Sjögren's syndrome - what is it? Causes, symptoms and treatment
Sjogren's syndrome (“dry syndrome”) is manifested by a decrease in the function of the exocrine glands; as a result of this pathology, severe dryness of the skin and mucous membrane of the vagina, trachea, nasopharynx, eyes, and oral cavity appears, and a decrease in the secretion of digestive enzymes produced by the pancreas is also observed.
Most often, this syndrome accompanies a number of autoimmune pathologies of connective tissue - dermatomyositis, scleroderma, and in such cases is called secondary Sjögren's syndrome. If the pathology develops independently, then the name sounds like primary Sjogren's syndrome, or Sjogren's disease.
Pathomorphology
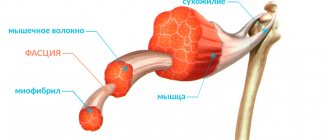
The main morphological feature is the infiltration of the exocrine glands with lymph and plasma cells. First of all, the lacrimal and salivary glands are affected, a little less often – the glands of the bronchi, digestive tract and vagina.
- Both large and small glands are affected. At first, at an early stage of the disease, only small ducts are involved in the process; as it progresses, its infiltrate spreads further to the gland tissue itself, as a result of which the glandular tissue atrophies and is replaced by connective tissue. In some cases, the infiltrates described above occur not only in the exocrine (external secretion) glands, but also in other organs and systems of the body, in particular in the muscles, lungs and kidneys. This ultimately leads to dysfunction of the affected organ.
- In 30-40% of patients, in the material taken by biopsy of the salivary glands, metaplasia (modification) of the cells lining the ducts is determined: myoepithelial islands appear.
The lobules of the affected glands are destroyed in some patients, while in others the lobular structure is preserved. The glands are either enlarged in size or within normal limits.
Interestingly, even in the absence of clear clinical symptoms of Sjögren's syndrome, a patient with any connective tissue disease will most likely show histological signs of inflammation of the salivary glands.
Quality of life of patients with Sjögren's disease during basic therapy
Quality of life of patients with Sjögren's disease during basic therapy
E.Yu. Gan, Ph.D., rheumatologist of the highest category at a self-supporting clinic1, assistant at the Department of Therapy2
L.P. Evstigneeva, Doctor of Medical Sciences, Head. Department of Rheumatology1, Associate Professor of the Department of Therapy2, Ch. freelance specialist rheumatologist of the Ministry of Health of the Sverdlovsk region
1Sverdlovsk Regional Clinical Hospital No. 1, Yekaterinburg
2Faculty of advanced training and professional retraining of the Federal State Budgetary Educational Institution of Higher Education "Ural State Medical University" of the Ministry of Health of Russia, Yekaterinburg
Quality of life of patients with Sjogren's disease with ongoing therapy with disease-modifying antirheumatic drugs
E.Yu. Gan, L.P. Evstigneeva
Sverdlovsk Regional Clinical Hospital N 1, Ural State Medical University; Ekaterinburg, Russia
Summary
Purpose of the study. Assessment of the association of the level of quality of life of patients with Sjögren's disease with therapy with disease-modifying antirheumatic drugs. Material and methods. The study was conducted on the basis of the regional rheumatology center of the consultative and diagnostic clinic of the Sverdlovsk Regional Clinical Hospital No. 1. The work is based on the results of a one-time study of 74 patients with Sjögren's disease (SD), distributed into three comparison groups, receiving various basic antirheumatic drugs chlorambucil, methotrexate and hydroxychloroquine . The diagnosis of BS was made using the European-American AECGC criteria (2002) [18]. To analyze the quality of life of patients with BS, the 36-Item Short Form Health Survey (SF-36) questionnaire was used [28]. Statistical data processing was carried out using the Statistica 7.0 program. Research results. Assessment of the quality of life of patients with BS, which is an integrative criterion of human health and well-being, revealed the absence of statistically significant differences (p > 0.05) on eight scales and two health components of the SF-36 questionnaire in the analyzed groups, which differed in the treatment with disease-modifying antirheumatic drugs drugs chlorambucil, methotrexate and hydroxychloroquine. Conclusion. The data obtained indicate an equivalent association of the level of quality of life of patients with BS with therapy with disease-modifying antirheumatic drugs (methotrexate, chlorambucil and hydroxychloroquine), and therefore hydroxychloroquine can be considered as an alternative basic therapy in patients with BS who have certain limitations and contraindications to prescribing methotrexate and chlorambucil.
