How common is non-alcoholic fatty liver disease? Forms of the disease
The content of the article
Non-alcoholic fatty liver disease, known as NAFLD, is the most common disease of this organ. However, knowledge about it, even among doctors, is still insufficient.
Non-alcoholic fatty liver disease
NAFLD is estimated to be very common. For example, among overweight and obese people, the percentage of patients with such pathology reaches 50%. These values are similar to or higher than the number of people with hypertension or type 2 diabetes.
Meanwhile, in 5% of patients the disease takes an advanced form called NASH, non-alcoholic steatohepatitis, and in 0.5% it progresses to fatal cirrhosis of the liver. The latter percentages seem small, but in terms of the total number of cases they are huge.
Therefore, NAFLD includes: “simple” steatosis and the more dangerous non-alcoholic steatohepatitis. When the latter form appears, a very serious complication can occur - liver cirrhosis.
Non-alcoholic steatohepatitis: from pathogenesis to therapy
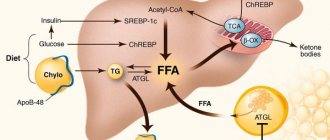
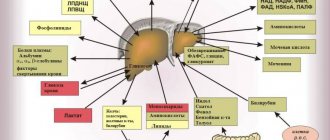
Non-alcoholic steatohepatitis (NASH) is an inflammatory infiltration of the liver parenchyma and stroma with the presence of focal necrosis. NASH is an intermediate link between successive stages of one pathological process (non-alcoholic steatosis and non-alcoholic steatofibrosis), a form of an independent metabolic disease - non-alcoholic fatty liver disease (NAFLD). Since the list of ICD-10 diseases does not have a single code reflecting the completeness of the diagnosis of NAFLD, currently the most often used is: K 76.0 – fatty liver degeneration, not classified in other categories. The term NASH was first formulated in 1980 by J. Ludwig et al., studying the nature of changes in the liver of patients with obesity and type 2 diabetes mellitus (DM), who had no history of alcohol intake in hepatotoxic doses, but A morphological study of liver tissue revealed signs characteristic of alcoholic liver disease (ALD) [1]. And the term NAFLD (non alcoholic fatty liver disease), introduced in 2000, is currently used as a general name for various dysmetabolic conditions of the liver, which are based on excessive intra- and extracellular accumulation of fat [2]. In this case, it is necessary to exclude chronic alcohol intoxication (when the consumption of alcohol-containing products in terms of pure ethanol is less than 20 g/day), hereditary hemochromatosis, HCV, HBV and HDV infections, increased levels of ceruloplasmin and α1-antitrypsin, and ensure the absence of autoimmune hepatitis. Epidemiology It must be remembered that there is a certain number of patients who do not drink alcohol, but have liver damage similar in histological structure to alcohol. Studies conducted in Japan and Italy have shown that the prevalence of fatty liver disease in the general population ranges from 3 to 58% (mean 23%). The high variability of data is probably caused by socio-economic differences in the studied settlements [3, 4]. In the United States, NASH is the most common disease. The percentage of obese people in the general population increased from 10 to 25% between 1961 and 1997 alone. In European countries, NASH is diagnosed in approximately 11% of patients who undergo liver biopsy due to elevated serum transaminases. In obese people, the prevalence of NASH is higher, amounting to 19%, and only 2.7% of cases of NASH are diagnosed at normal weight [5, 6]. In fact, the prevalence of NASH may be even higher among asymptomatic patients who do not drink significant amounts of alcohol if serological markers of viral hepatitis are absent. Thus, many patients with increased activity of liver enzymes in the blood and negative results of non-invasive studies may have NASH. There are reports of cases of NASH detected at the age of 10–20 years [3, 7, 8]. Pathogenesis The pathogenesis of NASH is based on peripheral insulin resistance [10–12]. Tyrosine kinase causes intracellular disruption of signal transmission after activation of the insulin receptor [11]. The exact mechanism of disruption of this metabolic pathway is not completely clear. Decisive, apparently, is the release of adipose tissue, especially mesenteric adipose tissue, tumor necrosis factor-α (TNF-α), as well as leptin and a number of other protein mediators. TNF-α reduces the regulation of the insulin receptor-substrate signal and thereby reduces the translocation of the glucose transporting protein GLUT-4 on the cell membrane. As a result, the amount of glucose utilized by the cell decreases. Peripheral insulin resistance leads to hyperinsulinism, which