA of the joints of the hands, pain in at least three joints, pain intensity in the analyzed joints > 40 mm on a visual analogue scale (VAS), X-ray stage I or II, the need to take NSAIDs, the absence of clinically significant disorders liver and kidney functions, signed informed consent. The study did not include patients with heart, renal and liver failure, type 1 diabetes mellitus, exacerbation of gastric and duodenal ulcers, as well as patients who received symptomatic slow-acting drugs at the time of inclusion in the study or 6 months before. actions.
Of the 60 patients included in the study, 51 patients (85%) completed the full course of treatment. 4 patients from group 1 and 5 patients from group 2 dropped out in the first month of treatment due to the occurrence of adverse events.
To assess the effectiveness of therapy, we studied the severity of pain, stiffness, dysfunction in joints using VAS, assessed the Dreiser [12] and Auscan [13] functional indices, the need for NSAIDs, and quality of life using the general SF-36 questionnaire.
Statistical processing of the material was carried out using a specialized statistical package SPSS 17.0. In groups, the arithmetic mean (M), standard deviation (σ), mean error of the arithmetic mean (m), and confidence interval were calculated. When comparing indicators in groups, Student's t-test was used. The study of the dynamics of the studied parameters during treatment was carried out using a paired Student's t test. In all cases, the null hypothesis was rejected at p < 0.05.
Results and discussion
The use of Arthrodarin (diacerein) had a positive effect on the symptoms of the disease (Table 2). The effect was observed 4–5 weeks from the start of taking Arthrodarin (diacerein) and increased throughout the entire treatment period. By the 16th week of continuous use of Arthrodarin (diacerein), positive dynamics of all clinical indicators were noted: severity of pain according to VAS (p < 0.01), stiffness (p < 0.01), joint function (p < 0.05), Dreiser index values (p < 0.01), Auscan (p < 0.01). In the group of patients receiving only NSAIDs, positive dynamics of the studied parameters were also noted. However, statistically significant changes in group 2 were observed only in relation to pain (p < 0.05) and the Dreiser index (p < 0.05). In addition, by the end of the 16th week, the values of all studied clinical parameters in the Arthrodarin (diacerein) group were lower than in the group of patients receiving only NSAIDs. For example, the use of Arthrodarin (diacerein) led to a reduction in pain according to VAS by 38.6%, while taking NSAIDs alone led to a reduction in pain by 11.2%. The value of the Dreiser index in the 1st group decreased by 40.6%, in the 2nd group - by 21.7%. The total value of the Auscan index in the Arthrodarin (diacerein) group decreased by 39.9%, in the NSAID group - by 21.7%.
During treatment with Arthrodarin (diacerein), the patients' need for NSAIDs significantly decreased. By the end of the 16th week of treatment, 53.8% of patients in group 1 and 12% in group 2 were able to completely stop taking NSAIDs (χ2 = 8.24; p = 0.004). The dose of NSAIDs taken was reduced by 50% or more in 26.9% of group 1 and 12% of group 2, and 19.2% of patients in group 1 and 76% of patients in group 2 (χ2 = 14.28; p = 0.000) continued to take NSAIDs at the same dose.
The achieved clinical effect, as shown by further observation for up to 9 months of patients receiving Arthrodarin (diacerein), persisted in 15 of 26 patients (57.7%) for another 2-3 months. Patients taking diclofenac only reported a return of symptoms within a few days after stopping treatment. During therapy with Arthrodarin (diacerein) and NSAIDs, the quality of life indicators of patients tended to improve in both groups (Table 3). However, a statistically significant (p < 0.05) improvement was observed only in patients of group 1 on the pain scale, the value of which improved by 28.9%.
Tolerability of Arthrodarin (diacerein) was satisfactory and comparable to NSAIDs. In group 1, side effects that led to discontinuation of the drug in the first month of treatment were detected in 4 patients (13.3%): severe diarrhea was observed in 3, an increase in the level of alanine aminotransferase (ALT) in 1 and aspartate aminotransferase (AST) in 2 times. When Arthrodarin (diacerein) was discontinued, the side effects resolved on their own. Side effects in group 2 were recorded in 5 patients (16.6%): 2 (6.6%) had abdominal pain, 3 (10%) had a 2-fold increase in ALT and AST.