Key words: Sjögren's disease, quality of life, hydroxychloroquine, chlorambucil, methotrexate.
Summary
Purpose of the study. Assessing the association between the life quality of patients with Sjogren's Disease and ongoing therapy with various disease-modifying antirheumatic drugs. Materials and methods. The study was conducted on the basis of the regional rheumatology center of the consultative diagnostic clinic of the Sverdlovsk Regional Clinical Hospital No. 1. This work is based on the results of a simultaneous study of 74 patients with primary Sjogren's Disease (SD), distributed in three comparison groups receiving various disease-modifying antirheumatic drugs chlorambucil, methotrexate and hydroxychloroquine. The diagnosis of SD was carried out according to European-American criteria AECGC (2002) [18]. In order to analyze the quality of life of patients with SD, the 36-Item Short Form Health Survey (SF-36) was used. Statistical data processing was carried out using Statistica 7.0 program. Results. Assessment of the quality of life of patients with SD, which is an integrative criterion of human health and well-being, revealed the absence of statistically significant differences (p > 0.05) on eight scales and two health components of the SF-36 questionnaire in the analyzed groups that differ in the treatment of disease-modifying antirheumatic drugs chlorambucil, methotrexate and hydroxychloroquine. Conclusions. The obtained data indicate an equivalent quality of life in SD patients treated with different disease-modifying antirheumatic drugs methotrexate, chlorambucil and hydroxychloroquine, and therefore hydroxychloroquine can be considered as an alternative basic therapy in patients with SD with certain limitations and contraindications methotrexate and chlorambucil.
Key words: Sjogren's disease, quality of life, hydroxychloroquine, chlorambucil, methotrexate
Introduction
At the present stage of development of medical science, systemic pathology of connective tissue is becoming common in the practice of a rheumatologist. One of the representatives of this group of diseases is primary Sjögren's syndrome (Sjögren's disease), accompanied by predominant damage to the lacrimal (with the development of sicca keratoconjunctivitis) and salivary glands (with the development of parenchymal sialadenitis) glands with possible involvement of various organs in the pathological process and the presence of laboratory and immunological activity [1 ]. The incidence of extraglandular manifestations in Sjögren's disease (SD), according to various authors, ranges from 30 to 60% [2, 3].
The prevalence of BS in the population is 0.5–1.0% and 2.0–4.8% among people over 50 years of age [1]. Moreover, BS ranks second in frequency after rheumatoid arthritis among autoimmune rheumatic diseases [4]. Women get sick nine times more often than men. The onset of BS usually occurs between the ages of 20 and 50 years. Menopausal women (40–50 years old) are more often affected [5, 6]. As is known, early onset of BS is usually associated with a more aggressive course of the pathological process, the development of systemic manifestations and high laboratory and immunological activity.
Pathogenetically, BS is based on hyperactivity of B cells followed by the production of autoantibodies. From a morphological point of view, BS is characterized by focal periductal lymphoplasmacytic infiltration with the formation of lymphoid follicles and the possible formation of “germinal” centers in the glandular tissue [2], which is often the background for the subsequent development of lymphoproliferative diseases (LPD), which rank second in the structure of mortality in described pathology [1, 7]. The incidence of lymphoma in patients with BS reaches 5–10% [7, 8, 9]. The most common are mucous-associated lymphoid tissue (MALT) lymphomas, which account for 60% of cases, nodal marginal zone lymphomas (NMZL) and diffuse large B-cell lymphoma (DLBCL) [9, 10].
Currently, three groups of predictors of the development of lymphoproliferation in BS have been developed: clinical, immunological and histological [7]. Clinical markers include persistent enlargement of the salivary glands, lymphadenopathy, cryoglobulinemic vasculitis, peripheral neuropathy, glomerulonephritis, Raynaud's phenomenon, and consistently moderate or high disease activity (according to the ESSDAI index) [11]. Immunological predictors of transformation of BS into LPZ include monoclonal gammopathy, cryoglobulinemia, high rheumatoid factor, C4 hypocomplementemia, high levels of SSA and SSB antibodies, as well as leukopenia. Histological risk factors for the development of LPD are a high focal count and the presence of “germinal” centers in the salivary gland biopsy. The focus in BS is understood as a focus of periductal lymphoid infiltration with a number of lymphocytes of 50 or more within 4 mm2 of gland tissue [7].
