The importance of restoring microflora
Normal intestinal microflora is a balance of beneficial and harmful bacteria that inhabit the human intestine. If harmful ones predominate, and beneficial ones are in the minority, this condition is called microflora imbalance and requires correction. This is what gastroenterologists do.
They recommend taking a stool test for dysbacteriosis and bacterial culture in case of noticeable disturbances in the intestines (bloating, diarrhea, nausea, constipation, pain, etc.).
Based on the test results, appropriate treatment is prescribed, after which the microflora is normalized and the person’s well-being improves. Normal intestinal microflora is important for the production of essential vitamins, strengthening the immune system and protecting against various diseases.
Various factors can worsen the condition of the intestinal microflora, these are:
- taking antibiotics and NSAIDs;
- passion for the Western diet (with the presence of fast foods);
- lack of fiber in the diet;
- taking painkillers;
- treatment with proton pump inhibitors;
- use of H2-histamine receptor blockers.
Some doctors believe that the intestinal environment does not need to be sterile. But harmful bacteria should make up no more than 15 percent of the total microflora. Only then will the imbalance persist.
Microflora can change depending on a person’s age, mood, well-being, climate, and season.
Microflora disturbances can cause the following diseases:
- oncology;
- asthma;
- colitis;
- autism;
- eczema;
- diabetes;
- obesity;
- multiple sclerosis;
- heart diseases.
That is why we must take care of the state of the intestinal microflora. Let's look at some useful tips on this matter.
Modern ideas about intestinal dysbiosis (dysbacteriosis)
Dysbiosis (dysbacteriosis) is a pathological condition in which the qualitative and quantitative composition of the intestinal microflora is disrupted compared to normal. For the colon, this term refers to a decrease in the number of beneficial microorganisms and an increase in the number of representatives of pathogenic flora. For the small intestine, in which in a healthy state the number of microorganisms is minimal, dysbiosis, on the contrary, is associated with an increase in the number of microorganisms. Small intestinal dysbiosis is called bacterial overgrowth syndrome (SIBO).
Causes of dysbiosis (dysbiosis) of the colon:
- unbalanced diet and insufficient fiber intake;
- water quality;
- alcohol abuse and smoking;
- antibacterial therapy and occupational exposure to antibiotics;
- long-term use of other medications;
- any acute and chronic infections;
- developmental anomalies, operations and their complications;
- primary and secondary immunodeficiencies;
- chronic gastrointestinal diseases and endocrine disorders;
- radiation;
- elderly age.
At the initial stage, colon dysbiosis has no noticeable symptoms and is reflected only in laboratory examination of stool samples. At a more advanced stage, noticeable manifestations appear.
Symptoms and manifestations of colon dysbiosis:
- bowel dysfunction - constipation or diarrhea;
- bloating and increased gas formation;
- stomach ache;
- food or drug allergies;
- metabolic disease;
- anemia, abnormal cholesterol levels;
- functional intestinal disorders;
- liver damage and others.
How to restore microflora
Complete diet
A person should eat a variety of foods per day, and not meager ones, consisting mainly of carbohydrates and fats. This is how most people eat, snacking, for lack of time, on fast foods, rolls, etc.. It is necessary to include fiber in the diet every day.
This:
- fresh vegetables;
- fruits;
- grain bread;
- legumes;
- greenery;
- nuts.
Orthodox fasting is useful for normalizing microflora. It has been observed that those who observe fasts suffer less from intestinal diseases, even cancer. Fasting excludes animal fats (meat, butter, eggs) and focuses on fresh vegetables and fruits, berries, and cereals.
It is known that inulin, which has a prebiotic effect, is found in the following products:
- garlic;
- onion;
- leek;
- asparagus;
- chicory;
- artichoke.
These products help improve intestinal microflora and defeat harmful bacteria. They must be included in your diet if you have intestinal problems.
Sources of bifidobacteria beneficial to humans are:
- apples;
- blueberry;
- artichoke;
- almond;
- pistachios.
They must be present on the table as often as possible.
Fermentation of products
It is useful to eat fermented ones, i.e. fermented products. As a result of the fermentation process, simple products become fantastically healthy because they change their composition thanks to bacteria. They help restore intestinal microflora.
The most common fermented products:
- sauerkraut;
- kefir;
- yogurt;
- tea mushroom.
It is customary for some peoples to ferment vegetables, even spicy ones. This dish of pickled spicy vegetables is called kimchi. Fermented soybeans are called tempeh.
Fermented dairy products contain lactobacilli, which are so necessary for normal microflora. People suffering from kidney disease simply need them, because... With such diseases, the microflora is disrupted.
Artificial sweeteners are harmful
Sugar substitutes (aspartame, saccharin) are harmful products. They have a destructive effect on the intestinal microflora. In addition, they increase blood glucose levels. Therefore, they must be excluded from the diet.
More prebiotics
Prebiotics contain beneficial bacteria that help evict harmful ones from the human intestines. They are found in vegetables, fruits, legumes, and grains. They should be eaten as often as possible. They also lower the level of triglycerides, cholesterol, and insulin in the body. This means that prebiotics reduce the risk of cardiovascular diseases, death from heart attack, and stroke.
Whole grains
The benefit of whole grains is that they contain fiber and indigestible carbohydrates, which are not absorbed in the small intestine, but enter the large intestine. There they break down and cause the growth of beneficial bacteria.
Whole grains contain:
- B vitamins;
- iron;
- zinc;
- proteins;
- carbohydrates.