Conclusion
Differences in the anatomical structure and function of joints, a variety of risk factors and outcomes suggest different effectiveness of the same therapeutic approaches for OA of different locations. For example, the correlation between symptoms and radiological changes in OA of the hand joints is even less pronounced than in gonarthrosis and coxarthrosis. However, evidence of the effectiveness of most symptomatic slow-acting drugs currently used for the treatment of OA of the joints of the hands is mainly extrapolated from the results of randomized controlled trials for OA of the knee and hip joints [14], due to the small number and limited range of clinical observations in OA hand joints [15]. The results obtained in our study showed that Arthrodarin (diacerein) has an analgesic and anti-inflammatory effect in patients with OA of the joints of the hands and retains the effect for 2-3 months after cessation of treatment. The use of the drug allows you to reduce the dose of NSAIDs used, and in some cases completely abandon them. Literature data on the tolerability of Arthrodarin (diacerein) are contradictory. In a meta-analysis of 19 controlled, randomized trials conducted by B. Rintelen et al. in 2006 [16], it was shown that Arthrodarin (diacerein) has similar tolerability as NSAIDs, without causing severe side effects. At the same time, in a Cochrane review that included 7 randomized clinical trials conducted by TS Fidelix et al. in 2006 [17], low adherence to treatment was noted due to side effects such as diarrhea (42%). In our study, the tolerability of Arthrodarin (diacerein) was satisfactory. Side effects were recorded in 13.3% of patients, including 10% of them who experienced diarrhea, which required discontinuation of treatment.
Thus, the data from the study demonstrate the presence of a symptomatic effect of Arthrodarin (diacerein) in OA of the joints of the hands. The drug Arthrodarin (diacerein) has a positive effect on the main clinical manifestations of OA of small joints of the hands: it reduces pain, stiffness, improves joint function, with satisfactory tolerability. Further longer studies are needed to confirm the findings.
Literature
- Boileau C., Kwan Tat S., Pelletier JP et al. Diacerhein inhibits the synthesis of resorptive enzymes and reduces osteoclastic differentiation survival in osteoarthritic subchondral bone: a possible mechanism for a protective affect against subchondral bone remodeling // Arthr. Res Ther. 2008. No. 10. R. 71.
- Felisaz N., Boumedien K., Ghayor C. et al. Stimulating effect of Diacerhein on TGF-β1 and b2 expression in articular chondrocytes cultured with and without IL-1 // Osteoarthr. Cart. 1999. No. 7. R. 255–264.
- Burkhard F. Leeb. Clinical Efficacy and Safety of Diacerein in Osteoarthritis - A Review // European Musculoskeletal Review. 2010; 5 (1): 23–29.
- Mengshol LA, Vincenti MP, Coon C. et al. Interleukin-1 induction of collagenase 3 (matrix metallproteinase 13) gene expressions in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor-kappaB: Differential regulation of collagenase 1 and collagenase 3 // Arthritis. Rheum. 2000. No. 43. R. 801–11.
- Yaron M., Shirazi I., Yaron I. Anti-interleukin-1 effects of diacerhein and Rhein in human osteoarthritis synovial tissue and cartilage cultures // Osteoarthr. Cart. 1999. V. 7. No. 3. R. 272–280.
- Balabanova R. M., Kaptaeva A. K. Arthrodarin - a new drug for pathogenetic therapy of osteoarthritis // Scientific and practical rheumatology. 2009. No. 2. P. 49–53.
- Balabanova R. M. The role of interleukin 1 in osteoarthritis and the possibility of blocking it // Modern rheumatology. 2011. No. 1. P. 58–62.