blocks mitochondrial β-oxidation. The adipose tissue hormone leptin is also important. Leptin resistance or deficiency leads to increased fat accumulation and impaired fatty acid β-oxidation in the liver. Also, in NAFLD, the level of the fat tissue hormone adiponectin decreases, and therefore intracellular signals, such as activation of MAP kinase and peroxisomal proliferator-proliferated nuclear receptor, are disrupted, which increases the accumulation of fat in the liver. Free fatty acids (FFA) have hepatotoxic effects. Normally, FFAs are neutralized through the following pathways: mitochondrial β-oxidation, production and secretion of very low-density lipoproteins (VLDL), synthesis of fatty acid binding protein, and synthesis of triglycerides. In NAFLD, various mechanisms of FFA neutralization are limited. Fatty liver, due to a second pathophysiological mechanism, may become the basis for the progression of liver pathology to NASH with fibrosis [13]. In this regard, it is important that FFAs can induce cytochrome P 450 2 E1 with the subsequent production of reactive oxygen species, which, by enhancing lipid peroxidation, lead to the activation of fibroneogenesis. Another mechanism is represented by an increased supply of endotoxins from the intestine to the liver. As with alcoholic liver damage, cytokines are released by Kupffer stellate cells. Cytokines, primarily TNF-α, contribute, on the one hand, to the pathogenesis of hepatitis, and on the other hand, to the development of peripheral insulin resistance [14, 15]. Among the causes leading to NASH, congenital and acquired metabolic disorders are considered: Wilson-Konovalov disease, metabolic syndrome, total parenteral nutrition, severe weight loss, as well as rare pathologies: abetalipoproteinemia, hypobetaliproproteinemia, tyrosinemia, peroxisome pathology, mitochondriaopathies. Polycystic ovary syndrome, celiac disease, and contact with solvents are important. It is known that previous surgical interventions such as gastric banding, extensive small intestinal resection, biliopancreatic or ileo-intestinal anastomosis also contribute to the development of NASH [14]. A number of drugs from various pharmacological groups (chloroquine, diltiazem, nifedipine, amiodarone, glucocorticoids, tamoxifen, estrogens, isoniazid, methotrexate, nucleoside analogues) cause NASH. Clinic and diagnosis of NASH The relevance of timely diagnosis and treatment of NASH is associated, on the one hand, with the fact that NAFLD, along with obesity, type 2 diabetes, arterial hypertension and dyslipidemia, is a component of the metabolic syndrome and is an independent risk factor for cardiovascular diseases. Moreover, according to accumulated data, NASH accounts for 20% of all cases of NAFLD. On the other hand, it was previously believed that NASH is benign and rarely progresses to decompensated liver cirrhosis (LC). But it has now been shown that cirrhosis can develop in 40% of cases of NASH, and the progression of NASH to cirrhosis is determined by the severity of inflammatory changes in hepatocytes [16]. In addition, the disease affects all age groups, incl. and children [16]. True data on the prevalence of NASH are scarce, which is due to its mild and asymptomatic course. Patients rarely present complaints, or they are not specific, even at an advanced stage of the disease. Often, the possibility of developing NASH is discussed when elevated transaminase levels are detected, hepatomegaly is detected during examination, or according to imaging studies [17]. Increased liver echogenicity on ultrasound examination was detected in 14% of 2574 randomly selected Japanese residents. Since ultrasound can only detect fat deposition and not inflammation, not all of these cases can be considered NASH [16]. Also, with excess body weight, there may be a discrepancy in ultrasound conclusions performed by different specialists due to an increase in the thickness of the subcutaneous fat layer, which entails technical difficulties in performing the study and makes it difficult to assess the echogenicity of the liver [2]. A definitive diagnosis of NASH can only be made by liver biopsy. According to autopsy data, NASH occurs in 18.5% of obese cases and in 2.7% of healthy individuals. In the USA, 20% of clinically healthy liver donors have fatty infiltration, and 7.5% have NASH. In Japan, fatty infiltration was detected in 9.2% of liver donors [16]. Histologically, fatty liver disease manifests itself as macrovesicular fat deposits in hepatocytes and infiltration of the liver tissue by neutrophils and mononuclear cells; in some advanced cases, signs of fibrosis or cirrhosis may be