From this article you will learn:
- what is isotretinoin,
- Acnecutane and Roaccutane - instructions, reviews,
- differences between these drugs.
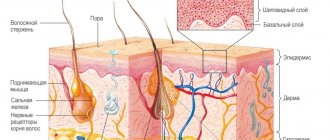
Isotretinoin is a retinoid that is also known as 13-cis-retinoic acid (Figure 1). This chemical compound is an isomer of tretinoin (trans-retinoic acid), which, like isotretinoin, is a structural analogue of vitamin A. Isotretinoin is best known as an oral systemic retinoid that is used to treat severe forms of acne. The latter include, for example, nodular and conglobate forms of acne, as well as forms of acne that are resistant even to oral antibiotics.
Systemic retinoids containing isotretinoin include drugs such as Acnecutane and Roaccutane. As we said above, they are intended for oral administration (available in capsule form). However, there are preparations with isotretinoin for external use that can be used to treat photoaging of facial skin. These drugs include those available on the Russian market - Retasol drugs, as well as retinoic ointment.
Isotretinoin (13-cis-retinoic acid) –
Reviews from dermatologists about Acnecutane and Roaccutane are the most positive, because in fact, these drugs with isotretinoin have made a real revolution in the treatment of severe forms of acne. They have improved the quality of life for many patients, including preventing the formation of scars. Isotretinoin therapy provides complete remission of the disease in almost all patients with acne, and after completion of the course of therapy, remission can last up to several months or even years.
However, therapy with systemic retinoids is associated with the risk of quite severe side effects, which we will discuss in detail below (they occur especially often if the dosage is incorrectly selected). Girls and women should take into account that these drugs have a pronounced teratogenic effect, and therefore they are prohibited both during pregnancy and breastfeeding. In addition, the entire period of use of the drug will require a two-level contraceptive system (both oral contraceptives and condoms).
How much do Roaccutane and Acnecutane cost in pharmacies -
For 2021, the price for Roaccutane will be from 1900 rubles - for a package of 30 capsules of 10 mg. It should be noted that this drug can now not be purchased in every pharmacy, which is apparently due to the current re-registration of the drug on the Russian market. For Acnekutan, the price for a package of 30 capsules of 8 mg will be from 1650 rubles, and for a package of 30 capsules of 16 mg - from 2600 rubles.
Analogs of Roaccutane include such drugs as Sotret (India) and Verocutan (Russia). The first one costs from 1350 rubles per pack of 30 capsules of 10 mg. As for the Russian drug Verokutan, it has currently disappeared from sale.
Roaccutane and Acnecutane (mechanism of action) –
The drug Roaccutane is produced by a pharmaceutical company (Switzerland), and the drug Acnecutane is produced by a pharmaceutical company (Croatia). The drugs contain isotretinoin and are practically equivalent to each other, however, in the production of the drug Acnekutan, the “Lidose” technology is used, which makes it possible to reduce the daily and course doses of isotretinoin, and therefore the risk of side effects during treatment. Lidose technology is a Belgian development.
Indications for use –
- severe forms of acne (nodular, conglobate, fulminant),
- forms of acne with a risk of scarring,
- acne that cannot be treated with topical medications or oral antibiotics.
These are the indications you can find in the official instructions for the drugs Roaccutane and Acnecutane.
However, according to numerous clinical studies and the most authoritative textbook on dermatology in the world, Fitzpatrick's Dermatology (we use the 8th edition), isotretinoin preparations are also very effective for the treatment of gram-negative folliculitis and facial pyoderma. Gram-negative folliculitis is exactly the complication that very often develops in patients with acne after using local and systemic antibacterial drugs. The mechanism of action of isotretinoin for acne -
The mechanism of action of isotretinoin has not been fully studied, but it is known that it quite strongly suppresses the activity of the sebaceous glands. It suppresses both the activity of sebocytes (these are cells in the sebaceous glands that secrete fatty secretions) and their proliferation, i.e. reproduction. Accordingly, as a result of a course of isotretinoin, both the secretion of sebum (secretion of the sebaceous glands) decreases and the sebaceous glands decrease in size.
Studies have shown that after completing the course of treatment with isotretinoin, in most patients the usual activity of the sebaceous glands returns only after 2-4 months, however, in some patients this effect can be expressed even up to 1 year. In addition, isotretinoin helps indirectly reduce the number of P. acnes bacteria, normalizes the processes of follicular keratinization, and also has anti-inflammatory activity.
