Story
People have been suffering from silicosis since ancient times. This is evidenced by silicotic changes in the lungs discovered during the study of Egyptian mummies, which may have been the cause of death. For a long time, Silicosis was identified with tuberculosis, calling it the consumption of stonemasons or miners, and only by the end of the 19th century. the idea of S. is formed as an independent lung disease caused by inhalation of dust. The term "silicosis" was proposed in 1870 by Visconti.
S. became most widespread at the end of the 19th and 20th centuries. Ch. arr. in connection with the development of the mining and engineering industries. According to researchers from a number of countries, the incidence of S. in the mentioned industries continued to remain high until the 60s of the current century. Thus, in England in 1955, the number of patients who received a pension due to S.’s disease was approx. 40 thousand. Approximately the same number of patients were registered in the USA and Germany in the same years. And only in recent decades has the incidence of S. in highly developed countries decreased. In the Soviet Union, due to the implementation of systematic preventive measures, the incidence rate of S. has constantly decreased; in recent years, new cases of the disease have not been identified in many enterprises.
Treatment of silicosis
It is prescribed after verification of the diagnosis, taking into account the stage of the disease, the state of the immune system and a number of other factors. Basic principles of silicosis treatment:
- Organization of high-quality nutrition with the inclusion of high-calorie foods in the diet, high in protein and vitamins. Drinking enough liquid.
- The use of expectorants for better expectoration.
- Therapeutic physical training together with oxygen therapy.
- Use antibiotics only for severe forms of the disease.
- Sanatorium-resort treatment in specialized sanatoriums.
Essential drugs
THERE ARE CONTRAINDICATIONS. CONSULT YOUR DOCTOR.
- Eufillin (bronchodilator drug). Dosage regimen: adults take 0.15 g orally after meals 1-3 times a day. The duration of the course of treatment is determined by the attending physician.
- Bromhexine (mucolytic and expectorant drug). Dosage regimen:
- Orally (syrup, tablets and dragees - for children over 6 years old, drops, oral solution), adults and children over 14 years old - 8-16 mg 3-4 times a day. If necessary, the dose can be increased for adults to 16 mg 4 times a day.
- In the form of inhalations (inhalation solution) for adults - 8 mg. Inhalations are carried out 2 times a day. The solution is diluted 1:1 with distilled water and heated to body temperature to prevent coughing.
- Bromhexine 8 - drops: orally, adults and adolescents over 14 years old - 23-47 drops 3 times a day.
- Parenterally (i.m., subcutaneously, i.v. slowly, over 2-3 minutes) - 2-4 mg 2-3 times a day. The solution for intravenous administration should be diluted with Ringer's solution or sterile water for injection.
- Ambroxol (expectorant). Dosage regimen:
- Tablets for adults and children over 12 years of age are prescribed orally, 1 tablet daily in the morning after meals (with a sufficient amount of liquid).
- The solution for oral administration and for inhalation is dosed using the supplied dosing cup. Adults are prescribed orally in the first 2-3 days, 4 ml 3 times a day, then 4 ml 2 times or 2 ml 3 times a day. When performing inhalations, the solution is inhaled using an inhaler. Adults and children over 5 years of age are prescribed 1-2 inhalations per day, 2-3 ml.
- The syrup is prescribed to adults in the first 2-3 days, 10 ml 3 times a day; then 10 ml 2 times or 5 ml 3 times a day.
Etiology
Silicosis develops when inhaling dust containing free silicon dioxide (SiO2), most often among workers in the mining industry (drillers, drifters, miners, fasteners, blasters, etc.), engineering (sandblasters, shot blasters, chippers, etc.), in the production of refractory materials , sand grinding, tunneling, granite processing. In nature, silicon dioxide (see) is more often found in three crystalline varieties - in the form of quartz, tridymite, cristobalite. As a rule, the disease occurs as a result of exposure to quartz dust and very rarely - condensation aerosols (see Aerosols) of silicon dioxide, which have a significantly less fibrogenic effect.
The incidence of Silicosis is directly dependent on the amount, dispersed composition of inhaled dust and the quartz content in it. Particles ranging in size from 0.5 to 5 microns have the most fibrogenic effect.
MPC of dust containing more than 70% crystalline silicon dioxide is 1 mg/m3, from 10 to 70% - 2 mg/m3, from 2 to 10% - 4 mg/m3; The maximum permissible concentration for dust containing more than 70% of amorphous silicon dioxide is 1 mg/m3, from 10 to 70% is 2 mg/m3. In previous years, the disease usually developed under unfavorable working conditions in the first 5-10 years of work.
Diagnosis of silicosis
- X-ray and tomography of the lungs: so-called silicotic nodules are visualized - round shadows of medium intensity with a diameter of 1 to 10 mm with clear contours. In the early period of the disease, these shadows are small, single, and symmetrical. As the disease progresses, their size and number increase.
- Study of external respiration function: development of ventilation disorders of a restrictive type, although PaO2 indicators are not disturbed for a long time.
- In the final diagnosis, crucial importance is attached to the analysis of working conditions and professional activities, provided they are documented, which will allow us to draw a conclusion about whether the symptoms identified during an X-ray examination are related to silicosis or their cause is different. This takes into account the data of all medical examinations carried out by an employee of an enterprise with hazardous working conditions, with the availability of the results of laboratory and instrumental research methods. A certificate is required about the frequency of cases of silicosis that were observed in other employees of this enterprise.