present. Among the laboratory parameters that most often change in NASH, the most common is a 2-3-fold increase in the activity of alanine aminotransferase (ALT) and aspartic transaminase (AST). In most cases, the AST/ALT ratio can differentiate between NASH (<1) and alcoholic liver damage (<2). Bilirubin levels are usually normal, but levels increase in 12–17% of cases. In 39–59% of cases, the activity of alkaline phosphatase moderately increases, and less often the content of albumin and prothrombin in the blood decreases. Hemosiderosis with increased iron content in the blood is possible. In primary NASH, changes in immunological parameters similar to those in autoimmune hepatitis often develop. For example, hypergammaglobulinemia is detected in 13–30% of cases. Although antibodies to smooth muscles are not detected, in 40% of cases ANA appears in a titer of 1:40 - 1:320. In almost 20% of cases, hyperlipidemia develops (due to triglycerides and cholesterol (CH)). Disorders of lipoprotein metabolism (especially type IV hyperlipidemia) are often (54% of cases) observed with excess body weight (50–200% above normal); usually these disorders are combined with NASH with less severe fibrosis. In 58% of cases, iron metabolism is disrupted (increased ferritin levels and transferrin saturation in plasma). Even with a 5-fold increase in plasma ferritin levels, histological signs of hemochromatosis do not develop. Since conventional liver function tests are nonspecific, their determination is not important in the diagnosis of NASH and its differentiation from non-inflammatory fatty liver disease [16]. Treatment Treatment of NASH is empirical, there are no generally accepted methods. General recommendations include following a low-calorie diet and combating physical inactivity. Laboratory and histological abnormalities, as well as liver size, may decrease with a gradual decrease in body weight. However, improvement is possible even against the background of persistent obesity. It has also been observed that rapid weight loss is accompanied by progression of NASH. In addition, the long-term positive effect of weight loss is difficult to assess, since this requires maintaining a reduced body weight, and this is rarely possible in patients with NASH and obesity. In case of decompensated cirrhosis as part of NASH, liver transplantation is effective, but NASH in the graft can recur, especially against the background of weight gain and dyslipidemia. Follow-up data after liver transplantation for NASH are scarce, but its recurrence has been described after 6–10 weeks. [16]. For drug therapy, drugs of various pharmacological groups are used. Considering the role of insulin resistance in the pathogenesis of NASH, the use of biguanides and thiazolidinediones is relevant, the effects of which are due to a decrease in gluconeogenesis and lipid synthesis in the liver, and an increase in insulin sensitivity, thereby helping to reduce obesity. Preparations are used that contain essential phospholipids, which are the main elements in the structure of the membrane of liver cell organelles and have a normalizing effect on the metabolism of lipids and proteins [18]. Small and short-term studies have shown that taking α-tocopherol (vitamin E), a combination of lecithin, vitamin C and low doses of vitamin E, β-carotene, selenium, and B vitamins slightly improves liver function [16]. Recently, the greatest therapeutic effectiveness in NASH has been identified with ursodeoxycholic acid (UDCA) preparations. In pilot studies, the use of UDCA (at a dose of 13–15 mg/kg/day) for 12 months. was accompanied by a significant improvement in liver tests, lipid metabolism, and a decrease in liver steatosis without a significant decrease in body weight [19, 20]. UDCA is an epimer of chenodeoxycholic acid, formed under the action of bacterial enzymes, and is a hydrophilic, non-cytotoxic bile acid. It is the least aggressive bile acid - a natural component of human bile and is found in an amount of 1-5% of the total amount of bile acids. Numerous experimental as well as ongoing clinical studies make it possible to highlight the diverse properties and effects of UDCA. The hepatoprotective effect develops due to the fact that UDCA is able to integrate into the phospholipid layer of the cell membrane, which contributes to its stability and increased resistance to damaging factors. The anticholestatic effect is determined by the displacement of a pool of toxic hydrophobic bile acids due to competitive uptake by receptors in the ileum from the enterohepatic circulation; stimulation of exocytosis in hepatocytes, which leads to a decrease in the concentration