They are sold whole and are also used to make whole grain bread. They are also used to make whole wheat flour.
Artificial Probiotics
There is controversy about probiotic drugs. There is evidence of some benefits that such drugs provide. But it has not been completely proven. Moreover, there are many fakes among the drugs. You need to be careful when choosing probiotics, following your doctor’s recommendations. We recommend paying attention to Maxilac.
Intestinal microflora and the importance of prebiotics for its functioning
The human intestinal microflora is a component of the human body and performs numerous vital functions. The total number of microorganisms living in various parts of the macroorganism is approximately two orders of magnitude greater than the number of its own cells and is about 1014–15. The total weight of microorganisms in the human body is about 3–4 kg. The largest number of microorganisms are found in the gastrointestinal tract (GIT), including the oropharynx (75–78%), the rest populate the genitourinary tract (up to 2–3% in men and up to 9–12% in women) and skin.
In healthy individuals, there are more than 500 species of microorganisms in the intestines. The total mass of intestinal microflora ranges from 1 to 3 kg. In different parts of the gastrointestinal tract, the number of bacteria is different; most microorganisms are localized in the large intestine (about 1010–12 CFU/ml, which is 35–50% of its contents). The composition of the intestinal microflora is quite individual and is formed from the first days of a child’s life, approaching the indicators of an adult by the end of the 1st - 2nd year of life, undergoing some changes in old age (Table 1). In healthy children, the colon is inhabited by representatives of facultative anaerobic bacteria of the genus Streptococcus, taphylococcus, Lactobacillus, nterobacteriacae, Candida, and more than 80% of the biocenosis is occupied by anaerobic bacteria, often gram-positive: propionobacteria, veillonella, eubacteria, anaerobic lactobacilli, peptococci, peptostreptococcus ki, and also gram-negative bacteroids and fusobacteria.
The distribution of microorganisms along the gastrointestinal tract has fairly strict patterns and closely correlates with the state of the digestive system (Table 2). Most microorganisms (about 90%) are constantly present in certain sections and are the main (resident) microflora; about 10% is facultative (or additional, accompanying microflora); and 0.01–0.02% accounts for random (or transient, residual) microorganisms. It is conventionally accepted that the main microflora of the colon is represented by anaerobic bacteria, while aerobic bacteria make up the accompanying microflora. Staphylococci, clostridia, Proteus and fungi belong to the residual microflora. In addition, about 10 intestinal viruses and some representatives of non-pathogenic protozoa are detected in the colon. There are always an order of magnitude more obligate and facultative anaerobes in the colon than aerobes, and strict anaerobes are directly adherent to epithelial cells, facultative anaerobes are located higher, followed by aerobic microorganisms. Thus, anaerobic bacteria (mainly bifidobacteria and bacteroides, the total share of which is about 60% of the total number of anaerobic bacteria) are the most constant and numerous group of intestinal microflora that performs basic functions.
The entire set of microorganisms and the macroorganism constitute a kind of symbiosis, where each benefits for its existence and influences its partner. The functions of the intestinal microflora in relation to the macroorganism are realized both locally and at the systemic level, with various types of bacteria contributing to this influence. The microflora of the digestive tract performs the following functions.
- Morphokinetic and energy effects (energy supply to the epithelium, regulation of intestinal motility, heat supply to the body, regulation of differentiation and regeneration of epithelial tissues).
- Formation of a protective barrier of the intestinal mucosa, suppression of the growth of pathogenic microflora.
- Immunogenic role (stimulation of the immune system, stimulation of local immunity, including the production of immunoglobulins).
- Modulation of P450 cytochrome functions in the liver and production of P450-like cytochromes.
- Detoxification of exogenous and endogenous toxic substances and compounds.
- Production of various biologically active compounds, activation of certain drugs.
- Mutagenic/antimutagenic activity (increased resistance of epithelial cells to mutagens (carcinogens), destruction of mutagens).
- Regulation of the gas composition of cavities.
- Regulation of behavioral reactions.
- Regulation of replication and gene expression in prokaryotic and eukaryotic cells.
- Regulation of programmed death of eukaryotic cells (apoptosis).
- Repository of microbial genetic material.
- Participation in the etiopathogenesis of diseases.
- Participation in water-salt metabolism, maintaining ionic homeostasis of the body.
- Formation of immunological tolerance to food and microbial antigens.
- Participation in colonization resistance.
- Ensuring homeostasis of symbiotic relationships between prokaryotic and eukaryotic cells.
- Participation in metabolism: metabolism of proteins, fats (supply of lipogenesis substrates) and carbohydrates (supply of gluconeogenesis substrates), regulation of bile acids, steroids and other macromolecules.
Thus, bifidobacteria, due to the fermentation of oligo- and polysaccharides, produce lactic acid and acetate, which provide a bactericidal environment, secrete substances that inhibit the growth of pathogenic bacteria, which increases the child’s body’s resistance to intestinal infections. Modulation of a child’s immune response by bifidobacteria is also reflected in a reduction in the risk of developing food allergies.
Lactobacilli reduce the activity of peroxidase, providing an antioxidant effect, have antitumor activity, stimulate the production of immunoglobulin A (IgA), suppress the growth of pathogenic microflora and stimulate the growth of lacto- and bifid flora, and have an antiviral effect.
Of the representatives of enterobacteria, the most important is Escherichia coli M17, which produces colicin B, due to which it suppresses the growth of Shigella, Salmonella, Klebsiella, Serration, Enterobacter and has a slight effect on the growth of staphylococci and fungi. E. coli also contribute to the normalization of microflora after antibacterial therapy and inflammatory and infectious diseases.