The latter, however, requires clarification, because at the first stage of drug therapy with Acnecutane or Roaccutane - on the contrary, acne may worsen. This effect usually lasts up to 2 weeks and its appearance does not require discontinuation of the drug, and to reduce the symptoms of acne exacerbation, reduced dosages of isotretinoin are usually used at the first stage of treatment.
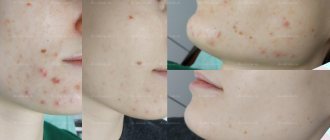
Isotretinoin: before and after photos
Important: Keep in mind that it usually takes at least 4 weeks to notice the first positive changes. In addition, during the first 1-2 weeks, your acne may worsen (this is normal with systemic use of isotretinoin). The full course of treatment will last from 16 to 24 weeks.
Why does acne occur?
The content of the article
Acne is a group of diseases associated with disorders of the sebaceous glands. The most popular form of acne appears to be acne vulgaris, which usually affects teenagers and some adults. As a result of hormonal changes, the sebaceous glands produce excessive sebum. In addition, we often encounter increased keratosis of cells that clog the excretory ducts, which contributes to the formation of acne and purulent pustules.
Acnecutane and Roaccutane: instructions for use
The drug Acnekutan is available in capsules (there are 2 forms of release - capsules of 8 and 16 mg). The drug Roaccutane also has 2 release forms - capsules 10 or 20 mg. A very important issue is determining the optimal daily dose in each specific clinical case. Never take these medications without the advice of a dermatologist and laboratory tests. According to the dermatology textbook Fitzpatrick's Dermatology, the recommended daily dose of isotretinoin is in the range of 0.5-1.0 mg/kg/day.
For Acnecutane, the average daily dosage will be slightly lower (than for Roaccutane), which is explained by the use of Lidose technology in its production. By the way, reviews of dermatologists on Acnecutan note that, apparently due to this circumstance, side effects are less likely to occur. At the 1st stage of therapy, it is customary to use slightly lower doses of isotretinoin than during the main period of treatment. For example, for Acnecutane the daily dose will usually be only 0.4 mg/kg/day at the 1st stage of therapy, and in the future we can increase it to 0.8 mg/kg/day. And for Roaccutane at stage 1, the daily dose will already be 0.5 mg/kg/day, and then it will need to be increased to approximately 1.0 mg/kg/day.
In addition to the standard daily dose recommended above in the range of 0.5-1.0 mg/kg/day, studies have described treatment regimens that use even lower daily doses (in the range of 0.1 to 0.4 mg/kg/day). day). It should be noted that such daily doses also show their effectiveness, but you must understand that in these cases the duration of remission after discontinuation of the drug will be shorter.
1) Recommended cumulative doses –
There is also the concept of a cumulative dose, which during the entire course of therapy can be: 1) for Acnecutane - 100-120 mg/per 1 kg of weight, 2) for Roaccutane - 120-150 mg/per 1 kg. Calculation of the cumulative dose per 1 kg of weight is very important for patients whose daily dosages change or there are breaks in treatment. In these cases, achieving the recommended cumulative dose per 1 kg of weight allows for the longest remission of the disease. In patients with severe lesions of the back and chest, the recommended daily dose can reach up to 2 mg/kg/day, because these areas are less sensitive to isotretinoin therapy.
2) Duration of treatment –
Complete remission of acne symptoms can be achieved after 16 to 24 weeks of taking isotretinoin. In each specific case, the duration of the course is individual and can only be determined by a dermatologist. Please note that an improvement in the condition of your acne may be observed, including within 1-2 months after stopping the drug. Therefore, the drug can be discontinued in some cases even before the inflammatory elements of acne completely disappear.
Approximately 10% of patients treated with isotretinoin require a second course of the drug, with the likelihood of repeat therapy increasing in patients younger than 16–17 years. A second course of therapy can be prescribed no earlier than 8 weeks (after the end of the first).
Important: in patients with severe forms of acne (especially granulomatous lesions), isotretinoin therapy often leads to a sharp exacerbation of acne. And in such patients, it is important not only to use lower dosages at the 1st stage of treatment, but also to conduct a short course of treatment with prednisolone (for 1-2 weeks, 40-60 mg/day). But if necessary, the course of prednisolone can be extended, and can cover the first 2 weeks of isotretinoin therapy (24stoma.ru).
→ Roaccutane instructions for use official. (PDF) → Acnecutane official instructions (PDF)
The introduction of oral isotretinoin into medical practice has brought about a revolution in the treatment of acne. This drug made it possible to successfully treat severe forms of nodular cystic and other acne that were not amenable to previously existing treatment methods.