Pathogenesis
The pathogenesis is still largely unclear. It has been established that S.'s development is impossible without phagocytosis (see) of quartz dust particles by macrophages (see), which die as a result of absorption of quartz crystals. Phagocytosis of dust particles is a necessary prerequisite for the occurrence of the disease. However, the reasons for the death of macrophages that absorb dust particles (coniophages) and the subsequent development of fibrosis remain unclear. According to modern It is believed that the cytotoxic effect of quartz particles on coniophage is based on complex biochemical, enzymatic and electron exchange mechanisms. Damage to the coniophage is associated with the appearance of hydroxyl groups on the surface of the quartz fracture, creating active centers of the crystal lattice. Changes in electronic structure and electronic potential, in turn, can lead to free radical activity (see Free radicals) and changes in the structural components of cell membranes. According to some data, a certain yet unknown fibrogenic factor may be released from dying macrophages, stimulating fibrogenesis and collagen formation. Along with this, there are facts indicating that immunopathological, including autoimmune, mechanisms also take part in S.’s pathogenesis. A certain role is assigned to individual predisposition, possibly related to the genetic characteristics of the body, including immune homeostasis.
Pathogenesis of silicosis
The mechanism of development of the disease is not fully understood. Three main theories of pathogenesis are considered - mechanical, toxic-chemical and immunological.
According to mechanical theory, microparticles act as abrasives. Damaged areas of lung tissue become scarred, displacing functional structures.
According to the toxic-chemical theory, quartz microparticles interact with cellular fluid, releasing silicic acid. Silicic acid has a detrimental effect on cells. Gradually, the pathological focus is separated by connective tissue.
The immunological theory is based on phagocytosis. Silica particles are taken up by macrophages. Silicon dioxide damages lysosomal membranes, causing macrophage death. Enzymes are released from the dead cell, which stimulate fibrosis.
Tissue proteins altered under the influence of silicon dioxide provoke autoimmune reactions, which causes additional damage and subsequent proliferation of scar structures.
Pathological anatomy
Silicosis is characterized by the development of specific progressive cell-dust and fibrous silicotic nodules in the lungs and regional lymph nodes against the background of diffuse interstitial changes, less often in the upper respiratory tract, trachea, bronchi, and extremely rarely in other organs. The silicotic nodule at the beginning of the process is distinguished by a concentric or vortex-like arrangement of argyrophilic, later dense hyalinized collagen fibers, completely displacing coniophages. These nodules can be miliary in diffuse form C. Their groups, merging, form large nodes of dense gray-whitish tissue in the lungs (nodular, nodular and tumor-like forms), sometimes with disintegration (silicotic cavities), sometimes with deposition of lime in the center or along the node periphery; nodules can also develop around blood vessels, squeezing them. On spodograms (see) or in the obliquely incident light of a dark field microscope, luminous quartz dust particles are found in silicotic nodules, especially along the periphery. With S., as a rule, adhesive pleurisy, hron, develop. predominantly atrophic bronchitis, diffuse interstitial pneumosclerosis (see), pulmonary emphysema (see). Patients die from cardiopulmonary failure with cor pulmonale and nutmeg liver or from associated tuberculosis.
Pathophysiology of silicosis
Alveolar macrophages take up inhaled free quartz particles and enter the interstitial and lymphatic tissues. Macrophages promote the release of growth factors (tumor growth factor TNF-beta), cytokines (tumor necrosis factor TNF-alpha, IL-1) and oxidants, stimulate collagen synthesis, parenchymal inflammation and fibrosis.
After death, macrophages release silica into the interstitial tissue around small bronchioles, leading to the formation of a pathognomonic silicotic nodule. Such nodules contain mast cells, macrophages, lymphocytes, and fibroblasts with disorganized clumps of collagen and scattered biconvex particles, clearly visible under polarized light microscopy. When they mature, the nodule centers develop into dense balls of fibrous tissue with the classic onion skin appearance, which are surrounded by a layer of inflammatory cells.
If the effects are short-term or not intense, the nodules remain discrete and do not cause functional changes in the lung - simple chronic silicosis. But if there is a high intensity or prolonged exposure time (complicated chronic silicosis), the nodules merge, which becomes the cause of progressive fibrosis and reduction in lung volume (VOL, VC). Sometimes they form large masses - progressive massive fibrosis.
In the case of acute silicosis caused by intense exposure to quartz dust over a short period of time, the alveolar spaces are filled with PAS-positive protein-like substrates, similar to those detected in silicoproteinosis. The alveolar septa are saturated with mononuclear cells. An occupational history of short-term exposure is required to differentiate silicoproteinosis from idiopathic changes.
Clinical picture
In 1930, at the 1st International Conference on Silicosis in Johannesburg, S.’s classification was adopted, taking into account both radiological data and wedge symptoms. This classification is widely accepted in a number of countries. Subsequent international classifications take into account and detail mainly the radiological (skialological) signs of the disease (the nature and density of shadows on the X-ray). In the classifications adopted in the USSR for all pneumoconiosis, including for S. (1958 and 1976), along with the X-ray wedge, signs of the disease were taken into account. The latest and currently valid classification provides for three stages (I, II, III) and three forms of S. (nodular, interstitial and nodular).
Rice. 1. Plain X-ray of the chest with nodular form of silicosis (direct projection): multiple small focal shadows in both pulmonary fields. Rice. 2. Plain X-ray of the chest with interstitial silicosis (direct projection): bilateral severe deformation of the pulmonary pattern, mainly in the middle and lower parts of the lungs.
The nodular form of Silicosis (Fig. 1) is characterized by the presence in the lungs of small, round, clearly defined shadows of the same type. Depending on the phase of development of the nodule (cellular, fibrous phase, phase of hyalinosis or calcification), the intensity and homogeneity of the nodular shadows changes. There are 3 types of nodules based on size: up to 1.5 mm (p), from 1.5 to 3 mm (q), from 3 to 10 mm (r).
In the interstitial form of S., the strengthening and deformation of the pulmonary pattern due to perivascular and peribronchial fibrosis are determined. According to the nature of the interstitial changes, fine linear reticular changes in the pulmonary pattern (s), stringy, irregular linear shadows (t), coarse stringy, irregular, in places cellular finely spotted darkenings (u) are distinguished. Linear-strand changes are observed on both sides and have a diffuse widespread nature, which is most pronounced in the middle and lower parts of the lungs (Fig. 2).