- Pavelka K., Trc T., Karpas K., Vitek P. et al. The efficacy and safety of diacerhein in the treatment of painful osteoarthritis of the knee: a randomized, multicenter, double-blind, placebo-controlled study with primary and points at two months after the end of a three-month treatment period // Arthritis. Rheum. 2007. V. 56. No. 12. R. 4055–4064.
- Bartels EM, Bliddal H, Schondorff PK et al. Symptomatic efficacy and safety of diacerhein in the treatment of osteoarthritis: a meta-analysis of randomized placebo-controlled trials // Osteoarthritis Cartilage. 2010. V. 18. No. 3. R. 289–296.
- Shin K. et al. Efficacy and Tolerability of Diacerein in Hand Osteoarthritis // Ann Rheum Dis, 2011; 70 (supp. l3): 389.
- Altman RD The classification of osteoarthritis//J Rheumatol Suppl. 1995. No. 43. R. 42–43.
- Dreiser RL, Maheu E, Guillou GB et al. Validarion of an algofunctional index for osteoarthritis of the hand // Rev. Rhum. (Engle ed.). 1995. V. 62 (6, suppl. 1). R. 43–53.
- Allen KD, Jordan JM, Renner VB Validity, factor structure, and clinical relevance of the AUSCAN Osteoarthritis Hand Index // Arthritis Rheum. 2006. V. 54. No. 2. R. 551–556.
- Brahmachari B., Chatterijee S., Ghosh A. Efficacy and safety of diacerhein in early knee osteoarthritis: a randomized placebo-controlled trial // Clin Rheumatol. 2009. V. 28. No. 10. R. 1193–1198.
- Alekseeva L. I., Chichasova N. V. Use of piascledine for osteoarthritis of the hands // Pharmateka. 2010. No. 10. pp. 48–55.
- Rintelen B., Neumann K., Leeb BF A meta-analysis of controlled clinical studies with diacerhein in the treatment of osteoarthritis // Arch. Intern. Med. 2006. V. 166. No. 17. R. 1899–1906.
- Fidelix TSA, Trevisani VFV Diacerhein for osteoarthritis. The Cochrane Database of Systematic Reviews. 2006.
E. A. Leushina O. V. Simonova, Doctor of Medical Sciences, Professor
State Budgetary Educational Institution of Higher Professional Education Kirov State Medical Academy of the Ministry of Health of the Russian Federation, Kirov
Contact information for authors for correspondence
Side effects
A person may experience symptoms such as::
During treatment with the drug, nausea may occur; vomiting and nausea;- Abdominal pain, bloating, flatulence, diarrhea, constipation;
- General ailments, weakness, insomnia, nightmares;
- Bronchospasm, cough, suffocation;
- Skin rash, itching, Quincke's swelling, anaphylactic shock;
- Toxic hepatitis.
If these conditions do occur, then you need to stop taking the medication.
Overdose
Did you know that...
Next fact
As a result of taking the medicine in a large dosage, the patient may experience symptoms and conditions such as:
- Leukopenia, anemia, thrombocytopenia, agranulocytosis;
- Drowsiness, blurred vision, insomnia;
- Head pain, dizziness, lightheadedness. If no action is taken, the patient may faint;
- Depression, blood pressure surges.
If the patient has taken the medicine in a large dosage, then he needs to stop taking the medicine. Next, the patient needs to call an ambulance. While the ambulance is traveling, you need to rinse your stomach.
Contraindications
Taking the medicine is contraindicated in the presence of hypersensitivity to individual components of the medicine. This drug can be prescribed with caution for enterocolitis, colitis .
Pregnancy
During pregnancy, this medicine should not be taken . This measure is associated with a negative effect on the fragile body of the fetus.
In addition, taking the medicine while breastfeeding is also strictly prohibited. If taking the drug is still necessary, then you need to abandon the natural method of feeding the child.
Video: “Conservative treatment of osteoarthritis of large joints: report”
special instructions
Effect on the ability to drive a vehicle : you should not drive after taking the pill. This is especially true for the first time you take the medicine.