In recent years, there has been a trend toward increased use of isotretinoin. It has proven effective not only for acne, but also for many other skin diseases. Indications for the use of the drug that are not officially recognized include hidradenitis suppurativa, ichthyosis, keratosis pilaris (Darier's disease), lichen planus, pityriasis versicolor pilaris, lupus erythematosus (cutaneous forms), acne rosacea, mycosis fungoides (T-cell lymphoma of the skin) . It is also prescribed prophylactically for nevoid basal cell carcinoma and for the prevention of skin cancer in patients with xeroderma pigmentosum. Isotretinoin is also used to treat cancer. It is effective as monotherapy, but, according to the results of most studies, the best effect is obtained when it is used simultaneously with other drugs, in particular with cytokines. In experimental studies, oral retinoids have been studied in myeloid leukemia, squamous cell carcinoma of the head and neck, breast cancer, cervical intraepithelial dysplasia, and cervical and kidney cancers. The number of new patients taking isotretinoin is constantly increasing. In the USA alone it amounts to 500 thousand people per year [1]. In the period from 1992 to 2000. consumption of isotretinoin in the country increased 2.5 times [2]. In 2000, the drug was prescribed to approximately 2 million patients. There is a trend towards increased use of isotretinoin for mild and moderate forms of acne: in the period from 1993 to 2000. the proportion of its prescriptions for severe acne decreased from 63% to 46%, while for mild and moderate acne it increased from 31% to 49% [2].
However, although the effectiveness of isotretinoin has been convincingly proven in clinical studies and widespread medical practice, its use is limited by side effects. At least 50 acute and chronic adverse reactions that occur during treatment with the drug have been described. Of greatest concern are teratogenic and central side effects.
Teratogenic effect and precautions during pregnancy
The ability of isotretinoin to cause severe congenital anomalies was first described in 1983, i.e. one year after its approval for marketing in the United States [3,4]. By this time, a teratogenic effect had been identified in other retinoic acid derivatives, so it was also expected for isotretinoin. Since its approval for medical use, the drug has been assigned an FDA category "X", which includes drugs that are absolutely prohibited during pregnancy, since the risk of their use outweighs any potential benefit.
Isotretinoin crosses the placenta relatively easily. Its teratogenic effect is associated with the slow elimination of the 13-cis isomer, the formation of 4-oxo derivatives during metabolism, ongoing isomerization, significant exposure of target tissues to trans-retinoic acid and insufficient interaction with cytoplasmic binding sites, which leads to higher intake drug into cell nuclei [7]. Its metabolite 4-oxoisotretinoin accumulates in the fetal liver, and small concentrations of retinoids are also found in the brain [5].
Various congenital disorders have been described in children whose mothers took isotretinoin during pregnancy, including pathology of the skull; ear lesions (reduction in size or absence of the auricle, narrowing or absence of the external auditory canal); eye lesions, including microophthalmia; violation of facial morphology; cleft palate; damage to the central nervous system, incl. abnormalities of the cerebral cortex, cerebellar pathology, hydrocephalus, microcephaly, hypoplasia of cranial nerves; cardiovascular abnormalities; pathology of the thymus and parathyroid glands [6,7] Cases of shortening of the limbs, similar to those with intrauterine exposure to thalidomide, have been described [8]. It is believed that birth defects are the result of disturbances in the morphology and motility of neural crest cells during a critical stage of fetal development [9]. In many cases, the anomalies are incompatible with life and lead to intrauterine death.
The teratogenic potential of the drug is quite high and, according to various studies, ranges from 15% to 45%, which is comparable to that for thalidomide [6,10]. Between 1982 and 1989 The manufacturer of isotretinoin received 151 reports of full-term pregnancy outcomes in women treated with isotretinoin. Moreover, congenital pathology was observed in 47% of cases.
The maximum risk of developing congenital anomalies is observed when isotretinoin is used in the first trimester of pregnancy, and they are registered even in the case of taking one capsule of the drug [11]. The frequency of spontaneous abortions during therapy is 20-30% [10]. The rate of induced abortion in women taking oral retinoids, according to an analysis conducted in Australia, was 1 in 319 courses of treatment [12]. Isotretinoin also increases the risk of preterm birth. A number of children exposed to the drug in utero showed a delay in the level of intellectual development according to the IQ test.
In July 1988, the United States began training pregnant women in the use of isotretinoin as part of the Pregnancy Prevention Program [13]. The program included the distribution of printed materials for patients, instructions for physicians on scheduling pregnancy tests, and information about enrolling patients in follow-up care. Women taking part in the program were required to sign a consent form indicating that they had been counseled. The implementation of the program led to significant positive results, but did not achieve the goal of zero pregnancy rates [13,14].