Rice. 3. Tomogram of the lungs in the nodular form of silicosis (direct projection): large nodular formations (indicated by arrows) in both lungs.
The nodular form of S. (Fig. 3) is characterized by fibrous formations. Based on the size of the nodular formations, they are divided into small nodular - the diameter of the nodes is from 1 to 5 cm (A), large nodular - from 5 to 10 cm (B) and massive - more than 10 cm (C). Nodules can be unilateral or bilateral, round or irregular in shape, with clear and fuzzy contours.
Stage I of the nodular form of S. is characterized by the presence of a small number of nodules against the background of interstitial fibrosis of the lungs (code designation: p - 1.2; q - 1; g - 1), and stage I with the interstitial form is characterized by moderate bilateral diffuse enhancement of the pulmonary pattern with visible linear reticular and linear-strand changes (s - 1.2; t - 1.2; u - 1.2).
Radiological signs that determine the transition of the process to stage II are mainly due to increased interstitial and nodular fibrosis of the lungs. At stage II of the nodular form, the pulmonary pattern is poorly differentiated, numerous symmetrical and evenly spaced small- or medium-nodular shadows are visible (p - 3; q - 3, d - 3). Stage II silicosis in the interstitial form is characterized by a high density of linear-mesh and coarse shadows per unit area with a more pronounced increase and the appearance of deformation of the pulmonary pattern (s - 3, t - 3, u - 3). At the same time, in most cases, small, irregularly shaped focal-nodular opacities are also detected; III, or the final, stage of nodular or interstitial S. is characterized by an increase in the number of fibrous nodules, their fusion, and the formation of shadows of massive fibrosis (A, B, C).
Patients with S., as a rule, have few complaints. Most often, they note moderate shortness of breath, cough (dry and with sputum) and chest pain. Wedge, S.'s manifestations increase as the fibrotic process in the lungs develops, but there is no complete parallelism with the x-ray picture. Cough and shortness of breath with S. can be associated not only with the severity of fibrosis, but also depend on the degree of bronchitis accompanying silicosis (see), which is predominantly atrophic in nature. The general condition of patients can remain satisfactory for quite a long time.
The chest is more often of the usual shape, but as emphysema increases, it expands with an increase in its anteroposterior dimensions. Typically harsh breathing, sometimes intermittent dry rales are heard, less often in the lower parts fine bubbling rales and pleural friction noise are heard. In stage III of silicosis, areas of shortened percussion sound and harsh breathing of a bronchial hue may be detected. As the disease progresses, a picture of cor pulmonale may develop (see).
Clinical blood tests for Silicosis often show no significant abnormalities. Of the biochemical studies, the most important is a blood test for protein fractions; S.'s progression may be accompanied by hypergammaglobulinemia. The respiratory function is often changed mildly, at the onset of the disease moderate obstructive disorders are possible, on which restrictive ones are usually layered as the process progresses.
S.'s course is progressive, with the development of fibrosis occurring at different times. There are S. slowly progressing, remaining at the same level sometimes for decades (usually interstitial); rapidly progressing - deterioration of the condition can occur in 7-8 years or faster; so-called late S.—developing many years after contact with dust has taken place. In the past, cases of acute S. were described under particularly unfavorable working conditions, when the disease developed quickly and was especially malignant. Currently time, acute S. does not occur in our country.
Silicosis (lecture)
The lung of a miner who suffered from silicosis and tuberculosis.
I.S. Ivanova
Central Institute for Advanced Medical Studies, Moscow
Silicosis
(lecture)
Silicosis
- one of the most common pneumoconiosis, which develops from inhalation of highly fibrogenic dust containing free silicon dioxide (SiO
2
). Silicon dioxide occurs in nature in the form of quartz, granite, gneiss, and sandstones. Many valuable minerals occur adjacent to or interspersed with quartz rocks, which determines the silica content in the mine workings. For the development of silicosis, many factors are important: the degree of dustiness (concentration) of the air, the content of free silicon dioxide, its density, electrical charge and other physical properties of dust particles, etc.
It is known that of the two varieties (crystalline and amorphous forms) of quartz, crystalline rocks have the greatest fibrogenic effect.
Pathogenesis.
The term “silicosis” was first proposed by the anatomist Visconti in 1870. From now on, the time for studying silicosis is practically counting down. Many works have been devoted to this problem, but until now there is no clear answer to the question of why free silicon dioxide has the most pronounced fibrogenic effect compared to other types of dust. In the past, various theories of the pathogenesis of silicosis were discussed, linking the aggressiveness of quartz dust with its physicochemical properties, but none of the proposed theories could explain the complexity of the developing pathology.
It has now been established that dust entering the respiratory tract is phagocytosed by macrophages, which transform into the form of coniophages. Histochemical methods have shown that the main cellular structures undergo degeneration under the influence of dust, and the cell itself undergoes decay. The damaging effect is associated with the activity of silanol (SiOH) and other hydroxyl groups that are formed when the quartz crystal lattice breaks. Phagocytosis of dust can be repeated many times; the rate of death of macrophages is determined by the aggressiveness of the active dust.
As a result of the death of coniophages, cellular structures are released, some of which have biological activity and stimulate the development of fibrosis. Importance is attached to the accumulation of lipid peroxidation products formed during the breakdown of macrophages from phospholipids under the influence of the catalytic activity of SiO 2
. Simultaneously with the stimulation of fibrosis formation, damage to fibroblast ultrastructures occurs, which is associated with qualitative changes in collagen and its hyalinization.
Not being an antigen, but having cytotoxic properties, SiO2
causes activation of cellular and humoral immune reactions. In patients with silicosis, there is an increase in various classes of immunoglobulins, and there is a tendency to decrease the absolute number of T-lymphocytes when hydrolytic and energy enzymes are activated in them. Activation of autoreactivity is observed, which is determined by the titer of antibodies and in cellular reactions to pulmonary antigen, collagen. Shifts in the immunological status are most pronounced in progressive forms of silicosis or when it is complicated by tuberculosis or rheumatoid arthritis.