Pediatric age : This drug should not be given to children under 18 years of age.
Elderly : Patients over 60 years of age may require dose adjustment.
Kidney damage : the drug may be prescribed, but with extreme caution.
Liver damage : taking the medicine is allowed, but with extreme caution.
Dispensing from pharmacies : a prescription from your attending physician is required.
Instructions for use DIAFLEX
If concomitant use of antibiotics is necessary, which may disrupt the intestinal microflora, temporary cessation of therapy should be considered.
The risk/benefit ratio of treatment should be assessed in patients with a history of enterocolitis (especially in patients with irritable bowel syndrome).
Concomitant dietary intake increases the bioavailability of diacerein (about 24%), while significant dietary deficiency reduces bioavailability.
Taking diacerein often results in diarrhea, which can cause dehydration and hypokalemia. If diarrhea develops, the patient should stop taking the drug and contact their doctor immediately to discuss alternative treatment. Caution should be exercised if the patient is concomitantly receiving diuretics as Dehydration and hypokalemia may develop. Particular caution should be observed in case of hypokalemia in patients receiving cardiac glycosides (digitoxin, digoxin).
The simultaneous use of laxative drugs should be avoided.
Caution should be given to patients with renal impairment.
The drug contains lactose, so patients with rare hereditary diseases such as galactose intolerance, lapp lactase deficiency or glucose-galactose malabsorption syndrome should not take Diaflex.
During post-registration monitoring, cases of increased activity of liver enzymes in the blood serum and symptomatic acute liver damage were identified. Before starting treatment, the patient should be asked about concomitant, current and history of liver diseases, and an examination should be carried out to identify violations of the functional state of the liver. Liver diseases are a contraindication to the use of diacerein. It is necessary to monitor laboratory and clinical manifestations of liver damage, and observe precautions when used simultaneously with other drugs with the risk of developing hepatotoxic reactions. Treatment with diacerein should be discontinued if an increase in liver enzyme activity is detected or the development of symptoms of liver damage is suspected. The patient should be informed of the signs and symptoms of hepatotoxicity and advised to consult a physician immediately if symptoms of liver damage are suspected.
Patients should be advised to limit alcohol consumption while using diacerein.
Use in pediatrics
The safety and effectiveness of the drug in pediatric practice have not been established. The use of the drug in children and adolescents is not recommended due to lack of data.
Impact on the ability to drive vehicles and operate machinery
Data on the effect of the drug on the ability to drive vehicles and operate machinery are not provided.
Analogs
If this drug does not suit you, your doctor may prescribe another medicine that has a similar composition and method of action.
Among the analogues we can distinguish drugs:
- Movagain;
- Arthroker;
Check out the list of DiacereinDon analogues;- Arthrodarin;
- Chondroitin-Acos;
- Structum;
- Diacerein-Mac;
- Diaflex Rompharm;
- Glucosamine;
- Sigan;
- Nimegesin;
- Nimulid;
- Artiflex;
- Nimika;
- Nimesil;
- Arthroker;
- Nemulex;
- Aponil.
Reviews
Almost everyone who took this drug noted the appearance of diarrhea, which appeared at various periods of therapy (usually this condition appeared with long-term use of the drug), some noted the appearance of pain in the liver and swelling of the legs.
Hepatotoxic effects were confirmed by laboratory tests . In this regard, it was decided to introduce some restrictions for elderly people and people who have liver disease on taking this medicine.
The discontinuation rate of this medication due to an adverse reaction is 25% . But, the laxative effect in people who suffer from frequent or chronic constipation is considered a positive effect. The presence of a lasting effect after treatment and an increase in the quality of life of patients gives the right to talk about the need to include this medicine in the list of drugs in the complex treatment of osteoarthritis.
If you have also taken Diacerein, we will be grateful if you leave your comment at the end of the article regarding your experience of treatment with this drug. There you can also find reviews from other site visitors.