of hydrophobic bile acids; induction of bicarbonate choleresis, which enhances the excretion of hydrophobic bile acids into the intestine. Immunomodulatory properties (by reducing the expression of HLA class I molecules on hepatocytes and HLA class II on cholangiocytes and reducing the production of pro-inflammatory cytokines), litholytic (by reducing the lithogenicity of bile due to the formation of liquid crystals with cholesterol molecules) and hypocholesterolemic (worsens the processes of cholesterol absorption) are also described in the intestine, its synthesis in the liver and excretion into bile) effects [21–23]. The positive effect of UDCA on the biochemical parameters of cytolysis and cholestasis in NASH has been described in many studies [24, 25], and the diverse effects of UDCA determine the use of the drug in a wide range of liver diseases [23, 26]. Currently, a new UDCA drug, Choludexan (World Medicine, UK), has appeared on the Russian market, each capsule of which contains 300 mg of UDCA. The growing interest in UDCA drugs, in particular Choludexan, is not accidental, since its pharmacotherapeutic effect is diverse. The properties of Choludexan are of particular value in comorbid vascular patients, the number of which is growing from year to year. Thus, the effectiveness of the use of UDCA for NASH in patients with coronary heart disease (CHD) was noted in several studies [27, 29]. A study conducted in 2006 in Ukraine examined the functional state of the liver of patients with ischemic heart disease combined with NSAH who received lipid-lowering therapy with statins and UDCA (Choludexan 300 mg) for 3 months. There was a decrease in the level of total cholesterol by 23-24%, triglycerides - by 40-41%, low-density lipoproteins - by 35-36%, VLDL - by 25%, atherogenic index - by 13-14%, an increase in the level of high-density lipoproteins – by 42%. A significant decrease in ALT activity (by 56%) was observed in patients receiving statins and UDCA [28]. The results of a study of the effectiveness of UDCA (Choludexan 300 mg) and statins in NASH and IHD indicate the validity of the use of drugs in order to achieve a lipid-lowering and cytoprotective effect, as well as the absence of adverse reactions when combined. In addition, despite the relatively benign course of NASH, in half of the cases there is progression of the pathological process and occasionally the formation of cirrhosis; the prescription of UDCA in patients with coronary artery disease and hyperlipidemia is justified [30]. So, UDCA (Choludexan 300 mg) in combination with statins probably has a potentiating hypolipidemic effect, leading to normalization of the lipid spectrum in patients with ischemic heart disease and NSAH. At the same time, the combination of drugs does not cause a deterioration in the metabolic function of the liver, which leads to the abolition of statin treatment. Choludexan is prescribed orally 1 time per day before bedtime or 2 times per day. The capsule is swallowed whole, without chewing, with a sufficient amount of liquid. When treating chronic liver diseases at a dose of 10–15 mg/kg/day, the duration of treatment ranges from several months to 2 years. It should be noted that, unlike other UDCA drugs, Choludexan has a more convenient dosage - 300 mg. As L. Vasiliev (2008) wrote in his article: “Let's face it: in the production of any medicine you should count not on a conscientious, but on a lazy patient, and the fewer capsules he needs to take per day, the greater the chances that he will complete the course of treatment to the end” [31]. It can be added that the advantage of a dosage of 300 mg lies in the convenience of calculating the dose of the drug per 1 kg of the patient’s weight, depending on the diagnosis (for NASH, Choludexan is used at a rate of 13–15 mg/kg/day from 6 months to several years). Thus, the range of pharmacotherapeutic effects of Choludexan is wide and, naturally, not limited to NASH. Indications for the appointment of Holudexan, in addition to NAG, are: uncomplicated gallstone disease (biliaric dummy; dissolution of cholesterol bile stones in the gall bladder if it is impossible to remove them with surgical or endoscopic methods; prevention of relapse of stone formation after cholecystectomy); chronic active hepatitis; toxic (including medicinal) liver damage; ABP; primary biliary CPU; primary sclerosing cholangitis; cystic fibrosis; atresia of the intrahepatic biliary tract, congenital atresia of the bile duct; Generative dyskinesias with proven effectiveness of the UDHK for all these diseases. Literature 1. Ludwig J., Viggiano Tr Non -alcoholic SteatoHepatis: Mayo Clinic Experiences with a Hitherto Unnamed Disease // Mayo Clin Proc. 1980. Vol. 55. R. 434–438. 