Pathological anatomy.
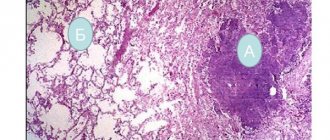
Morphological changes in the lungs with silicosis are characterized by the proliferation of fibrous tissue along the bronchi, vessels, around the lobules and alveoli. A specific morphological substrate - silicotic nodules - appears on a section in the form of miliary and larger foci of gray or gray-black color. The histological structure of typical nodules is represented by a concentric or vortex-like (regular) arrangement of collagen fibers, sometimes with hyalinization, especially in the central zone. Silicotic nodules are characterized by the presence of a large amount of collagen with a relatively small number of dust particles.
As silicosis progresses, conglomerative formations (nodes) are formed, most often localized in the upper-posterior segments of the lungs. In silicotic nodules and nodes, calcification and individual foci of necrosis due to ischemia can be detected. Simultaneously with damage to the lung parenchyma, fibrous changes are formed in the lymph nodes of the roots of the lungs, sometimes also with calcification phenomena along their periphery - “egg shell”; an adhesive process develops in the pleura; signs of emphysema form, first around the nodules like compensatory hyperpneumatosis, and in severe cases - in the form of bullae.
Clinic.
Silicosis can occur when performing various types of work. The main silica-hazardous professions are: miners in mines for the extraction of various metals (drillers, drifters, blasters, etc.); foundry workers (sand blasters, shot blasters, chippers, core makers, pourers, beaters, molders); workers involved in the production and use of refractory and ceramic materials, tunnelers; granite processors, sand grinders. In addition to the listed groups, miners extracting iron ore, silicates and coal have contact with quartz-containing dust components, which is due to the composition of the rocks. Pneumoconiosis developing during these works is designated as siderosilicosis, silicosilicosis, anthracosilicosis. These pneumoconiosis can also be classified as pneumoconiosis from fibrogenic dust. Free silicon dioxide plays an important role in their formation. However, the presence of other dust components (iron, silicates, coal) can somewhat weaken the fibrogenic effect of quartz. Since the listed pneumoconiosis has a more favorable course compared to silicosis.
Silicosis is a disease with a gradual, often invisible to the patient, onset. The length of work preceding the onset of the disease can vary widely from 1 to 20 or more years, which depends on the aggressiveness of the dust, the intensity of exposure and individual predisposition.
| ◄ ► |
Silicotic nodules, stage I-II pneumoconiosis
Silicotic nodules, connective tissue changes
Silicotic nodules, connective tissue changes
Anthracosilicosis stage I
Anthracosilicosis stage I, interstitial form, right-sided chronic pneumonia
Anthracosilicosis stage I-II, right-sided chronic pneumonia
Stage II silicosis, bilateral chronic pneumonia
Silicosis stage II-III
Siderosilicosis stage III
Siderosilicosis stage II, miliary pulmonary tuberculosis
The clinical picture of silicosis is distinguished, especially when the process is mild, by scant objective and subjective symptoms. Active complaints usually appear later than changes in the lungs form. A detailed survey can identify complaints characteristic of any chronic pulmonary disease. The most typical complaints are shortness of breath, which increases as the process progresses. Often there are stabbing pains in the chest of unclear localization, a feeling of heaviness in the subscapular areas. The cough is usually mild and there is little sputum.
The general condition can remain satisfactory for a long time. Body temperature in uncomplicated cases is normal. The shape of the chest, even in severe cases of the disease, is not changed. Percussion detects a small box-shaped sound in the lower lateral parts of the chest. In more pronounced cases, a shortening of the percussion sound is detected over the area of large fibrous nodes, alternating with a boxy tint of the sound over areas of bullous emphysema. In such cases, they talk about “mosaic” percussion sound, but in clinical practice this phenomenon is rarely detected. As the disease progresses, breathing becomes harsh in the upper sections and weakened in the lower sections. In uncomplicated cases, wheezing is not audible. Only in some patients can one hear small moist rales or crepitus and pleural friction noise in the lower parts. With silicosis, changes in the peripheral blood are not observed. Progressive forms are accompanied by changes in protein fractions towards an increase in the coarse fraction of globulins. The concentrations of haptoglobulin, fibronogen, neuraminic and diphenylamic acids often increase, and the level of C-reactive protein increases. All of these shifts indicate shifts related to connective tissue processes.
The development of silicosis is characterized by varying degrees of respiratory failure. Mostly, pulmonary ventilation disturbances occur of a reactive type. It should be noted that the severity of respiratory disorders often does not correlate with the degree of fibrotic changes in the lungs. Sometimes, even in severe cases of the disease, very little respiratory function is impaired. And only in the final stages do signs of progressive pulmonary and then pulmonary heart failure appear.
The main method for diagnosing silicosis is x-ray examination. There are three forms of radiological changes: nodular (small round darkening), interstitial (small irregular darkening) and nodular (large round or irregular darkening), which corresponds to the morphological changes in silicosis.
Nodular silicosis on a radiograph appears in the form of clearly defined monomorphic shadows, symmetrically located in both lungs. They predominantly occupy the middle and lower sections of the lungs. There is a small-nodular process with a nodule diameter of up to 1.5 mm, a medium-nodular process with a nodule size of 1.5 to 3.0 mm, and a large-nodular process with a nodule diameter of 3 to 10 mm. These changes are encoded with the Latin letters p
,
q
,
r
respectively. The number or density of nodules is indicated by the numbers 1-2-3.
Interstitial silicosis can manifest as linear reticular changes (type s
), severe changes (type
t
), rough changes (type
u
).