Diacerein in the treatment of osteoarthritis of the knee joints: results of a comparative study
Osteoarthritis is one of the most common joint diseases, occurring mainly in middle and older age groups. As the world continues to experience an increasing number of older people, osteoarthritis is becoming an increasingly significant health problem. In recent years, the pathogenesis of osteoarthritis has been actively studied, with a significant role being given to pro-inflammatory mechanisms of disease development and, in particular, cytokine regulation. In this regard, the very definition of this nosological form is discussed - most authors suggest using the term “osteoarthritis,” which we will use in the future. The main clinical manifestation of osteoarthritis (OA) is pain in the affected joint, the intensity of which depends both on the severity of inflammatory changes and on the stage of OA (structural disorders of cartilage tissue, subchondral bone and soft tissue surrounding the joint). The disease is characterized by both significant heterogeneity in terms of clinical manifestations and varying rates of progression of structural abnormalities, therefore the main tasks currently facing clinicians and researchers are the identification of a group of patients with rapidly progressing variants of OA and the development of an optimal effective therapeutic algorithm. The role of pro-inflammatory cytokines in the development of osteoarthritis
Despite significant progress in understanding the pathogenetic mechanisms of OA development, cause-and-effect relationships in the process of its development and progression have not been fully established. There is a consensus understanding that OA is a heterogeneous disease with various risk factors for its development and its further course. In this case, the most important task is to establish the mechanisms of rapid progression of OA of large joints (with severe structural damage to articular tissues) and the possibilities of pharmacological correction of such disorders, since this category of patients, as a rule, often requires surgical treatment after 5–7 years . In a healthy joint, cartilage and bone homeostasis is maintained due to the balance of anabolic and catabolic processes, which are regulated by pro- and anti-inflammatory mediators, including interleukins (IL-1, -2, -6, etc.), tumor necrosis factor-α ( TNF-α) and others [1]. In OA, hyperproduction of IL-1 leads to a shift in the balance towards catabolic and anti-anabolic processes, resulting in a decrease in the synthesis of cartilage matrix components, increased degradation and remodeling of the subchondral bone. In particular, the ability of IL-1 has been proven to enhance the formation of catabolic factors by chondrocytes - matrix metalloproteinases (MMP) and nitric oxide (NO), as well as to reduce the expression of specific enzymes - tissue inhibitors of metalloproteinases (TIMP)), inhibiting the activity of MMP [ 2, 3]. IL-1 also has the ability to induce the synthesis of enzyme activators, one of which is urokinase-like plasminogen activator. Plasminogen, in turn, activates pro-MMP, which leads to subsequent degradation of the cartilage matrix [2]. It has been proven that IL-1 indirectly induces apoptosis of chondrocytes and synoviocytes, and also plays a key role in the induction of inflammatory reactions, which results in impaired functional activity of chondrocytes and a decrease in the volume of cartilage tissue in OA [1]. Along with stimulating the production of other pro-inflammatory cytokines, IL-1, through a positive feedback mechanism, induces increased own production by producer cells. Thus, the signal transmitted to the IL-1 receptor causes activation of the transcription factor and nuclear factor κB (NF-κB), which, in turn, activate the expression of the IL-1 gene, which leads to further formation of the cytokine with the development of a whole cascade of pathophysiological reactions : cartilage degradation, subchondral bone remodeling and progression of inflammatory changes [4]. Thus, taking into account the pathogenetic significance of IL-1 in the induction and maintenance of the inflammatory process in OA by influencing the mechanisms of chondrocyte apoptosis, bone remodeling, reducing the activity of anabolic processes in cartilage tissue, leading to degradation of the extracellular matrix and progression of the disease, it should be noted that drugs , affecting the production and activity of IL-1, may potentially help slow down the progression of OA.