Enterococci (Enterococcus avium, faecalis, faecium) stimulate local immunity by activating B lymphocytes and increasing the synthesis of IgA, the release of interleukins-1β and -6, γ-interferon; have antiallergic and antimycotic effects.
Escherichia coli, bifidobacteria and lactobacilli perform a vitamin-forming function (participate in the synthesis and absorption of vitamins K, group B, folic and nicotinic acids). In its ability to synthesize vitamins, E. coli is superior to all other bacteria of the intestinal microflora, synthesizing thiamine, riboflavin, nicotinic and pantothenic acids, pyridoxine, biotin, folic acid, cyanocobalamin and vitamin K. Bifidobacteria synthesize ascorbic acid, bifidobacteria and lactobacilli promote the absorption of calcium and vitamin D , improve iron absorption (due to the creation of an acidic environment).
The process of digestion can be divided into its own (remote, cavity, autolytic and membrane), carried out by enzymes of the body, and symbiotic digestion, which occurs with the assistance of microflora. The human intestinal microflora is involved in the fermentation of previously unresolved food components, mainly carbohydrates, such as starch, oligo- and polysaccharides (including cellulose), as well as proteins and fats.
Proteins and carbohydrates that are not absorbed in the small intestine in the cecum undergo deeper bacterial breakdown - mainly by Escherichia coli and anaerobes. The end products resulting from the bacterial fermentation process have varying effects on human health. For example, butyrate is necessary for the normal existence and functioning of colonocytes and is an important regulator of their proliferation and differentiation, as well as the absorption of water, sodium, chlorine, calcium and magnesium. Together with other volatile fatty acids, it affects the motility of the colon, in some cases speeding it up, in others slowing it down. When polysaccharides and glycoproteins are broken down by extracellular microbial glycosidases, monosaccharides (glucose, galactose, etc.) are formed, among other things, the oxidation of which releases at least 60% of their free energy into the environment as heat.
Among the most important systemic functions of microflora is the supply of substrates for gluconeogenesis, lipogenesis, as well as participation in protein metabolism and recycling of bile acids, steroids and other macromolecules. The conversion of cholesterol into coprostanol, which is not absorbed in the colon, and the transformation of bilirubin into stercobilin and urobilin are possible only with the participation of bacteria in the intestine.
The protective role of saprophytic flora is realized both at the local and systemic levels. By creating an acidic environment, thanks to the formation of organic acids and reducing the pH of the colon to 5.3–5.8, symbiont microflora protects a person from colonization by exogenous pathogenic microorganisms and suppresses the growth of pathogenic, putrefactive and gas-forming microorganisms already present in the intestine. The mechanism of this phenomenon is the competition of microflora for nutrients and binding sites, as well as the production by normal microflora of certain substances that inhibit the growth of pathogens and have bactericidal and bacteriostatic activity, including antibiotic-like ones. Low molecular weight metabolites of saccharolytic microflora, primarily volatile fatty acids, lactate, etc., have a noticeable bacteriostatic effect. They are able to inhibit the growth of salmonella, Shigella dysentery, and many fungi.
Also, intestinal microflora strengthens the local intestinal immunological barrier. It is known that in sterile animals a very small number of lymphocytes are detected in the lamina propria; in addition, these animals exhibit immunodeficiency. Restoration of normal microflora quickly leads to an increase in the number of lymphocytes in the intestinal mucosa and the disappearance of immunodeficiency. Saprophytic bacteria, to a certain extent, have the ability to modulate the level of phagocytic activity, reducing it in people suffering from allergies and, conversely, increasing it in healthy individuals.
Thus, the microflora of the gastrointestinal tract not only forms local immunity, but also plays a huge role in the formation and development of the child’s immune system, and also maintains its activity in an adult. Resident flora, especially some microorganisms, have fairly high immunogenic properties, which stimulates the development of the intestinal lymphoid apparatus and local immunity (primarily by enhancing the production of the key link in the local immune system - secretory IgA), and also leads to a systemic increase in the tone of the immune system, with activation of cellular and humoral immunity. Systemic stimulation of the immune system is one of the most important functions of microflora. It is known that in germ-free laboratory animals not only the immune system is suppressed, but also the involution of immunocompetent organs occurs. Therefore, with disturbances in intestinal microecology, deficiency of bifid flora and lactobacilli, and unhindered bacterial colonization of the small and large intestines, conditions arise to reduce not only local protection, but also the resistance of the body as a whole.
Despite their sufficient immunogenicity, saprophytic microorganisms do not cause immune system reactions. Perhaps this happens because the saprophytic microflora is a kind of repository of microbial plasmid and chromosomal genes, exchanging genetic material with host cells. Intracellular interactions are realized through endocytosis, phagocytosis, etc. With intracellular interactions, the effect of exchange of cellular material is achieved. As a result, representatives of the microflora acquire receptors and other antigens inherent to the host. This makes them “friends” for the immune system of the macroorganism. As a result of this exchange, epithelial tissues acquire bacterial antigens.
The issue of the key participation of microflora in providing antiviral protection to the host is discussed. Thanks to the phenomenon of molecular mimicry and the presence of receptors acquired from the host epithelium, the microflora becomes capable of intercepting and eliminating viruses that have the appropriate ligands.
Thus, along with the low pH of gastric juice, the motor and secretory activity of the small intestine, the microflora of the gastrointestinal tract belongs to the nonspecific factors of the body’s defense.