Nodular silicosis is characterized by the presence of conglomerates of varying sizes, often symmetrically located. Silicotic nodes, in the absence of complications, have a round shape and an almost homogeneous structure. They are often located symmetrically in the upper-posterior segments of the lungs. There are small nodular silicosis (type A) - the diameter of the nodes is from 1 to 5 cm, large nodular (type B) - with a diameter of nodes from 5 to 10 cm and massive (type C) - with a total diameter of nodes more than 10 cm.
In addition to changes in the pulmonary parenchyma, with silicosis there are changes in the roots of the lungs: they are fibrously compacted, contain dense lymph nodes, sometimes with elements of calcification. The clarity of the contours of the root allows us to talk about their “chopping off”, as a sign of silicosis. Silicosis is characterized by involvement of the pleura in the process: thickening of the interlobar pleura, pleuro-diaphragmatic and pleuro-pericardial adhesions.
In addition to designating identified radiological changes using coding, the traditional identification of three stages of silicosis according to the severity of the process is currently used.
Stage I silicosis manifests itself in the form of linear-mesh changes (1.2 s
), or nodular changes with a small number of nodules (1.2
p
; 1.2
q
; 1.2
r
).
Stage II silicosis is characterized by nodular changes with significant distribution and a large number of nodules (3 p
;
3 q
;
3 r
).
Stage III includes nodular forms of silicosis (A, B, C). The given division of silicosis into stages is very arbitrary, and therefore the issue of a possible revision of the idea of the stage of the disease is currently being discussed.
Silicosis is a disease prone to progression. According to the flow, they distinguish: slowly progressive forms, when the increase in fibrous changes and the transition from one to another stage takes ten or more years, and in some cases, long-term observation indicates the stability of the process, and rapidly progressive, in which an increase in changes occurs in a relatively short period of time ( 5-10 years).
Slow progression is most typical for interstitial or internodular forms of silicosis, which most often develop upon contact with dust with a low content of free SiO2
, not exceeding 10-20%.
Nodular forms of silicosis that develop when exposed to dust with a high content of free SiO2
, exceeding 30-40%, rapid progression is often observed up to stage III.
The development of the silicotic process most often occurs during work in dusty production conditions. However, upon contact with quartz-containing dust, silicosis may develop after stopping work in dusty conditions, sometimes after 10-15 years or more. This is the so-called late form of silicosis. More often, late silicosis develops in people who have had a short history of working in conditions of significant dust. The provoking moment for the development of the process may be acute pneumonia or tuberculosis. With this form, there is often a tendency to calcification of the lymph nodes of the roots of the lungs and the silicotic nodules themselves.
It should be emphasized that cases of late-diagnosed silicosis should be distinguished from cases of late silicosis, which may be due to the lack of X-ray examination during the period of work in dust production.
In addition to the usual forms, atypical variants of the course of silicosis are known, which complicate diagnostic issues.
Thus, there are known cases of unilateral asymmetric silicosis in the form of nodular formations, often mistaken for a tumor. It can be assumed that the basis for the development of such forms is a congenital defect of the lung, which is associated with its greater vulnerability. When exposed to low concentrations of low-aggressive dust, silicosis may develop with a predominant lesion of the hilar lymph nodes against the background of very small linear-network changes in the parenchyma - lymphoglandular form of silicosis.
The form of acute silicosis described in the past has not occurred in recent years. The development of acute silicosis has been associated with exposure to particularly aggressive dust with a high content of SiO 2
. The disease was very severe, with high fever and progressive pulmonary failure. The development of the process was assumed to involve immune mechanisms. According to the nature of the described morphological changes in the lungs, a process develops that resembles the picture of fibrosing alveolitis.
Complications.
The severity of silicosis in many cases determines the addition of complications, one of which is tuberculosis. Most often, tuberculosis complicates nodular and nodular forms of silicosis. There is a clear dependence of the frequency of complications on the severity of fibrosis. Thus, with stage I silicosis, complicated forms of silicosis account for 15-20%, with stage II silicosis – 25-30%, and with stage III silicosis – up to 75-80%.
The development of silicotuberculosis is associated mainly with the reaction of old tuberculosis foci, mainly in the lymph nodes of the roots. Tuberculosis against the background of silicosis can manifest itself in all forms of pulmonary secondary tuberculosis. Most often, focal tuberculosis is associated, somewhat less often - infiltrative. The development of silicotuberculous bronchoadenitis, single and multiple silicotuberculomas is possible. Disseminated and fibrous-cavernous tuberculosis and exudative pleurisy are less common. In cases where tuberculosis occurs against the background of pronounced nodular forms of silicosis, determining the form of the associated tuberculosis is practically impossible. In these cases, the disease is regarded as small- or large-nodular or massive silicotuberculosis. The clinic of silicotuberculosis has a number of features that distinguish it from tuberculosis:
- There is no acute onset of the process.
- In the first period, signs of tuberculosis intoxication are little expressed or do not appear at all (fever, weakness, sweating, changes in the blood).
- After an asymptomatic first period, especially if anti-tuberculosis therapy is not started in a timely manner, the disease enters the second phase, corresponding to an exacerbation of the tuberculosis process with infiltrative, sometimes destructive phenomena, dissemination, severe intoxication, and weight loss.
- Rare bacilli isolation.
- Less common than with tuberculosis, hemoptysis.
- As a result of the therapy, it is more often necessary to note not a cure for tuberculosis, but only stabilization of the process, transfer to an inactive form with fibrous compaction or calcification of foci.
Associated tuberculosis has an adverse effect on the course of the main silicotic process, in many cases contributing to its progression.
In connection with all that has been said, it is clear that the task of early detection of complicated forms and timely initiation of therapy seems very important. In addition to generally accepted diagnostic methods, dynamic X-ray examination helps to establish the diagnosis of silicotuberculosis: in contrast to monomorphic diffuse nodular dissemination, polymorphic focal shadows are noted, localized mainly in the apices of the lungs, in the subclavian zones, and asymmetry of the lesion often appears.