Diacerein – a component of pathogenetic therapy of osteoarthritis
One of the symptomatic slow-activ drugs for the treatment of OA (SYSADOA - Symptomatic slow-activ drug in osteoarthritis) is diacerein, the mechanism of action of which is to inhibit the production and pathophysiological effects of IL-1. Numerous studies have demonstrated that significant positive effects from its use occur within 2–4 weeks. from the start of taking the drug with clinically significant differences from placebo at 4–6 weeks. [5, 6]. An improvement in the symptoms of the disease is also observed some time after the end of the course of therapy (the so-called “aftereffect”), which is an important positive factor influencing the long-term treatment strategy for OA (taking into account the chronic course of the disease). Diacerein has a unique mechanism of action that distinguishes it from other drugs for the treatment of OA. In particular, numerous studies have shown effective inhibition by diacerein of the production and activity of IL-1 and other catabolic cytokines that are expressed in OA, which was accompanied by a decrease in the degree of degradation of cartilage tissue [7, 8]. This was also facilitated by a decrease in the activity of plasmin, MMP and other proteases that have a negative effect on the cartilage matrix. In addition, in a study by N. Felisaz et al. (1999) demonstrated that diacerein has the ability to increase the expression of transforming growth factors (TGF-β1 and -β2) in cultured chondrocytes, which led to an increase in the number and activity of cells with increased production of hyaluronan, collagen and proteoglycans [9]. Thus, diacerein, having a pro-anabolic effect on cartilage, can be considered as an important pharmacological agent in the complex therapy of OA. This point of view is confirmed by the results of a study by M. Dougados et al. (2001), who assessed the structure-modifying effect of diacerein in patients with primary coxarthrosis [10]. The authors found that radiographic progression (narrowing of the joint space of the hip joint by at least 0.5 mm) in the group of patients taking diacerein was significantly less pronounced and developed later than in the group of patients taking placebo. An important advantage of diacerein is also the established fact of the presence of an aftereffect when using it, which was demonstrated in a double-blind, randomized, placebo-controlled study by K. Pavelka et al. [eleven].
Diacerein in the treatment of OA of the knee joints
The purpose
of this study was to study the clinical effectiveness, tolerability and safety of the drug diacerein (Diaflex) in comparison with chondroitin sulfate sodium in patients with OA of the knee joints during continuous use for 12 months, as well as to evaluate the “after-effect” effect. drugs.
Material and methods
The study included 30 patients (28 women and 2 men) with OA of the knee joints stage II-III (according to Kellgren - Lawrence), meeting the criteria of the American College of Rheumatology (ACR, 1986). The average age of the patients was 60.4±6.2 years, the duration of the disease was 5.9±4.5 years. The study was conducted in accordance with the basic principles of good clinical practice. Participation in the study was allowed only after the patient voluntarily signed an informed consent and received permission from the local ethics committee. When including patients in the study, the following criteria were taken into account: • men and women aged 45–75 years; • primary tibiofemoral OA of the knee joint (according to the AKR criteria, 1986); • pain when walking > 40 mm on the visual analogue scale (VAS); • radiographic stage II–III (according to Kellgren – Lawrence). Exclusion criteria: • secondary gonarthrosis (including with rheumatoid arthritis, gout, after an intra-articular fracture, etc.); • intra-articular injections of glucocorticoids, hyaluronic acid preparations for 3 months. before the start of the study; • aseptic necrosis of the condyles of the femur and/or tibia; • previous surgical interventions on the knee joint; • concomitant severe diseases: stage III arterial hypertension, unstable angina, cardiovascular failure, type 1 diabetes mellitus, severe liver and kidney damage; • acute gastric ulcer (duodenal ulcer) during the last month before the study. The criteria for patient exclusion during the study were ineffectiveness of therapy (preservation or intensification of pain requiring a change in treatment tactics), development of serious adverse events, patient refusal from the study, or violation of the study protocol. The total duration of the study was 15 months. (12 months – taking medications and 3 months – observation). A total of 8 visits were planned: B.0 – screening, washout period (3–7 days) in case of previous use of non-steroidal anti-inflammatory drugs (NSAIDs); B.1 – randomization, initiation of therapy; B.2 – B.7 – scheduled visits after 2 weeks, 1.5, 3, 6, 9 and 12 months. after the start of therapy, respectively, and B.8 - final examination after 3 months. after the end of therapy (assessment of the aftereffect of drugs). To assess the effectiveness and safety of therapy, the following criteria were used: pain intensity according to VAS (mm), “Get up and walk” test (in seconds), WOMAC index (pain, stiffness, functional impairment), EQ-5D health questionnaire, need for analgesics , assessment of the effectiveness of therapy by the doctor and the patient, assessment of the tolerability of the therapy. All patients were randomized into 2 groups of 15 patients each: 1st group took diacerein (Diaflex) at a dose of 50 mg 2 times a day for 12 months, 2nd group took sodium chondroitin sulfate (Chondroxide) at a dose of 500 mg 2 rubles/day If the patient had pain in the first 7 days of therapy, paracetamol was allowed to be prescribed up to 2 g/day, and then on demand. The characteristics of the groups of examined patients are presented in Table 1. Almost all examined patients were diagnosed with comorbid conditions (Table 2).