An important function of microflora is the synthesis of a number of vitamins. The human body receives vitamins mainly from the outside - from food of plant or animal origin. Incoming vitamins are normally absorbed in the small intestine and partially utilized by the intestinal microflora. Microorganisms inhabiting the intestines of humans and animals produce and utilize many vitamins. It is noteworthy that the microbes of the small intestine play the most important role for humans in these processes, since the vitamins they produce can be effectively absorbed and enter the bloodstream, while vitamins synthesized in the large intestine are practically not absorbed and are inaccessible to humans. Suppression of microflora (for example, with antibiotics) also reduces the synthesis of vitamins. On the contrary, creating conditions favorable for microorganisms, for example, by eating a sufficient amount of prebiotics, increases the supply of vitamins to the macroorganism.
The most studied aspects at present are those related to the synthesis of folic acid, vitamin B12 and vitamin K by intestinal microflora.
Folic acid (vitamin B9), when supplied with food, is effectively absorbed in the small intestine. Folate, synthesized in the colon by representatives of normal intestinal microflora, is used exclusively for its own needs and is not utilized by the macroorganism. However, folate synthesis in the colon may be important for the normal state of colonocyte DNA.
Intestinal microorganisms that synthesize vitamin B12 live in both the large and small intestines. Among these microorganisms, the most active in this aspect are representatives of Pseudomonas and Klebsiella sp. However, the capabilities of the microflora to fully compensate for hypovitaminosis B12 are not enough.
The ability of the intestinal epithelium to resist carcinogenesis processes is associated with the content of folate and cobalamin in the lumen of the colon, obtained from food or synthesized by microflora. It is assumed that one of the reasons for the higher incidence of colon tumors, compared to the small intestine, is the lack of cytoprotective components, most of which are absorbed in the middle sections of the gastrointestinal tract. Among them are vitamin B12 and folic acid, which together determine the stability of cellular DNA, in particular the DNA of colon epithelial cells. Even a slight deficiency of these vitamins, which does not cause anemia or other serious consequences, nevertheless leads to significant aberrations in the DNA molecules of colonocytes, which can become the basis of carcinogenesis. It is known that insufficient supply of vitamins B6, B12 and folic acid to colonocytes is associated with an increased incidence of colon cancer in the population. Vitamin deficiency leads to disruption of DNA methylation processes, mutations and, as a consequence, colon cancer. The risk of colon carcinogenesis increases with low consumption of dietary fiber and vegetables, which ensure the normal functioning of the intestinal microflora, which synthesizes trophic and protective factors for the colon.
Vitamin K exists in several varieties and is required by the human body for the synthesis of various calcium-binding proteins. Vitamin K1, a phyloquinone, comes from plant foods, and vitamin K2, a group of menaquinone compounds, is synthesized in the human small intestine. Microbial synthesis of vitamin K2 is stimulated by a lack of phyloquinone in the diet and is quite capable of compensating for it. At the same time, vitamin K2 deficiency with reduced microflora activity is poorly corrected by dietary measures. Thus, synthetic processes in the intestine are a priority for providing the macroorganism with this vitamin. Vitamin K is also synthesized in the colon, but is used primarily for the needs of microflora and colonocytes.
Intestinal microflora takes part in the detoxification of exogenous and endogenous substrates and metabolites (amines, mercaptans, phenols, mutagenic steroids, etc.) and, on the one hand, is a massive sorbent, removing toxic products from the body with intestinal contents, and on the other hand, it utilizes them in metabolic reactions for their needs. In addition, representatives of saprophytic microflora produce estrogen-like substances based on bile acid conjugates, which influence the differentiation and proliferation of epithelial and some other tissues by changing gene expression or the nature of their action.
So, the relationships between micro- and macroorganisms are complex, occurring at the metabolic, regulatory, intracellular and genetic levels. However, normal functioning of microflora is possible only with a good physiological state of the body and, first of all, normal nutrition.
The nutrition of microorganisms inhabiting the intestines is provided by nutrients coming from the overlying sections of the gastrointestinal tract, which are not digested by their own enzymatic systems and are not absorbed in the small intestine. These substances are necessary to provide the energy and plastic needs of microorganisms. The ability to use nutrients for one’s life depends on the enzymatic systems of various bacteria.
Depending on this, bacteria with predominantly saccharolytic activity are conventionally isolated, the main energy substrate of which is carbohydrates (typical mainly for saprophytic flora), with predominantly proteolytic activity, using proteins for energy purposes (typical for most representatives of pathogenic and conditionally pathogenic flora), and mixed activity. Accordingly, the predominance of certain nutrients in food and disruption of their digestion will stimulate the growth of various microorganisms.
Carbohydrate nutrients are especially necessary for the functioning of normal intestinal microflora. Previously, these food components were called “ballast”, suggesting that they do not have any significant significance for the macroorganism, but as microbial metabolism was studied, their importance became obvious not only for the growth of intestinal microflora, but for human health in general. According to the modern definition, prebiotics are partially or completely indigestible food components that selectively stimulate the growth and/or metabolism of one or more groups of microorganisms living in the large intestine, ensuring the normal composition of the intestinal microbiocenosis. Colon microorganisms meet their energy needs through anaerobic substrate phosphorylation, the key metabolite of which is pyruvic acid (PVA). PVC is formed from glucose during glycolysis. Further, as a result of the reduction of PVC, from one to four molecules of adenosine triphosphate (ATP) are formed. The last stage of the above processes is referred to as fermentation, which can take different paths with the formation of different metabolites.