A survey of women participating in the Pregnancy Prevention Program in 1989-1993 showed that almost all of them were aware of the harm that isotretinoin can cause the developing fetus [13]. Among sexually active women, 99% reported using contraception. Despite this, 3.4 pregnancies have been documented for every 1000 treatments. Approximately 75% of registered pregnancies were terminated, and 32 resulted in live births. Among the 30 children about whom information was available, developmental anomalies were noted in 7.
In a more recent study from California, 23 pregnancies were reported over a 2-year period [15]. Information about their outcomes was available in 14 cases. Of these, in 4 cases there was a miscarriage, 5 pregnancies were terminated, one newborn had a congenital pathology, 4 children were born healthy.
Study results indicate that 14-33% of women who reported pregnancy during treatment did not undergo pregnancy testing or did not wait for test results and were pregnant before isotretinoin was prescribed, and 12-16% of women became pregnant during the period after initiation of treatment. but before the onset of the next menstruation [16,17]. In addition, approximately 64% of women were unable to avoid behaviors associated with a high risk of conception [17]. In most cases, pregnancy occurred in those who used one method of contraception.
The total number of pregnancies occurring during isotretinoin treatment remains unknown. In September 2000, the drug's manufacturer reported 1,995 cases to the FDA [18]. It is believed that this figure, although unexpectedly high, is actually an underestimate due to the voluntary nature of the Pregnancy Prevention program. It is estimated that only 40% of women taking isotretinoin during pregnancy participate in the program [15]. Currently, the number of pregnancies occurring during treatment appears to be increasing due to the increase in the number of women taking the drug for various indications.
In May 2000, it voluntarily made significant changes to the instructions for use of isotretinoin. A number of the most serious warnings are placed in a frame (the so-called “black box”) to attract the attention of consumers. They say that women should:
- use the drug only in the presence of severe acne that is resistant to other types of treatment;
- understand the instructions for using isotretinoin well;
- perform all pregnancy tests;
- receive oral and written warnings about the risk of teratogenicity;
- receive oral and written information about the need to use two types of contraception during treatment;
- have negative pregnancy test results at the first determination and a second determination performed on the 2nd day after the next menstrual period, or 11 days after the last sexual intercourse;
- receive information about the opportunity to participate in the isotretinoin research program and view the manufacturer's videotape.
Due to the long half-life of the drug, measures to prevent pregnancy should be observed for a month after stopping treatment. Later after discontinuation of isotretinoin, the incidence of congenital anomalies, spontaneous and medical abortions does not differ significantly from those for the general population [19].
Women taking isotretinoin should not donate blood during treatment and for one month after its cessation, since transfusion of their blood to pregnant women carries a risk to the recipient's fetus [3].
The high lipophilicity of isotretionine determines its penetration into breast milk and the risk of adverse effects on the child.
Side effects from the central nervous system and sensory organs
In recent years, the central side effects of isotretinoin—depression and associated suicide—have been of particular concern [20]. A possible link between isotretinoin use and depression or suicide was suspected many years ago. Psychiatric side effects began to be reported to the FDA shortly after the drug entered the pharmaceutical market [21]. Between 1982 and 2000 The Agency received 431 reports of depression, suicidal motivation, suicide attempts, or suicide in patients receiving isotretinoin [22]. Among the suicides (37 cases), the vast majority (84%) were men, with an average age of 17 years. Eight of them had a history of mental illness [22].
Depression was included as a side effect of the drug in 1985. In February 1988, the FDA added a black box warning for psychiatric disorders, including suicide [18]. However, the role of isotretinoin in the development of mental disorders remains unproven.
It is known that approximately 30-50% of people aged 12-20 years experience psychological reactions due to acne, ranging from mild anxiety to severe depression, decreased self-confidence and self-esteem, and impaired social adaptation. In most patients, successful treatment of the underlying disease also leads to an improvement in psychosocial condition. However, in some patients, depression develops during the treatment period and even several months after its completion, which makes it possible to suspect that they have other problems that they previously associated exclusively with their appearance.
Concern among physicians and patients about the association between isotretinoin and adverse psychiatric reactions, supported by the media, prompted the manufacturer to conduct a special population-based cohort study that analyzed information contained in the Canadian Saskatchewan Health and Research Database. UK General Practice Database [23]. In total, the study included data from about 8,000 patients treated with isotretinoin and more than 14,000 controls treated with antibiotics. The study results do not suggest an increased risk of depression, suicide or other mental disorders with isotretinoin use. FDA officials came to the same conclusion after analyzing all available data [22,24]. Similar results were recently obtained in Australia [25].