The overall 5-year survival rate for developing lymphomas is currently 92%. At the same time, the survival rate is better in MALT lymphomas (94.12%), the prognosis is worse in NMZL (87.50%) and DLBCL (75.00%) [7].
At the present stage of development of medical science, therapeutic tactics in relation to patients with BS raises many questions and discussions, which is due to the lack of certainty regarding the etiological and pathogenetic aspects of the development of the disease, as well as a very heterogeneous picture of the course of the disease in different patients.
The goal of the therapy is to reduce the severity of clinical manifestations of BS, reduce laboratory and immunological activity and prevent lymphoid proliferation. Ultimately, treatment is aimed at achieving remission of the disease, slowing down the rate of its progression, reducing the risk of complications, primarily lymphoproliferative processes, and improving the quality of life of patients with BS as an integrative indicator of human health and well-being, which is a significant and objective criterion for the success of the therapy [1 , 7, 12].
Since today the etiological aspects of BS remain completely unclear, the main role in achieving the goals and objectives of therapy is played by local therapy of dry syndrome, basic pathogenetically based treatment with disease-modifying antirheumatic drugs, genetically engineered biological drugs, of which rituximab is in the first place [ 1, 13, 14].
Russian clinical guidelines recommend the use of cytotoxic drugs (chlorambucil and cyclophosphamide) as the basic antirheumatic therapy for BS due to their effectiveness in relation to the clinical and laboratory activity of the disease and the prevention of the development of lymphoproliferative processes [1, 14]. At the same time, due to the high risk of adverse events and side effects, the range of indications for cyclophosphamide therapy is limited primarily by the involvement of vital organs in the process (lungs, kidneys, nervous system, vasculitis). Glucocorticoids in small doses are recommended for patients with recurrent sialadenitis and minimal systemic manifestations, such as articular syndrome, as well as as part of combination therapy with chlorambucil and cyclophosphamide [14].
In real domestic clinical practice, methotrexate is often used in the treatment of patients with BS due to its potential effectiveness aimed at relieving articular syndrome and reducing the laboratory activity of the disease, which is also considered as a possible alternative in the treatment of BS in foreign publications [2].
In a pilot study assessing the effectiveness of methotrexate in BS at a dose of 0.2 mg per 1 kg of body weight per week, positive dynamics were shown in subjective sensations of dry eyes and mouth, but according to objective criteria (Schirmer test and sialometry) there was no dynamics. There was no effect on the level of immunoglobulin G and erythrocyte sedimentation rate (ESR) [15].
At the same time, the above antirheumatic drugs (cyclophosphamide, chlorambucil, methotrexate) have high cytotoxic activity, associated not only with their effectiveness, but also with the frequent development of adverse events and side effects (toxic hepatitis, cytopenia, viral and bacterial infections, mutagenic and teratogenic effects and etc.), which often casts doubt on the possibility and feasibility of carrying out the discussed active basic therapy. These issues are of particular relevance in case of existing initial cytopenia (usually leukopenia) or a tendency to its development, the presence of hepatitis of various origins, accompanied by an increase in liver transaminases, chronic infections, comorbid pathology, with low clinical and laboratory activity of BS, as well as when planning pregnancy. This problem is partially solved by adding glucocorticoids (GC) to the treatment. However, this category of drugs often has its own contraindications and side effects, such as erosive and ulcerative processes of the gastrointestinal tract, arterial hypertension, diabetes mellitus, osteoporosis, cataracts, etc. In this case, it is necessary to carefully weigh the benefit-risk ratio when selecting and planning therapy.
In connection with these circumstances, it is of interest to consider the possibility of an alternative use of hydroxychloroquine as a basic therapy for BS, the prescription of which would be justified in patients with low clinical and laboratory activity of the process, the presence of contraindications to therapy with GCs and cytostatic drugs, side effects and complications as a result of their use , as well as in cases where the potential risk exceeds the expected benefit.