2. Butorova L.I. Non -alcoholic fatty disease of the liver as a manifestation of metabolic syndrome: epidemiology, pathogenesis, clinical manifestations, principles of diagnosis, modern treatment possibilities. M., 2012. 3. Carneiro de Moore M. Non -alcohol steatogepatitis // Clinical prospects in gastroenterology, hepatology. 2001. No. 2. S. 12–15. 4. Fadeenko G.D. Fat liver: etiopathogenesis, diagnosis, treatment // Consumer of the Gastroenterology. 2003. No. 3 (13). S. 9–17. 5. Severov M. Non -alcoholic fatty liver disease // doctor. 2002. No. 10. S. 23–26, 1212. 6. BELLENTANI S., Tinbelli C. Epidemiology and Risk Factors for Fatty Liver. In: Leuschner U. James OFW, Dancygier H (Eds.). SteatoHepatitis (Nash and Ash), Kluwer Academic Publishers, Dordrecht, 2001, 3-10. 7. Sherlock S., blew J. Diseases of the liver and biliary tract (lane from English). M., 1999. S. 486–497. 8. Yakovenko E.P., Grigoryev P.Ya., Agafonov N.A., Yakovenko A.V. and other metabolic liver diseases: problems of therapy // Pharmacle. 2003. No. 10. P. 47–52. 9. BELLENTANI S., SACCOCOCIO G., Masutti F. et al. Prevalence of and Risk Factors for Hepatic Steatussis in Northern Italy // Ann Intern Med. 2000. Vol. 132. R. 112–117. 10. Chitturi S., Abeygunaskera S., Farrell GC et al. Nash and Insulin Resistance: Insulin Hepersecretion and Specific Association with the Insulin Resistance Syndrom // Hepatology. 2002. N 35. P. 373–379. 11. Neuschwander-Tetri Ba, Caldwell Sh Nonalcoholic SteatoHepatis: Summary of Anasld Single Topic Conference // Hepatology. 2003. N 37. P. 1202–1219. 12. Sanyal Aj, Campbell-Sargent C., Mirshahi F., Rizzo Wb et al. Nonalcoholic SteatoHepatis: Association of Insulin Resistance and MitoChondrial Abnor-Malities // Gastroenterology. 2001. N 20. P. 1183–1192. 13. Oneta CM, Dufour JF Non-alcoholic Fatty Liver Disease: Treatment Options Baed on Pathogenic Considerations // Swiss Med.wkly. 2002. N 132. P. 493–505. 14. Bueverov A.O. Hepatitis. Rational diagnosis. M.: GEOTAR-MEDIA, 2010. 15. Tilg H., Diehl Am Cytokines in Alcoholic and Nonalcoholic SteatoPatis // N. English. J. Med. 2000. N 343. P. 1467–1476. 16. Macnelly P.R. Secrets of Gastroenterology (Publishing House 2nd). M.: Bino, 2005. 17. Pavlov Ch., Bakulin I. Non -alcohol steatogepatitis: clinical features and principles of treatment // doctor. 2007. No. 10. P. 24–28. 18. Stepanov Yu.M., Filippova A.Yu. Statosis of the liver and non -alcohol steatogepatitis: a modern view of pathogenesis, diagnosis and treatment // Health of Ukraine. 2004. No. S. 19. Ceriani R., Brunati S., Morini L. et al. Effect of ursodeoxycholic Acid Plus Diet in Pathents with Nonalcoholic SteatoPatitis (Abstract) // Hepatology. 1998. Vol. 28.No. 894. R. 386. 20. Guma C., Viola L., Thome M. et al. Ursodeoxycholic Acid in the Treatment of Nonalcoholic SteatoPatitis: Results of a Prospective Clinical Controlled Trital (Abstract) // Hepatology. 1997. Vol. 26.No. 1036. R. 387. 21. Bakulin I.G., Sandler Yu.G. The possibilities of using hepatoprotectors in the practice of a general practitioner // Consilium Medicum. 2010. T. 12. No. 8. 22. rational pharmacotherapy of diseases of the digestive organs / under the general ed. V.T. Ivashkina. M.: Litterra, 2007. 23. Bueverov A.O. Possibilities of the clinical use of ursodeoxichole acid // Consilium Medicum. 2005. No. 7 (6). 24. Holoman J., Glasa J., Kasar J. et al. Serum Markers of Liver Fibrosis in Patients with Non-AlcoCholic SteatoPatitis (NASH). Correration to Morphology and Effect of Therapy // J Hepatol. 2000. Vol. 32. R. 210. 25. Nadinskaya M.Yu. A study of the use of ursodeoxichole acid in hepatology from the perspective of medicine based on scientific evidence // Consilium Medicum. 2003. No. 6. S. 318–322. 26. Lazaridis KN, Gores GJ, Lindor KD Ursodeoxycholic Acid Mechanisms of Action and Clinical Usery Disorders // J Hepatol. 2001. Vol. 35. R. 134–146. 27. Fedorov I.G., Baykova I.E., Nikitin I.G., Storozhakov G.I. Non -alcoholic steatogepatitis: clinic, pathogenesis, diagnosis, treatment // breast cancer. 2004. No. 2. P. 46–49. 28. Dolzoko M.N. A patient with coronary heart disease and chronic steatogepatitis: how to conduct a hypophydramic correction? // Ukrainian medical journal. 2007. No. 1 (57). 29. Shipulin V.P., Dolzoko M.N. Chronic steatogepatosis: a prospective study of the functional state of the cardiovascular system // Crimean Medical Journal. 2006. No. 3. S. 12–16. 30. Rational pharmacotherapy of digestive diseases / Ed. V.T. Ivashkina, T.L. Lapina. M.: Laterra, 2006. 552 p. 31. Vasiliev L. Healthy as ... Bear // Pharmacy. 2008. No. 1.