To further investigate the potential risk of psychiatric side effects, in 2001 the manufacturer developed a form that asks patients to indicate symptoms of depression or psychosis in their immediate family or themselves prior to starting treatment with isotretinoin. In addition, it emphasizes the need to promptly report to the physician all psychiatric symptoms that occur during treatment [1,18].
A prospective analysis of side effects in 124 patients treated with isotretinoin at a dose of 1 mg/kg for 4 months showed that depression was observed in 4% of cases and tended to persist throughout the course of therapy [26].
A special prospective comparison study of antibiotics and topical agents, including 215 participants (mean age 20 years), failed to detect a statistically significant difference between groups in depression scores (p = 0.62) and quality of life [27]. There was also no correlation shown between the dose of isotretinoin and the severity of depression. Obviously, larger studies are needed to make a final conclusion about the absence of a cause-and-effect relationship between the use of this drug and depression.
In 2002, the pharmaceutical company sent US doctors a “Dear Doctor” letter, notifying them of changes in the instructions for use of the drug [29]. They included warnings about the potential for aggressive and violent behavior under the influence of isotretinoin and information for doctors and patients based on the results of a new study in pediatrics. It showed that 29% of children treated with isotretinoin experienced back pain, and 22% developed atralgias. In addition, two potentially adverse drug interactions were added to the instructions: with systemic corticosteroids and phenytoin.
In addition to depression, isotretinoin can cause other central nervous system symptoms. These include: insomnia or pathological drowsiness, fatigue, excitability, loss of appetite and interest in work or study.
In rare cases, isotretinoin (mainly when combined with tetracyclines) can cause pseudotumor cerebri. Due to the risk of increasing intracranial pressure, tetracyclines should not be taken concomitantly with isotretinoin [27]. Patients should be warned about the symptoms of pseudotumor - severe headache, nausea, vomiting and visual disturbances [1].
An analysis of 1741 reports containing information about ophthalmic adverse reactions of isotretinoin revealed 38 different symptoms of eye damage [30]. The most common among them were dry eyes (40%), blurred vision (including twilight vision), photophobia, blepharoconjunctivitis, discomfort in the eye, clouding of the cornea, increased sensitivity to contact lenses, keratitis, increased osmotic pressure of tear fluid, development of cataracts and congenital eye pathology [26,31]. Ophthalmic side effects can develop within a few days or several years after starting to use the drug. In a special study that included 40 patients receiving isotretinoin at a dose of 0.5-1 mg/kg/day for 2 months, 42.5% of participants had conjunctival colonization with Staphylococcus aureus [32]. However, this study also showed that when the drug is used in low doses for short courses, visual impairment is usually not serious and completely disappears within a month after the end of therapy [32].
Cases of hearing loss have also been reported in patients receiving isotretinoin [33].
Other adverse reactions
The most common side effects of oral isotretinoin, occurring in more than 90% of patients, are cheilitis or xerosis of varying severity. Skin adverse reactions also include dermatitis (50%), itching (
In a number of patients, during treatment with isotretinoin, carbohydrate metabolism is disrupted [26,33]. In case of an overdose of the drug, hypervitaminosis A may develop [38].
Recommendations for the correct use of isotretinoin
Despite the huge number of potential side effects of isotretinoin, practice shows that when used correctly it is a highly effective and fairly safe drug [39]. Before prescribing a drug, it is necessary, first of all, to weigh the benefit/risk ratio for a particular patient. Its use should be considered in severe forms of acne or in cases where acne is associated with severe psychosocial disorders. The use of the drug for milder forms of acne should be resorted to only in cases where repeated long courses of antibacterial therapy have not brought the desired result, when relapses develop after several successful courses of treatment with standard means, in patients with a tendency to form scars.
It should be remembered that absolute contraindications to the use of the drug are pregnancy, breastfeeding, hypervitaminosis A, and hypersensitivity. Treatment with isotretinoin is not recommended for persons with hepatic and renal insufficiency, diabetes mellitus and hyperlipidemia. Relative contraindications for taking retinoids are also pseudotumor cerebri, inflammatory bowel disease, hepatitis and childhood.
Women of childbearing age should be given detailed instructions about the risk and frequency of teratogenic effects and asked to sign a form confirming that they understand the risk of conception during treatment before being prescribed the drug. The drug is prescribed only after receiving a negative pregnancy test result and against the background of effective contraception using two methods. Since androgens play an important role in the pathogenesis of acne, when treating with isotretinoin, contraceptives containing a progestin with androgenic action, for example, 19-nortestosterone derivatives, should not be used. It is advisable to start treatment on the second or third day of the next menstrual cycle. The pregnancy test should be repeated 4 weeks after the end of therapy. The drug must be taken under the close supervision of a specialist. Clinicians who have not received specific training in the use and monitoring of oral retinoid therapy should not prescribe these drugs.