Homofermentative lactic fermentation is characterized by the predominant formation of lactic acid (up to 90%) and is typical for lactobacilli and streptococci of the colon. Heterofermentative lactic fermentation, during which other metabolites (including acetic acid) are also formed, is characteristic of bifidobacteria. Alcoholic fermentation, leading to the formation of carbon dioxide and ethanol, is a metabolic side effect in some members of Lactobacillus and Clostridium. Certain types of enterobacteria (E. coli) and clostridia obtain energy as a result of formic acid, propionic acid, butyric acid, acetone butyl or homoacetate types of fermentation.
As a result of microbial metabolism in the colon, lactic acid, short-chain fatty acids (C2 - acetic; C3 - propionic; C4 - butyric/isobutyric; C5 - valeric/isovaleric; C6 - caproic/isocaproic), carbon dioxide, hydrogen, water are formed. Carbon dioxide is largely converted into acetate, hydrogen is absorbed and excreted through the lungs, and organic acids (primarily short-chain fatty acids) are utilized by the macroorganism. The normal microflora of the colon, processing carbohydrates that are not digested in the small intestine, produces short-chain fatty acids with a minimal amount of their isoforms. At the same time, when microbiocenosis is disrupted and the proportion of proteolytic microflora increases, these fatty acids begin to be synthesized from proteins mainly in the form of isoforms, which negatively affects the condition of the colon, on the one hand, and can be a diagnostic marker, on the other.
In addition, various representatives of the saprophytic flora have their own needs for certain nutrients, explained by the characteristics of their metabolism. Thus, bifidobacteria break down mono-, di-, oligo- and polysaccharides, using them as an energy and plastic substrate. At the same time, they can ferment proteins, including for energy purposes; They are not demanding when it comes to getting most vitamins from food, but they do need pantothenates.
Lactobacilli also use various carbohydrates for energy and plastic purposes, but they do not break down proteins and fats well, so they need amino acids, fatty acids, and vitamins from outside.
Enterobacteriaceae break down carbohydrates to produce carbon dioxide, hydrogen and organic acids. At the same time, there are lactose-negative and lactose-positive strains. They can also utilize proteins and fats, so they have little need for external supply of amino acids, fatty acids and most vitamins.
It is obvious that the nutrition of saprophytic microflora and its normal functioning fundamentally depends on the supply of undigested carbohydrates (di-, oligo- and polysaccharides) for energy purposes, as well as proteins, amino acids, purines and pyrimidines, fats, carbohydrates, vitamins and minerals - for plastic exchange. The key to supplying bacteria with the necessary nutrients is rational nutrition of the macroorganism and the normal course of digestive processes.
Although monosaccharides can be easily utilized by colon microorganisms, they are not considered prebiotics.
Under normal conditions, the intestinal microflora does not consume monosaccharides, which must be completely absorbed in the small intestine. Prebiotics include some disaccharides, oligosaccharides, polysaccharides and a fairly heterogeneous group of compounds, which also contains poly- and oligosaccharides, which is designated as dietary fiber. Prebiotics in human milk include lactose and oligosaccharides.
Lactose (milk sugar) is a disaccharide consisting of galactose and glucose. Normally, lactose is broken down by lactase in the small intestine into monomers, which are almost completely absorbed in the small intestine. Only a small amount of undigested lactose in children in the first months of life enters the large intestine, where it is utilized by the microflora, ensuring its formation. At the same time, lactase deficiency leads to an excess of lactose in the colon and a significant disruption of the composition of the intestinal microflora and osmotic diarrhea.
Lactulose, a disaccharide consisting of galactose and fructose, is not found in milk (women's or cow's milk), but can be formed in small quantities when milk is heated to boiling point. Lactulose is not digested by gastrointestinal enzymes, is fermented by lacto- and bifidobacteria and serves as a substrate for energy and plastic metabolism, thereby promoting their growth and normalizing the composition of microflora, increasing the volume of biomass in the intestinal contents, which determines its laxative effect. In addition, the anti-candidal activity of lactulose and its inhibitory effect on salmonella have been shown. Synthetically produced lactulose (Duphalac) is widely used as an effective laxative with prebiotic properties. As a prebiotic for children, Duphalac is prescribed in low doses that do not have a laxative effect (1.5–2.5 ml 2 times a day for 3–6 weeks).
Oligosaccharides are linear polymers of glucose and other monosaccharides with a total chain length of no more than 10. Based on their chemical structure, galacto-, fructo-, fucosyl-oligosaccharides, etc. are distinguished. The concentration of oligosaccharides in human milk is relatively low, no more than 12–14 g/l, however, their prebiotic effect is quite significant. It is oligosaccharides that are today considered as the main prebiotics of human milk, ensuring both the formation of normal microflora of the child’s intestines and its maintenance in the future. An important fact is that oligosaccharides are present in significant concentrations only in human milk and are absent, in particular, in cow's milk. Consequently, prebiotics (galacto- and fructosaccharides) should be added to the composition of adapted milk formulas for artificial feeding of healthy children.
Polysaccharides are long-chain carbohydrates mainly of plant origin. Inulin, which contains fructose, is present in large quantities in artichokes, tubers and roots of dahlias and dandelions; utilized by bifidobacteria and lactobacilli, promotes their growth. In addition, inulin increases calcium absorption and affects lipid metabolism, reducing the risk of developing atherosclerosis.