Of the 30 patients included in the study, 26 (86.7%) completed the full course of treatment; During the study, 2 patients dropped out of each group: from the 1st - due to the development of side effects (diarrhea, skin rash), from the 2nd - as a result of low effectiveness of therapy. Statistical processing of the material was carried out using standard methods, including comparison in groups using the Wilcoxon method for quantitative indicators and χ2 for qualitative indicators.
results
The main complaints of patients suffering from OA are pain in the affected joint and subsequent disruption of its functional activity, and the disease itself is non-lethal, therefore very important criteria for evaluating combination therapy are the degree of pain relief and improvement in the functional activity of patients, which ultimately has an impact on the quality of life of patients. It was also important to study not only the time of onset of the analgesic effect, but also its duration. The results of the study indicate that patients taking the study drugs experienced a significant reduction in pain, which was expressed in a decrease in the VAS index (Table 3). It should be noted that, despite the absence of significant differences in the groups depending on the duration of therapy, the VAS (pain) score remained <30 throughout the entire period of active drug use. Patients with concomitant OA of the hands (forming Heberden's nodes were diagnosed in 4 patients of the 1st group and 2 of the 2nd group) noted a significant decrease in pain in the distal interphalangeal joints already 7–10 days from the start of taking Diaflex.
To assess the functional activity of patients, the “Get up and walk” test was used, which was performed on all patients before the start of treatment, during therapy and after 3 months. after completion of the course of pharmacotherapy. In accordance with the test protocol, we used a chair with armrests and a stopwatch. The patient was asked to get up from the chair, walk 5 m, walk around an object located on the floor, return and sit on the chair. The time was recorded with a stopwatch. This test well reflects the patient’s real capabilities in everyday life, since depending on the severity of the initial pain in the affected knee joint and the intensity of the pain in general, the patient’s functional abilities can be assessed. According to the presented data (Fig. 1), over time, in the examined patients, the time to perform this test was reduced, which indicates an increase in functional activity and a decrease in the severity of starting pain. There was no significant difference between the groups. It should be noted that the results of this test remained stable even after the end of pharmacotherapy (period B.7 - B.8).
When analyzing the total WOMAC index (pain, stiffness, functional joint insufficiency), patients in group 2 showed an almost linear decrease throughout the entire observation period, while in patients in group 1 (who took Diaflex), the dynamics of this indicator were significantly different (Fig. 2). So, in the first 6 months. therapy, there was a pronounced decrease in the WOMAC index, followed by its stabilization and the formation of a “plateau” at a fairly low level until visit 8. When studying individual indicators of the WOMAC index (assessment of pain, functional joint insufficiency) in the examined patients in 2 groups, the same trend was observed – a more pronounced clinical effect, including the speed of its development, when using Diaflex (Fig. 3, 4). Thus, it can be stated that the analgesic effect when using Diaflex is more pronounced and develops earlier than when using sodium chondroitin sulfate.