Most of the side effects of isotretinoin are dose-dependent, so it should be used in the minimum effective dose, however, to reduce the likelihood of relapse, the course dose of the drug should reach at least 120 g/kg. The recommended initial dose is 0.5-1 mg/kg per day. It should be divided into 2 doses. The dose can be increased to 2 mg/kg/day. During the treatment period, it is recommended to determine the level of liver enzymes, triglycerides and cholesterol. A significant increase in triglyceride concentrations in some cases led to the need to discontinue treatment. However, an analysis of data on 907 patients receiving isotretinoin for 5-9 months showed that triglyceridemia above 400 mg% is quite rare - in 1.5% of patients [40]. No pronounced increase in liver enzyme activity leading to drug discontinuation was observed in patients included in the analysis. The data obtained allowed the authors of the analysis to suggest that routine laboratory monitoring (with the exception of pregnancy tests) is not indicated for healthy young patients.
It should be remembered that isotretinoin has clinically significant interactions with food and drugs. Under the influence of food, its bioavailability increases by 2 times. Taking the drug with food reduces fluctuations in bioavailability. Simultaneous use with isotretinoin of other drugs for the treatment of acne - drugs with keratolytic or exfoliative effects, as well as ultraviolet therapy is not recommended. Patients should avoid other types of ultraviolet radiation, such as insolation. During the treatment period and for six months after its completion, due to the risk of developing dermatitis, hair removal using wax applications is not recommended.
Isotretinoin should not be co-administered with other vitamin A derivatives (risk of exacerbating symptoms of hypervitaminosis A). As mentioned above, the combined use of the drug with tetracyclines, corticosteroids and phenytoin should be avoided.
If the above recommendations are followed, the benefits of using isotretinoin in most cases significantly outweigh the risks [40]. The main reasons for the development of side effects of isotretinoin and unplanned pregnancies during the treatment period are non-compliance with these recommendations by medical professionals and violation of precautions to prevent conception by patients [41,42]. For example, in the United States, at least 47% and likely 72% of patients do not receive standard therapy (eg, a topical retinoid or oral antibiotic) before isotretinoin initiation [41]. Doctors often ignore the possibility of pregnancy in girls under 16 years of age, and therefore patients in this age group often do not receive the necessary consultations [42]. According to foreign studies, no more than half of doctors perform a pregnancy test before prescribing the drug, and in cases where such a test is performed, preference is given to a less sensitive urine test [43]. A study from the Motherisk program in Canada found that distributing educational materials to patients significantly improved contraceptive adherence [44]. If conception does occur during the treatment period, women should be asked to consider terminating the pregnancy, again explaining the potential danger to the fetus.
One of the latest measures taken by the FDA to improve the safety of isotretinoin use was the introduction at the end of 2002 of restrictions on the import into the country of 10 potentially toxic prescription drugs, the use of which requires special control [45]. Persons attempting to bring these drugs into the United States are denied entry. This list also includes isotretinoin.
In addition, a new microionized dosage form of the drug has now been developed, which, having a profile of adverse reactions similar to standard isotretinoin, is less likely to cause some adverse effects, for example, lesions of the skin and mucous membranes, hypertriglyceridemia [46].
Side effects of isotretinoin -
The severity of side effects always depends on the daily dose of isotretinoin. Most of the side effects will be similar to chronic hypervitaminosis syndrome “A” and, accordingly, they will be associated with the skin and mucous membranes. Below you can see statistics on the most common side effects associated with the skin and mucous membranes.
Frequency of side effects (per number of patients) –
- cheilitis (inflammation of the lips) – in 100% of patients,
- facial dermatitis – 46.1%,
- dry nasal mucosa – 24.8%,
- dry skin – 21.4%,
- skin itching – 14.5%,
- increased cholesterol levels – 9.3%,
- dermatitis of the hands – 6.0%,
- dry conjunctiva of the eye – 3.4%,
- bleeding of the nasal mucosa – 2.6%.
This study of side effects was published in the scientific journal “Bulletin of Dermatology and Venereology 2017”. The study was conducted at the Federal State Budgetary Educational Institution of Higher Education "KSMU" of the Ministry of Health of Russia. Considering that Acnecutane was used as a drug with isotretinoin, which is taken in slightly lower doses (compared to Roaccutane), then the statistics of side effects for Roaccutane should therefore be somewhat worse.