Another complication of silicosis, which is observed more often in the interstitial form of the process, is the addition of a banal nonspecific infection with the development of chronic bronchitis with its characteristic obstructive respiratory dysfunction. And although additional bronchitis does not have a significant effect on the course of the fibrotic process, the increase in respiratory failure has a significant impact on the severity of the patient’s condition.
A complication characteristic of severe forms of silicosis should be considered spontaneous pneumothorax, which develops as a result of rupture of emphysematous bullae. Spontaneous pneumothorax is not a common complication of silicosis. Clinical manifestations correspond to limited parietal forms. Some patients have a tendency to repeated pneumothorax, which is apparently due to the individual characteristics of the body.
Particularly noteworthy are cases of complications, or more correctly, a combination of silicosis with rheumatoid arthritis, known as Kaplan syndrome, or silicoarthritis. Silicosis in these forms is often represented by nodular formations with the presence of multiple rounded shadows along the periphery of the lungs. Histological examination of these nodes reveals silicotic nodules with a zone of cell proliferation with vasculitis and a “palisade-like” arrangement of fibrocytes characteristic of rheumatoid lesions. Silicosis and rheumatoid arthritis greatly enhance each other in clinical manifestations; silicosis is often characterized by a progressive course, which indirectly indicates the common pathogenetic basis of these diseases.
Treatment and prevention.
There are currently no effective treatments for pulmonary fibrosis, including silicosis.
. In recent years, the antifibrogenic effect of some polymer drugs, in particular polyvinyl oxide, has been assessed, the therapeutic effect of which is associated with the inactivation of quartz due to its adsorption on the polymer and the protection of cell membranes from its cytotoxic effect. And although the experiment yielded encouraging results, clinical trials did not confirm its positive effect on the course of the silicotic process.
The most important element of medical care for workers in silicosis-hazardous professions is the implementation of preventive preventive measures. Along with engineering, technical and sanitary-hygienic measures aimed at reducing the possible impact of aggressive dust, medical prevention occupies a special place. The most important in this regard are careful professional selection when hiring for jobs in silicosis-hazardous professions, taking into account all contraindications, regular and high-quality periodic medical examinations using a full range of necessary additional examination methods in order to early identify signs of dust exposure.
Therapeutic and preventive measures of a preventive nature in groups at increased risk of developing silicosis and in the treatment of patients with silicosis without significant impairment of respiratory functions are largely similar. Therapy should be aimed at improving the functional state of the bronchopulmonary system and eliminating dust. A nutritious diet rich in proteins and vitamins is recommended. To improve the condition of the mucous membrane of the respiratory tract and cleanse it from dust particles, it is recommended to conduct courses of therapeutic inhalations using saline-alkaline solutions, solutions of phytoncides, and herbal decoctions.
To eliminate respiratory disorders, physical therapy with the inclusion of respiratory complexes is indicated. Among the means of hardening, hydroprocedures are recommended, especially therapeutic showers (circular shower, Charcot shower).
Physiotherapeutic treatment is advisable: vibration massage of the chest muscles, iontophoresis with calcium chloride, diodynamic currents, ultrasound on the chest.
Oxygen therapy is indicated, including the use of hyperbaric oxygenation.
For rapidly progressing forms of silicosis, glucocorticosteroid hormones are used in courses of 1.0-1.5 months. with a daily dose of 20-25 mg. There is evidence of a positive effect when immunocorrective drugs (levomizole, interferon) are included in the complex of therapy, especially in cases of complicated bronchitis. In this case, simultaneous prophylactic administration of anti-tuberculosis drugs (tubazid) is mandatory.
The treatment of patients with silicotuberculosis requires special attention. It is recommended to carry out active anti-tuberculosis therapy for a period of 6 to 12 months. The transition to an intermittent method of treatment is permissible only after stabilization of the process. For infiltrative forms, it is advisable to combine antibacterial therapy with the use of glucocorticoid hormones. After completing the main course, anti-relapse courses of treatment are used.
Treatment of other complications (chronic bronchitis, rheumatoid arthritis) is carried out using conventional treatment methods used for these diseases.
Work ability examination
The diagnosis of silicosis is the reason for transfer to work away from exposure to dust and irritating substances, with the exception of significant physical activity and adverse meteorological factors.
The criterion for determining the disability group and the percentage of disability is the form, stage of the disease, the presence of complications, the degree of pulmonary insufficiency and prognosis.
The conclusion on employment in each specific case must be made taking into account the severity of the process, the nature and working conditions, the patient’s age, his length of service, education and work skills.
Complications
S.'s course is aggravated by the addition of complications, the most severe of which is tuberculosis. S. complicated by tuberculosis is called silicotuberculosis (see Tuberculosis of the respiratory system).
S.'s complications also include infrequently occurring acute and chronic ones. pneumonia (see), bronchiectasis (see), bronchial asthma (see). Immune and autoimmune processes that develop in severe forms of S. can lead to systemic lesions such as rheumatoid arthritis (see) and other collagenoses. Sometimes in the lungs, when S. is combined with rheumatoid arthritis (so-called silicoarthritis), a peculiar picture is observed - the formation of multiple round opacities (see Kaplan syndrome); in this case, rheumatoid factor can be detected in the blood (see).
Pulmonary silicosis, symptoms
For a long time the disease does not manifest itself in any way. Characteristic signs of the disease are detected much later. Symptoms of pulmonary fibrosis include:
- diffuse respiratory failure;
- dry cough gradually turns into wet with an abundance of phlegm;
- stabbing pain in the chest area;
- hard breathing;
- dry wheezing;
- feeling of lack of air;
- tachycardia;
- cyanosis.
Late stages are complicated by bronchitis, asthma, pneumonia, tuberculosis, pneumothorax, rheumatoid arthritis, and lung cancer.
Diagnosis
The diagnosis is established on the basis of the wedge, symptoms, prof. anamnesis and X-ray data. research, the Crimea plays a major role in the diagnosis of S. The x-ray of the lungs reflects all the anatomical elements characterizing silicotic pneumofibrosis. Along with this, S. is characterized by symmetrical expansion and fibrous compaction of the roots of the lungs, sometimes with shell-like calcification of lymph nodes and an adhesive pleural reaction.