Dietary fiber is a large heterogeneous group of polysaccharides, the best known of which are cellulose and hemicellulose. Cellulose is a straight-chain polymer of glucose, and hemicellulose is a polymer of glucose, arabinose, glucuronic acid and its methyl ester. In addition to serving as a substrate for the nutrition of lacto- and bifid flora and indirectly as a supplier of short-chain fatty acids for colonocytes, dietary fiber has other important effects. They have a high adsorption capacity and retain water, which leads to an increase in osmotic pressure in the intestinal cavity, an increase in the volume of feces, and accelerated passage through the intestines, which causes a laxative effect.
In average quantities (1–1.9 g/100 g of product) dietary fiber is found in carrots, sweet peppers, parsley (in roots and greens), radishes, turnips, pumpkin, melon, prunes, citrus fruits, lingonberries, beans, buckwheat, pearl barley, Hercules, rye bread.
A high content (2–3 g/100 g of product) of dietary fiber is typical for garlic, cranberries, red and black currants, chokeberries, blackberries, oatmeal, and bread made from protein-bran flour.
The largest amount (more than 3 g/100 g) is found in dill, dried apricots, strawberries, raspberries, tea (4.5 g/100 g), oatmeal (7.7 g/100 g), wheat bran (8. 2 g/100 g), dried rose hips (10 g/100 g), roasted coffee beans (12.8 g/100 g), oat bran (14 g/100 g). Dietary fiber is missing from refined foods.
Despite the obvious importance of prebiotics for the nutrition of microflora, the well-being of the gastrointestinal tract and the whole organism, in modern conditions there is a deficiency of prebiotics in the diet in all age groups. In particular, an adult should eat approximately 20–35 g of dietary fiber per day, while in real conditions a European consumes no more than 13 g per day. A decrease in the proportion of natural feeding in children of the first year of life leads to a lack of prebiotics contained in human milk.
Thus, prebiotics ensure the well-being of the colon microflora, colon health and are an essential factor in human health due to their significant metabolic effects. Overcoming the deficiency of prebiotics in modern conditions is associated with ensuring rational nutrition for people of all age categories, from newborns to the elderly.
Literature
- Ardatskaya M. D., Minushkin O. N., Ikonnikov N. S. Intestinal dysbiosis: concept, diagnostic approaches and ways of correction. Possibilities and advantages of biochemical examination of stool: a manual for doctors. M., 2004. 57 p.
- Belmer S.V., Gasilina T.V. Rational nutrition and the composition of intestinal microflora // Issues of pediatric dietology. 2003. T. 1. No. 5. P. 17–20.
- Doronin A.F., Shenderov B.A. Functional nutrition. M.: GRANT, 2002. 296 p.
- Kon I. Ya. Carbohydrates: new views on their physiological functions and role in nutrition // Issues of pediatric dietology. 2005. T. 3. No. 1. P. 18–25.
- Boehm G., Fanaro S., Jelinek J., Stahl B., Marini A. Prebiotic concept for infant nutrition//Acta Paediatr Suppl. 2003; 91:441:64–67.
- Choi SW, Friso S., Ghandour H., Bagley PJ, Selhub J., Mason JB Vitamin B12 deficiency induces anomalies of base substitution and methylation in the DNA of rat colonic epithelium//J. Nutr. 2004; 134(4):750–755.
- Edwards CA, Parrett AM Intestinal flora during the first months of life: new perspectives//Br. J. Nutr. 2002; 1:11–18.
- Fanaro S., Chierici R., Guerrini P., Vigi V. Intestinal microflora in early infancy: composition and development //Acta Paediatr. 2003; 91:48–55.
- Hill MJ Intestinal flora and endogenous vitamin synthesis//Eur. J. Cancer. Prev. 1997; 1:43–45.
- Midtvedt AC, Midtvedt T. Production of short chain fatty acids by the intestinal microflora during the first 2 years of human life//J. Pediatr. Gastroenterol. Nutr. 1992; 15:4:395–403.
S. V. Belmer , Doctor of Medical Sciences, Professor A. V. Malkoch , Candidate of Medical Sciences, Russian State Medical University, Moscow
Metabolic changes
As part of the MetaHIT project, dedicated to the study of the intestinal metagenome, that is, the total genomes of all intestinal inhabitants, 124 Europeans were examined [11]. The total number of genes in the intestinal microbiome was 150 times (!) greater than the number of human genes. But it's worth noting that excess fatty foods led to a reduction in bacterial diversity: obese people had an average of six fewer types of bacteria than people with normal body weight. The results of metagenomic analysis divided the experiment participants into two groups: carriers of a “small genome” (low gene count) and carriers of a “large genome” (high gene count). A small genome is a metagenome in which there are relatively few genes of various bacterial species: the difference between the “small” and “large” genomes in the number of genes reached an average of 40%. In most individuals with a poor intestinal metagenome, Bacteroides predominated, and in those with a rich intestinal metagenome, Methanobrevibacter predominated. At the same time, the two described categories of people differed greatly in the representation in their microbiota of groups that form a pro-inflammatory (Bacteroides, Ruminococcus gnavus) or anti-inflammatory (Faecalibacterium prausnitzii, Roseburia inulinivorans) background. The former were much more often found in individuals with a poor metagenome.
The results of the MetaHIT project clearly indicate that the abundance of a person's intestinal microflora correlates with its metabolic markers, with bacterial genes playing almost a greater role in the pathogenesis of obesity than our own.