Why is the disease called insidious? No signs or symptoms
The disease does not show any symptoms for a very long time (and sometimes not at all). And those that the patient himself experiences, and that the doctor could detect during the examination of the patient (signs).
In simple steatosis, if symptoms occur, they are very nonspecific, meaning they can also occur in a number of other diseases besides NAFLD. Experts typically report two of these symptoms:
- fatigue;
- weak, usually dull pain in the upper right corner of the abdomen.
The condition is similar to other "progressive" diseases: hypertension and type 2 diabetes. But unlike non-alcoholic fatty liver disease, these diseases usually do not develop complications before they can be diagnosed.
In the case of NASH, i.e. a more dangerous form of NAFLD that causes liver degeneration and fibrosis), symptoms may be more typical. More often than with simple steatosis, the following appear:
- dull or aching pain in the right upper abdomen;
- a more intense feeling of tiredness, sometimes it can be severe fatigue;
- unexplained weight loss;
- weakness.
A complication of NASH, liver cirrhosis, is also accompanied by a number of symptoms:
- jaundice, i.e. yellowing of the skin and whites of the eyes;
- skin itching;
- swelling of the entire leg or limited swelling around the ankles or feet;
- nausea, loss of appetite and/or weight loss;
- enlarged abdomen due to fluid accumulation (ascites);
- confusion.
Ascites
But let us repeat once again - these symptoms appear late and concern not the most common “simple” fatty steatosis, but a more dangerous form - NASH and cirrhosis of the liver.
How is NAFLD diagnosed?
The question arises of how to detect this disease in general, since in most cases it is asymptomatic.
NAFLD is diagnosed mainly based on ultrasound of the abdominal cavity, including the liver. Interestingly, fatty liver disease in most cases is discovered by chance when an ultrasound is performed for other reasons, for example, to detect gallstones or to diagnose abdominal pain.
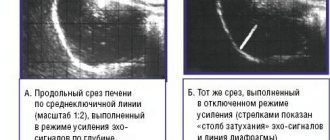
Fatty liver is very characteristic on ultrasound images. Radiologists say that the liver “glows” on the screen and is hyperechoic (its echo is greater than that of the surrounding tissue).
It is important to understand that fatty liver disease can also occur with other conditions or diseases, such as alcohol abuse, certain medications, or hepatitis C.
Early detection of fatty tissue using ultrasound is very important. Early diagnosis of NAFLD helps prevent exacerbation of the disease - NASH, as well as prevent the development of complications, the most important of which is cirrhosis of the liver. Therefore, gastroenterologists recommend regular ultrasound examinations of the liver (abdominal cavity), even if there are no symptoms. This is especially true for patients from risk groups.
Ultrasound of the liver (abdominal cavity)
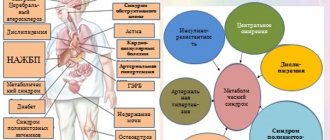
A group or risk factor is a statistical concept that limits a group of people with a particular condition or disease where the likelihood of developing a particular disease (in our case NAFLD) is higher than in the general population. Therefore, if the risk of developing non-alcoholic fatty liver disease in the entire population is 20-25%, then in type 2 diabetes mellitus this figure reaches 75%. Therefore, type 2 diabetes is a risk factor for the development of NAFLD.