Differential diagnosis
carried out with other pneumoconiosis and pulmonary diseases of other etiologies, accompanied by pulmonary dissemination syndrome. For differential diagnosis of S., general and prof. are especially important. anamnesis and gig. characteristics of working conditions. When differential diagnosis with disseminated tuberculosis (see. Respiratory tuberculosis), sarcoidosis (see), Hamman-Rich syndrome (see Hamman-Rich syndrome), the peculiarities of the course of each of these forms, their clinical and radiological forms, the absence of a typical prof. anamnesis. In cases that are difficult to diagnose, diagnostic bronchoscopy with transbronchial biopsy can be recommended.
Classification
There are three forms of silicosis:
- nodular;
- diffuse sclerotic;
- mixed.
The nodular form is expressed by the appearance of silicotic granulomas, which are connective tissue formations, in the respiratory organs. They are characterized by concentric or vortex-like localization.
They can merge into large tumor-like nodes. If such a node necrotizes (dies) and lyses (liquefies), followed by a breakthrough into the bronchial cavity, a silicotic cavity (cavity) is formed in its place.
The diffuse sclerotic type of pathology is characterized by fibrinization of interalviolar, peribronchial and perivascular tissues. This form is complicated by emphysema, bronchiectasis, and the formation of pleural adhesions.
For the mixed form, a combination of diffuse sclerosis and nodular granulomas is typical.
The course of the pathology is:
- sharp;
- chronic;
- progressive;
- accelerated.
Acute is a disease that manifests itself after less than two years of work in hazardous conditions and is characterized by a rapid increase in symptoms.
The chronic (classical) form appears after 10-15 years of contact with industrial dust. In the classic course, there is no clear clinical picture, and patients often do not attach importance to cough and shortness of breath during physical exertion.
The progressive variant is characterized by impaired ventilation of the lungs, increasing shortness of breath, and the presence of complications (bronchitis, tuberculosis, abscesses).
The accelerated course is similar to the classic one, but is expressed by more pronounced symptoms due to combination with autoimmune reactions or tuberculosis. Manifests after 5-10 years of work in harmful conditions.
Treatment
Treatment should be comprehensive, aimed at improving the general condition of the body, preventing complications and progression of diseases. Adequate nutrition with sufficient vitamin content, breathing exercises, and treatment in sanatoriums and dispensaries are indicated. In the early stages of S., physiotherapeutic methods are used: iontophoresis with novocaine, calcium chloride, less often UHF on the chest, ultrasound. According to indications, bronchodilators (aminophylline, ephedrine, etc.), cardiac medications, and oxygen therapy are prescribed. If there are pronounced immunopathological manifestations accompanying S., careful administration of small doses of glucocorticoids under the protection of antibiotics is permissible; delagil and plaquinil are less commonly prescribed. Currently, the question of the possibility of using certain polymer preparations for S. is being studied. Timely treatment of complications of silicosis and, above all, silicotuberculosis is important. In this case, the main method of treatment is chemotherapy with modern anti-tuberculosis drugs (see Tuberculosis). In some cases, surgical intervention is indicated.
Diagnostics
When the first signs of malaise and respiratory problems appear, you must immediately make an appointment with a general practitioner or occupational pathologist. The specialist will conduct a consultation and initial examination and, if necessary, prescribe the following diagnostic studies: chest x-ray, ultrasound of the sternum, determination of the vital capacity of the lungs and their maximum ventilation, forced expiratory volume, identification of the respiratory rate and the maximum speed of the air stream during exhalation.
Prevention
Prevention consists primarily of hygiene and medical care. measures aimed at reducing dust formation and protecting the respiratory tract from dust (see Pneumoconiosis, prevention; Primary prevention). Preliminary and periodic medical examinations are carried out, health groups and physical therapy classes are organized, preventive wet and salt-alkaline inhalations and stays in dispensaries are indicated. For the purpose of secondary prevention, it is necessary to transfer the patient to working conditions that exclude contact with dust.
Development of the disease[edit]
- Silicon dioxide particles penetrate into the alveoli and remain in them.
- Macrophage (white blood cells) try to absorb and remove dangerous particles from the lungs.
- Silicon dioxide causes macrophage cells to rupture and release the substance into the lung tissue, thereby causing scarring (fibrosis).
- Scars begin to grow around the silica particles, leading to the formation of nodules.
With silicosis, changes are found not only in the lungs, but also in the upper respiratory tract, bronchi, pleura, lymph nodes and pulmonary vessels, as well as in some other organs[4]. Changes occur in the mucous membrane of the nasal concha, larynx and trachea. Often with silicosis, especially in the later stages of the disease, cor pulmonale is detected. Typical silicotic nodules can in rare cases be found in the kidneys, bone marrow of long bones, liver and spleen.
Recommendations
1951
[1]
The study of external respiratory function is important for diagnosing and establishing the severity of silicosis. To do this, measure the vital capacity of the lungs (VC), forced expiratory volume (EFV), and maximum pulmonary ventilation (MVV) (spirometry).
At the first stage of silicosis, the state of health is usually satisfactory, sometimes there is shortness of breath when doing heavy work, an intermittent dry cough, tingling in the chest ( due to changes in the pleura due to its microtrauma and the formation of adhesions in the pleural tissue when dust particles enter it through the lymphatic system of the lungs
). In the absence of complications, chest pain and shortness of breath may be absent. cough occurs due to irritation of the mucous membrane by dust. The first stage may be manifested by unpronounced changes in indicators of external respiration function.
In the second stage, shortness of breath is stronger and can appear even with mild exertion. Chest pain increases, cough is dry or with a small amount of mucous sputum. Indicators of external respiration function deteriorate. Breathing becomes more harsh, and pleural friction noise is often heard.