Among those with a “small genome” (23% of all participants), there were more overweight people. This group as a whole was characterized by disturbances in the tissue response to the action of insulin, which led to an increase in its concentration in the blood. Such people also showed a statistically significant decrease in the content of so-called “good cholesterol” - high-density lipoproteins that transport cholesterol from various tissues to the liver for further transformation and utilization. There was also a tendency to increase blood levels of triglycerides, free fatty acids and the hormone leptin, high concentrations of which are considered an independent risk factor for the development of cardiovascular pathologies and thrombosis. (Major risk factors also include specific lipid profiles, high blood pressure, chronic inflammation, and smoking.)
A number of studies have shown that in people whose diet is dominated by plant components, the microbiome is dominated by bacteria that break down polysaccharides - and these are precisely representatives of the phylum Bacteroidetes, some of which protect the host from the development of local and systemic inflammation. At the same time, lovers of plant foods reduce the number of Firmicutes, as well as Enterobacteriaceae, which are often called “pathobionts”: they are capable of creating an inflammatory environment due to the lipopolysaccharide of their outer membrane and increased permeability of the intestinal epithelium, which leads to large-scale penetration of lipopolysaccharide molecules into the bloodstream and provocation metabolic endotoxemia, and possibly a craving for regular overeating. By the way, this is exactly how events develop against the backdrop of a high-fat diet. A vegetarian diet, on the contrary, is associated by most studies with a reduced risk of developing metabolic syndrome and related “diseases of civilization” [7].
Active decomposition of plant fiber by the corresponding bacteria of the large intestine leads to the formation of monosaccharides and short-chain fatty acids (SCFA). The latter - especially butyric acid - are necessary not only for the intestinal microflora, but also for the macroorganism. For example, they reduce the pH of the intestinal contents, thereby displacing a number of pathobionts from the community, and most importantly, they provide energy to enterocytes, protect them from cancer transformation and suppress inflammatory signaling pathways. But even here, not everything is so simple: on the one hand, an excess of SCFA can enhance lipogenesis and therefore contribute to the development of obesity, on the other hand, some studies have shown a beneficial effect of SCFA on the lipid profile and blood glucose levels. This positive effect may be mediated by the binding of SCFAs to cellular G protein-coupled receptors GPR41 and GPR43, which entails hormonal changes leading to a feeling of fullness and increased tissue sensitivity to insulin. In general, the consequences of the production of large amounts of SCFAs by microflora are influenced by a lot of factors - from the type and amount of food “raw materials” to variations in bacterial composition, which are inherent in the early stages of the organism’s development. On the other hand, dietary fiber prevents metabolic disorders regardless of the composition of the microflora. Moreover, the preventive effect of a predominantly plant-based diet regarding the development of atherosclerosis was shown by studies related to the biotransformation of L-carnitine: it is some intestinal bacteria, quite useful from other points of view, that convert the L-carnitine contained in red meat into atherogenic substances [7], [ 12].
Another competition article tells a fascinating story about the colonization of the human body by bacteria and the leading role of colonizers in the formation of “correct” immune reactions of the host: “The intestinal microbiome: the world within us” [13]. - Ed.
Intestinal bacteria are able to reduce the level of triglycerides in the blood, improve glucose and lipid metabolism also through direct participation in the circulation of bile acids, and reduce fat reserves by activating the already mentioned lipoprotein lipase inhibitor [10]. But it is still difficult to draw any conclusions: sometimes the results of experiments are too contradictory. A new review will help to get an idea of the contradictions and their causes, and most importantly, of the possible mechanisms connecting the activity of the microbiota with the host metabolism [10].
What to do?
If your body, despite proper nutrition, experiences intestinal dysbiosis, then you need probiotics. They contain significantly more beneficial bacteria than fermented milk products.
The drug "Bifidum BAG" contains bifidobacteria of the species B. bifidum and B. longum, which form the basis of the human intestinal microflora. The bacteria titer is 1011 – the same number of bacteria is contained in 1 ton of kefir.
“Bifidum BAG” is recommended for dysbacteriosis, during and after treatment with antibiotics, for disorders of the gastrointestinal tract, allergic diseases, and decreased immunity. "Bifidum BAG" normalizes intestinal microflora, disturbed due to unfavorable conditions.
Antibiotics and growth hormones in products
However, even if a modern person adheres to proper nutrition, this does not mean that there will be no microflora disturbances in his body. The products we consume every day - milk, meat, eggs - contain small amounts of antibiotics and growth hormones, which are used in raising animals. These substances accumulate in the human body over time and poison it. Even in small quantities, antibiotics lead to the death of beneficial bacteria in the human body.
Is there an alternative to antibiotics?
The vast majority of antibiotics used in medicine . They have become so firmly established in everyday life that many doctors, out of habit, prescribe them even for minor reasons (say, for a simple cold). And people who are accustomed to trusting people in white coats regularly follow all the recommendations. With all the ensuing consequences that are described above. However, it is encouraging that recently there has been a tendency to abandon the use of synthetic antibiotics “for all occasions.” Today there is an understanding that if, in the treatment of a particular disease, an artificial antibiotic is not a mandatory drug and, in principle, one can do without its use, then it is better to turn to an alternative - antibiotics of plant origin. Moreover, the medicinal properties of plants have been known for a very long time.