According to European recommendations, ultrasound examination of the abdominal cavity should be carried out for people with:
- obesity;
- risk factors for metabolic disorders - increased waist circumference, hyperglycemia [increased blood sugar levels], hypertriglyceridemia [increased triglyceride levels in the blood], decreased HDL cholesterol, hypertension;
- persistent increase in alanine aminotransferase (ALT).
1.General information
Steatosis, or fatty infiltration, is an accumulation of lipid compounds in hepatocytes (parenchymal, functionally specialized liver cells). Hepatitis is an inflammatory process in the liver. Accordingly, steatohepatitis is a disease that combines the signs and patterns of both pathological processes. The clarification “non-alcoholic” (syn. “pseudo-alcoholic”, “diabetic”, etc.) is necessary because the histological picture of non-alcoholic steatohepatitis (NASH) is identical to fatty degeneration of liver cells in alcoholics, however, it develops in individuals for whom it is reliably known that there is no abuse of such kind.
This nosological unit was identified relatively recently (1980) and is actively being studied by hepatologists around the world, since many issues have not been clarified to this day. However, quite extensive statistical material has been accumulated. In particular, the pronounced endemicity (regional dependence) of the incidence of NASH is known: if in Japan its prevalence in the general population is slightly more than 1%, then in Western countries, according to various estimates, the incidence ranges from 7% to 11%. It has also been reliably established that the probability of “starting” the steatohepatitis process depends on age (persons aged 45-55 years old fall into the risk category) and gender (women get sick three times more often).
It should be understood that the data presented are purely statistical trends, although quite strong; in real clinical practice, NASH is found in patients of any gender and age.
A must read! Help with treatment and hospitalization!
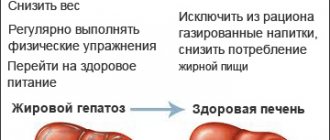
How to treat NAFLD?
There is no miracle cure for this disease. In order for adipose tissue to regress, it is necessary to follow an appropriate low-calorie diet and regularly engage in physical activity at least 5 days a week for 30 minutes a day.
Regular physical activity
It is very important to be constantly monitored by a gastroenterologist so as not to miss complications.
4.Treatment
A generally accepted and universally effective treatment protocol for NASH has not been developed to date. However, in this case, etiopathogenetic therapy (aimed at eliminating the root causes of the disease) most often involves eliminating the above risk factors.
Thus, an individually developed diet is prescribed and other measures are taken to gradually normalize body weight. The intake is canceled or, if necessary, an alternative to medications that are potentially harmful to the liver, which are objectively indicated for the patient, is selected. Hepatoprotectors are prescribed, and according to indications - antibiotics, statins and other drugs.
Important Diet Guidelines for Non-Alcoholic Fatty Liver Disease
What to avoid in your diet:
- Eliminate saturated fats and red meat, and full-fat dairy products.
- It is important to avoid trans- and hydrogenated (hardened) fats, sugar, and alcohol.
- Eliminate all processed grain products (that is, do not use white flour or white rice in your diet).
Control of sodium content in the diet is mandatory - do not exceed 1500 mg of table salt per day.
Controlling sodium in your diet
Be careful with supplements and medications, many have contraindications for liver disease. Before taking them, you should definitely consult a gastroenterologist.
Useful products for patients with non-alcoholic liver disease:
- Extra virgin olive oil - for frying and three tablespoons per day orally.
- Fruits and vegetables (but watch the salt content!).
- High fiber foods such as whole grain bread and brown rice.
- Oily fish several times a week.
- Skinless chicken and only lean pork.
- Vegetable protein (legumes) without restrictions.
- Coffee – no more than 3 cups of filtered coffee per day.
Sources
- Hartleb M, Wunsch E, et al: Management of patients with non-alcoholic fatty liver disease.
- Gundermann KJ et al. Activity of soybean essential phospholipids (EPL) in liver diseases.
ONLINE REGISTRATION at the DIANA clinic
You can sign up by calling the toll-free phone number 8-800-707-15-60 or filling out the contact form. In this case, we will contact you ourselves.
If you find an error, please select a piece of text and press Ctrl+Enter