At the third stage - despite respiratory failure and fibrotic process in the lungs - the general condition of patients may remain relatively satisfactory for some time. Shortness of breath appears at rest, intense pain in the chest, coughing intensifies, the amount of mucus produced increases, and sometimes attacks of suffocation are observed. Dry and moist rales and pleural friction noise are often heard. All indicators of pulmonary ventilation are significantly reduced. Signs of chronic pulmonary heart disease appear due to overload of the right atrium and right ventricle (pumping blood through damaged lungs), hypertension of the pulmonary circulation.
When making a diagnosis, X-ray data, the nature and degree of respiratory dysfunction, and the clinical picture are taken into account. However, facts indicate that not very advanced fluorography of the lungs in the 1960s[5], and more modern ones in the 2010s[6], are not always able to detect the disease in the initial stages (when the best conditions exist for preserving life and health and human ability to work). Specifically, the autopsies of 29 miners who died during the accident[6]; and a comparison of their respiratory organs with the respiratory organs of accidentally deceased people (who were not exposed to dust in the mine) showed that with a successful medical examination (including fluorography), the employee could have changes in the lungs that clearly indicate a pneumoconiotic process. The authors proposed additional methods for diagnosing the initial stages of the disease. Similarly, autopsies of 10 accidentally killed molybdenum mine miners who were exposed to dust containing 88% quartz for 3-5 years showed:
Conclusions. ... 3. A histological study of the lungs of workers who died from accidental causes and were considered practically healthy during life revealed changes characteristic of silicosis.
— [5]
This is consistent with the case described in [7]: the detection of a round formation during a periodic medical examination of a miner with 24 years of experience was regarded as lung cancer. Analysis of the tissue removed during the operation showed that it was a chondromatous hamartoma ( benign tumor - approx.
), and coal dust deposition. An in-depth examination revealed focal pneumoconiosis.
Forecast
In the absence of life-threatening complications and when the worker is transferred to dust-free production areas, the progression of the process with the predominance of interstitial changes may stop, but there remains an increased risk of developing inflammatory and specific lung lesions. The nodular form of S. often progresses even after the worker’s contact with dust ceases. Patients with Silicosis most often die from pneumonia, cardiovascular failure or tuberculosis.
Bibliography:
Velichkovsky B. T. and Katsnelson B. A. Etiology and pathogenesis of silicosis, M., 1964, bibliogr.; Genkin S. M. Clinic of silicosis, M., 1948, bibliogr.; Dvizhkov P. P. Pneumoconiosis, M., 1965; Molokanov K. P. Rehabilitation for pneumoconiosis, M., 1977; Occupational diseases, ed. E. M. Tareeva and A. A. Bezrodnykh, p. 47, M., 1976; Senkevich N. A. Clinical forms of silicosis and silicotuberculosis, M., 1974.
N. A. Senkevich; Yu. P. Likhachev (pat. an.), E. A. Grigoryan (rent.).
Prevention of silicosis
The most effective measures must be taken in the workplace where a person works. This includes the process of insulation, ventilation, dust removal from production premises, and the use of abrasives that do not contain quartz. Breathing masks provide some benefit, but do not provide adequate protection.
Monitoring of exposed workers using questionnaires, spirometry, and chest radiography is recommended. In those workers who inhale quartz, future development of non-tuberculous mycobacterial infections and pulmonary tuberculosis should be suspected. Miners are primarily at risk. People exposed to quartz but without silicosis have a 3 times higher risk of developing tuberculosis compared to others. Miners diagnosed with silicosis have a risk of tuberculosis 20 times or more higher than other people. They are also more likely to have pulmonary and extrapulmonary manifestations.
Patients exposed to silica and with a positive tuberculin test and negative sputum culture for tuberculosis should receive standard chemoprophylaxis with isoniazid. Therapy is the same as in other cases of tuberculosis treatment. Relapses of the disease in question more often occur in those who have silicotuberculosis. In some cases, longer courses are needed than are usually prescribed.
Preventing disease[edit]
Since the disease is incurable and irreversible
, then the only way to prevent it is to prevent excessive amounts of quartz from entering the respiratory system:
- The use of effective ventilation[8][9][10], air showers and other means to reduce the inhalation of dust containing quartz; remote control of machines.
- The use of sand-replacing materials during abrasive blasting, for example, cooper slag.
- Automation of technological processes and use of remote control
- Effective monitoring of the degree of air pollution using personal samplers, including personal PDM dust meters operating in real time [11] (which makes it possible to timely detect exceeding MPCs and take adequate corrective measures.
Respirators do not provide reliable protection from dust - “Coal” No. 3 2015
The use of respirators does not provide protection against silicosis. Causes:
- Due to insufficient attention to reducing dust in the air, dust concentrations can be very high.
- In the USSR and the CIS, miners are usually given half-mask respirators without forced air supply to protect them from dust. Their protective properties are unstable and low - in the USA, legislation prohibits their use when dust levels are > 10 MAC (see Expected degree of respirator protection). The quality of many masks developed half a century ago is very low. When you install a plastic bag instead of a filter in the F-62Sh (with a knitted seal) and moderate tension on the straps, you can breathe, all the air passes through the gaps between the mask and the face.
- Repeated measurements of the effectiveness of half-mask respirators in England and the USA during the actual work of miners have shown that workers cannot use them continuously - whenever required due to increased air pollution (need to talk, etc.). Therefore, in practice, the use of half masks reduces the pollution of inhaled air by an average of 3-8 times, which, given high air pollution, is not enough to reliably protect health.
Because different people have different shapes and sizes of faces, the same respirator fits differently on different workers' faces. If the face fits the mask, then there will be little unfiltered air leakage through the gaps between the mask and the face (the main reason for the low average efficiency) and ( this
worker) a respirator will provide reliable protection. The fact that respirators sometimes reliably protect some workers is confusing and makes it difficult to establish the overall ineffectiveness of the RPE used[12].