Less common side effects –
- hair thinning,
- myalgia (muscle pain),
- from the eyes - xerophthalmia, night blindness, conjunctivitis, keratitis (corneal clouding) and optic neuritis,
- hearing loss (both transient and permanent),
- severe headaches, lethargy, fatigue,
- risk of depression, suicide, psychosis and aggressive behavior,
- from the gastrointestinal tract - nausea, esophagitis, gastritis, colitis, acute pancreatitis, acute hepatitis,
- an increase in cholesterol levels with a decrease in high-density lipoprotein levels - in the first 4 weeks from the start of therapy,
- impaired bone mineralization (with a repeated course of therapy, osteoporosis may be diagnosed and the risk of fractures increases).
Isotretinoin and pregnancy -
All drugs containing isotretinoin have a strong teratogenic effect. Patients should use 2 reliable methods of contraception at once (both oral contraceptives and condoms). Contraception should be started at least 1 month in advance and continued for at least 1 month after completion of isotretinoin therapy. The patient must have a negative pregnancy test result within 11 days before starting the drug, plus regular monthly testing throughout the course. If the drug is taken by a man, then there is no risk to the fetus.
Isotretinoin and pregnancy
A fairly serious side effect of oral administration of retinoids is their teratogenic effect, that is, causing developmental defects in the fetus. Therefore, oral retinoid therapy is contraindicated in women of childbearing potential unless they are using contraception.
You also cannot make the decision to conceive immediately on the day you stop taking the drug, since you should wait until all the substance is eliminated from the body.
For isotretinoin, this period is the period of passing the next full menstrual cycle, and for acitretin (another drug from the retinoid group) it is as much as 2 years! However, this risk does not apply to male therapy, since isotretinoin in sperm does not cause developmental defects in the fetus.
The use of isotretinoin for the correction of wrinkles –
But the retinoid isotretinoin can be used not only systemically (orally).
There are a small number of drugs with isotretinoin for external use, which were initially used exclusively for the treatment of acne and pimples, but later these drugs began to be used to correct the symptoms of photoaging. As in the case of the retinoid tretinoin, preparations with isotretinoin also help to increase skin elasticity and reduce the depth of wrinkles. What effects do external forms of isotretinoin cause in the skin:
- Peeling effect - the thickness of the superficial stratum corneum of the epidermis decreases (due to exfoliation of dead skin cells). This evens out skin tone and texture, leaving skin looking more youthful and radiant—like you've had a few superficial chemical peels.
- Increasing the thickness of the deep layers of the epidermis - isotretinoin affects stem keratinocytes located at the basement membrane, increasing the rate of their division and differentiation. This leads to an increase in the thickness of the deep layers of the epidermis, consisting of living keratinocytes. As a result, the hydrophobicity of the epidermis increases, which contributes to less evaporation of moisture from the surface of the skin. In addition, it prevents skin photoaging.
- Stimulation of the production of collagen and hyaluronic acid - isotretinoin affects not only the epidermis, but also the dermis. It promotes the proliferation (reproduction) of fibroblasts, and also significantly stimulates their activity, which leads to an increase in their production of collagen, elastin and endogenous hyaluronic acid. This leads to an increase in the thickness of the dermis, a decrease in the depth of wrinkles, and an increase in skin elasticity. It is known that thicker skin is less susceptible to the aging process.
Optimal concentrations of external forms of Isotretinoin –
There are a number of clinical studies where scientists have determined the optimal concentration of isotretinoin for the treatment of photoaging.
1) “Armstrong RB, Lesiewicz J, Harvey G et al. Clinical panel assessment of photodamaged skin treated with isotretinoin using photographs. Arch Dermatol 1992; 128:352–6.” 2) “Sendagorta E, Lesiewicz J, Armstrong RB. Topical isotretinoin for photodamaged skin. J Am Acad Dermatol 1992; 27:S15–18.”
In these studies, the concentration of Isotretinoin was increased from 0.05% at the beginning of the study to 0.1% at the end of the study. Despite the increase in concentration, the drug was well tolerated by patients without causing significant skin irritation. As a result of the treatment of photoaging skin with 0.1% Isotretinoin, the skin condition gradually improved throughout the 36-week treatment, and a decrease in the depth of wrinkles and fine lines was achieved.