The overall assessment of the effectiveness of therapy with the study drugs also correlated with the presented data (Fig. 5). Thus, “significant improvement” and “improvement” were stated by 11 patients taking Diaflex, and “no effect” or “deterioration” by 2 (in group 2 – 9 and 4 patients, respectively). During the use of the study drugs, the overall need for paracetamol in patients of both groups decreased, while the analgesic was completely canceled in 7 (46.7%) patients taking Diaflex, and in 3 (20%) taking chondroitin sulfate sodium.
It can be stated that patients taking Diaflex generally assessed the effectiveness of therapy better than patients in group 2, which may have been associated with subjective sensations from faster pain relief. At the same time, as noted earlier, when comparing the final results of various indicators both at the end of the therapeutic phase (B.7 - 12 months) and during the follow-up period (B.8 - 15 months) there were no significant differences between groups were not received. Tolerability of the study drugs was satisfactory. Over the entire period, 4 patients dropped out of the study: 2 patients from group 1 - due to intolerance to the study drug and 2 patients from group 2 - due to ineffectiveness of therapy (deterioration of condition requiring intra-articular injections - patients were excluded at visits B.6 and B.7 respectively). Two patients of group 1 were excluded from the study at visits 2 and 4 (B.2 and B.4) due to the development of diarrhea (reducing the dose of the drug to 50 mg/day, as recommended in such cases in the instructions for use of Diaflex, was not provided for by the protocol), also 3 (20%) patients from this group noted a slight darkening of the urine (metabolites of the drug are predominantly excreted in the urine).
Conclusion
The results of the study indicate that long-term use of symptomatic slow-acting drugs (SYSADOA) - Diaflex and chondroitin sulfate sodium is an effective method of treating OA of the knee joints, taking into account their effect on pain and functional activity of patients. Since OA is essentially a non-lethal disease, various currently existing therapeutic methods (pharmacological and non-drug), which have minimal side effects with a high therapeutic index, can be considered as the most promising in these patients for long-term use. An analysis of the results of a 12-month use of Diaflex and chondroitin sulfate sodium in patients with knee OA showed their effectiveness in relieving the clinical symptoms of the disease (pain, stiffness, limitation of movements) against the background of discontinuation of NSAIDs and a reduction in the dose (or complete withdrawal) of paracetamol. However, taking into account the data of the study, the use of Diaflex may be preferable in patients with OA of the knee joint due to a more rapid increase in the clinical effect, as well as in patients with polyosteoarthritis with developing Heberden nodes due to the good analgesic effectiveness of the drug against small hand joints. Taking Diaflex in the examined patients was accompanied by a positive clinical effect after 2–3 weeks. from the start of taking the drug and increased throughout the entire treatment period. So, already by the 4th week. The patients showed positive dynamics of all clinical symptoms studied: the severity of pain, including starting pain, stiffness, and joint function. The presented results are consistent with literature data indicating that diacerein significantly improves the symptoms of the disease compared to placebo, has a pronounced aftereffect and does not cause severe adverse events, and therefore it is recommended as a drug for long-term therapy of OA [12].
conclusions
1. The use of symptomatic slow-acting drugs - Diaflex and chondroitin sulfate sodium in patients with OA of the knee joints has demonstrated high clinical effectiveness, safety, good tolerability, and a rare occurrence of adverse reactions. 2. Diaflex demonstrated a faster (after 2–4 weeks) clinical effect on OA symptoms, including those affecting small joints of the hands, which is probably due to the peculiarities of the drug’s mechanism of action. 3. Prescribing drugs from the SYSADOA group allows minimizing the development of side effects in comorbid patients by reducing the dose (duration of prescription) of NSAIDs and other drugs. 4. Therapeutic courses lasting 6 months are more preferable. or more, and the presence of an aftereffect in these drugs allows for course therapy for OA. 5. A more pronounced aftereffect was observed when using Diaflex, which confirms the increase in the positive dynamics of the WOMAC index even after the end of the 12-month course of therapy. We associate the results obtained with the mechanism of action of diacerein.