Many of them - for example, elecampane, licorice and bearberry - contain glycosides. Many of these organic compounds (they are also called theol glycosides or phytoncides) are similar in their action to antibiotics . Their use has proven effective in the fight against harmful microorganisms, but there are no side effects, not to mention addiction and complications. Experts recommend: if treating your disease in an acute form is impossible without synthetic antibiotics, then use natural antibiotics for its chronic course. Having, of course, agreed on this issue with your doctor, because medications should also not be taken uncontrolled. In addition to the above-mentioned plants, onions and garlic, horseradish and cranberries, apples, citrus fruits and dogwoods . Garlic also contains the substance allin. Interacting with food enzymes, it turns into allicin, which is known for its pronounced bactericidal properties.
Pathogenic microorganisms do not feel very comfortable in the vicinity of phytoalexins - substances that are a type of phytoncides . Their amazingness is that at first they are simply absent from plants. But as soon as pathogenic microbes appear “on the horizon,” the process of their synthesis immediately begins. Phytoalexins can be formed in potatoes, carrots, peppers and a number of other vegetable crops.
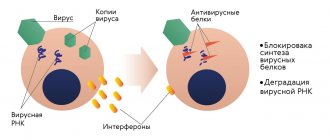
Another effective alternative to artificial antibiotics is called bacteriophages . They have not yet found widespread use, but in medical circles they are sure that everything is moving towards this. Bacteriophages are some types of viruses that have a selective effect on certain bacteria. That is, when they treat specific diseases, only causative microorganisms will be destroyed. Since each illness has its own bacteriophage, there is no need to fear damage to beneficial intestinal microflora. Bacteriophages, unlike conventional antibiotics, do not have any effect on internal processes in the body and certainly do not disrupt their course. But there are also disadvantages: it is difficult to select “their” viruses for certain pathogenic bacteria. And treatment with bacteriophages usually takes longer than a traditional course of antibiotics. But both doctors and patients are inclined to believe that these disadvantages can be easily accepted and lived with. The main thing is that bacteriophages do not cause damage to the human body, which is already weakened due to the underlying disease.
"Bio/mol/text"-2016
This work was published in the “Free Topic” category of the “bio/mol/text” competition 2016.
The general sponsor of the competition, according to our crowdfunding, was entrepreneur Konstantin Sinyushin, for which he has great human respect!
The audience award was sponsored by.
The sponsor of the publication of this article is Alexey Petrovich Semenyaka.
The role of intestinal microflora in the regulation of many body processes has long been known. For example, it forms a protective barrier for the intestinal mucosa, stimulates the immune system [1], neutralizes toxins, produces vitamins, digests fiber, and much, much more. But science, as we know, does not stand still, and new data appear. For example, the relationship between the state of the intestinal microflora and obesity, as well as the development of type 2 diabetes mellitus (DM) is now being considered!
Fermented products
In the times of our ancestors, a significant proportion of the diet consisted of fermented foods. For the winter, sauerkraut was prepared in large quantities, cucumbers and tomatoes were salted in barrels. And this is no coincidence - sauerkraut products (and especially sauerkraut) are of great benefit to our body.
Fermentation is a cooking method based on lactic acid fermentation. Fermented foods contain lactic acid bacteria, which help maintain intestinal microflora. At the same time, lactic acid accumulates in the fermented product, which is secreted by lactic acid bacteria participating in the process and is a natural preservative.
The main thing is to prepare such products using natural fermentation, without adding vinegar and sugar.
Impact of antibiotics on microflora
Antibiotics are indispensable in the treatment of a large number of serious diseases of the respiratory tract, ENT organs, genitourinary system, and infectious intestinal diseases. Therefore, one can hardly expect that medical practice will abandon their use - and this is not necessary! But, since these substances do not have a selective effect, destroying both pathogens and beneficial bacteria, their side effects are often no less pronounced than the therapeutic effect. Much to our regret.
The most common side effect is considered to be indigestion. Stool upset and so-called antibiotic-associated diarrhea (AAD) . The frequency of its occurrence directly depends on the class of drugs taken and varies within 30%. The development of AAD directly stems from dysbiosis , which occurs due to the destruction of beneficial intestinal microflora. As a result, the human body is left without its natural helpers, which contribute to our absorption of useful substances, take part in the synthesis of vitamins, regulate stool and improve intestinal motility. One of the main targets of antibiotics are lacto- and bifidobacteria, which die in large numbers from such drugs. Instead, colonies of opportunistic microorganisms begin to grow, releasing their metabolic products - toxins. abdominal pain and a number of other unpleasant symptoms occur Colitis is also common in such cases .
The metabolic function of the intestine , or rather the microflora inhabiting it, seriously Carbohydrates are poorly digested, which leads to their poor absorption by the body. The process of breaking down fiber into SCFAs - short-chain fatty acids - is disrupted . As a result, the intestinal cells begin to experience a lack of nutrition, and the trophism of its mucous membrane deteriorates. Water absorption and bile acid metabolism are also impaired. Since the latter actively stimulate the production of intestinal secretions, secretory diarrhea occurs.
Under the influence of antibiotic drugs, colonization resistance of beneficial intestinal microorganisms decreases. They become unable to suppress the growth of harmful bacteria with the same effectiveness. In competition with the latter, lacto- and bifidobacteria noticeably lose their positions and gradually pathogenic microflora begins to “host” the receptors of the intestinal mucosa. Result: local immunity due to decreased production of immunoglobulin A and lysozyme is noticeably reduced . If the mechanism of the destructive effect of antibiotics on beneficial microflora is not stopped, then inflammatory processes in the colon mucosa and its inflammation become so pronounced that restoring the original state of health becomes a very difficult task, requiring a lot of effort and time.