3) The study “Maddin S, Lauharanta J, Agache P et al. Isotretinoin improves the appearance of photodamaged skin: results of a 36-week, multicenter, double-blind, placebo-controlled trial. J Am Acad Dermatol 2000; 42:56–63.” In this study, a combination of 0.05% Isotretinoin plus SPF sunscreen was used to treat photoaging. The result was that the condition of skin with visible photodamage was significantly improved, which was recorded using profilometry.
Conclusions: if you are interested in preventing skin photoaging, it is best to use a 0.05% concentration in combination with sunscreen. If you want to achieve an increase in skin elasticity and a decrease in the depth of wrinkles, then the main treatment should be carried out using a 0.1% concentration (it is better to use a 0.05% concentration for the first month so that the skin gets used to retinoids).
Isotretinoin has a delayed effect - it will take at least 8-12 weeks before you notice any positive changes, although the first positive effect associated with improved skin tone and texture will be noticeable after 4-6 weeks. It must be admitted that abroad isotretinoin is used for the correction of photoaging much less frequently than other types of retinoids - tretinoin or pure retinol.
Preparations with isotretinoin for external use –
- “Retinoic ointment” (Fig. 8) – is available with an isotretinoin concentration of 0.05% or 0.1%. The cost will be from 300 rubles for a 15 g tube. About a tenth of the volume is ethyl alcohol, so you should not use this drug if you have dry and/or sensitive skin. In principle, the manufacturer writes in this regard that this drug is intended for the treatment of acne in patients with oily skin.
- "Retasol" (Fig. 9) - is a solution for external use, with an isotretinoin concentration of 0.025%. But keep in mind that this drug also contains alcohol, but in less quantity than retinoic ointment. In addition, the drug contains propylene glycol, which may cause irritation in patients with sensitive skin. Cost from 400 rubles per 50 ml bottle.
Isotretinoin: instructions for use
These instructions for using the drug are equally suitable for the treatment of acne and for facial skin rejuvenation techniques, for which information will be given below.
1) Wash your face thoroughly with a mild cleanser. 2) It is advisable to wait 20-30 minutes for the skin to dry thoroughly. 3) Squeeze out a pea-sized amount of the preparation and rub evenly. 4) Avoid contact of the drug with the mucous membranes of the eyes, lips, and nose. 5) After applying the drug, wash your hands thoroughly. 6) Use Isotretinoin 1 time per day (before bed). 7) Be patient - you will see the first results after 4 weeks when treating acne, and after 8-12 weeks for skin rejuvenation. The average treatment duration is 16-24 weeks for acne, and up to 36 weeks for improving the appearance and firmness of the skin.
The average treatment duration is 16-24 weeks for acne, and up to 36 weeks for improving the appearance and firmness of the skin.
Features of application –
Do not use more of the drug than recommended or more often than prescribed, because This will not speed up the effect, but will cause more redness, peeling and itching. In addition, isotretinoin should not be used if the skin in the area of application is damaged. During the treatment period, it is necessary to avoid exposure to sunlight, especially during periods of high solar activity (otherwise you may get hyperpigmentation of the treated skin areas).
Always apply sunscreen with SPF 50 before going outside. In summer, wear wide-brimmed hats to shield your face from the sun.
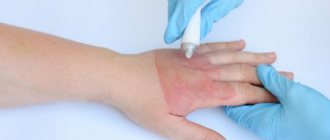
Side effects of topical forms of isotretinoin -
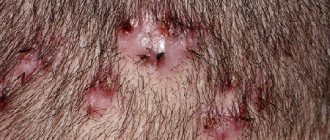
It should be noted that isotretinoin may affect different people differently. In most patients they are completely absent or mild. The most common side effects are redness, dryness, flaking, itching and burning of the skin, and increased sensitivity to sunlight. You can see the side effects from the use of retinoids in Fig. 10-11.
We hope that our article: Acnecutane and Roaccutane reviews was useful to you!
Sources:
1. Textbook of dermatology “Fitzpatrick's Dermatology” (8th edition), 2. American Academy of Dermatology (USA), 3. “Isotretinoin in the treatment of acne” (Tlish M., Shavilova M.), 4. “Cosmetic dermatology” (Bauman L.).
Forms of acne treatment
There are many forms of acne therapy available today, although it often takes some time to find one that's right for you. The first step is to take appropriate skin care with products designed for the skin of people struggling with this problem.
Another treatment for acne is primarily the disinfectant benzoyl peroxide, which inhibits keratosis cells with azelaic acid or antibiotics.
A special group of drugs consists of retinoids, and among them isotretinoin. Although topical treatment with these agents is quite common, oral administration (due to numerous side effects) is reserved exclusively for the most severe cases of acne, such as comedones, pimples, purulent fistulas and acne scars.