In foreign recommendations, the modern approach to the choice of treatment tactics for patients with BS is based on the course of the disease and is focused on the leading clinical syndrome: articular syndrome (arthralgia, arthritis), “dry” syndrome (xerostomia, xerophthalmia), fatigue syndrome (fatigue) and systemic syndrome (vasculitis, involvement of the lungs, kidneys, nervous system) [3]. At the same time, in case of BS, the possibility of using various disease-modifying antirheumatic drugs, including hydroxychloroquine, is being discussed.
In this regard, more and more studies and publications in foreign publications are devoted to studying the effectiveness and safety of hydroxychloroquine therapy for patients with BS. According to the literature, the main points of use of hydroxychloroquine for BS are joint syndrome and fatigue syndrome [3, 16, 17, 18]. Research results on the effectiveness of hydroxychloroquine are mixed. The Sjogren's Syndrome Foundation (SSF) guidelines recommend hydroxychloroquine as first-line treatment for articular syndrome in patients with HD [20]. The EULAR guidelines (2019) [13] report two RCTs with no statistically significant improvement in clinical symptoms such as dry mucous membranes, fatigue and pain [21, 22]. However, in the study by J.-E. Gottenberg, in the absence of dynamics in relation to the clinical manifestations of BS, positive laboratory dynamics of laboratory activity were found [21]. A study by A. Kruize revealed a trend towards a decrease in ESR and a significant decrease in the level of immunoglobulins G and M in the blood [23]. The literature also contains data on the effectiveness of hydroxychloroquine in BS on pathogenetic and, as a consequence, clinical characteristics [24]. Thus, G. Mumcu et al. revealed a statistically significant decrease in the level of B-cell activation factor (BAFF) and an increase in the level of sialometry in patients with BS during therapy with hydroxychloroquine. Based on the results of the study, the authors concluded that hydroxychloroquine may have an effect on increasing salivation, as well as reducing the degree of BS activity by reducing the level of BAFF, which is one of the leading aspects in the pathogenesis of BS [24].
From a practical point of view, it seems interesting to evaluate the effectiveness of BS therapy with leflunomide. Thus, van Woerkom et al. A study was conducted, the results of which showed, during therapy, a decrease in fatigue and the level of immunoglobulins G, A and M, rheumatoid factor, and a tendency to increase the Schirmer test. Five of the 15 patients underwent repeat biopsy of the minor salivary gland, four of them showed a decrease of 1 focus/4 mm2 [25].
A study of combination therapy with hydroxychloroquine and leflunomide has been conducted, the results of which are expected to be published [2, 26].
In recent years, studies on the effectiveness of genetically engineered biological drugs have been published and systematized [13], most of which assessed the effect of rituximab. Russian scientists have described a positive experience of treating a patient with BS with rituximab and belimumab in the form of combination therapy [27].
In addition to the subjective and objective clinical signs of BS, laboratory-immunological and histological activity of the disease, reflecting the severity of the pathological process, an extremely important integrative indicator of health is the level of quality of life of patients with BS [28]. In recent years, there have been many publications indicating a decrease in the quality of life of patients with BS compared to the healthy population [20, 29]. However, there is practically no data regarding the assessment of the relationship between the quality of life of patients with BS and the basic drug therapy they receive, which was the reason for conducting this study.
Purpose of the study: to assess the association of the level of quality of life of patients with Sjögren's disease with therapy with disease-modifying antirheumatic drugs.
Material and methods
The study was conducted on the basis of the regional rheumatology center of the consultative and diagnostic polyclinic of the State Autonomous Institution of Health Care "Sverdlovsk Regional Clinical Hospital No. 1". The work is based on the results of a cross-sectional study of 74 patients with Sjogren's disease, divided into three comparison groups, receiving various disease-modifying antirheumatic drugs chlorambucil, methotrexate and hydroxychloroquine.
Criteria for inclusion of patients in the study: age not older than 75 years, voluntary consent to participate in the examination, absence of mental illness, oncological pathology, severe concomitant somatic diseases, severe encephalopathy and other autoimmune diseases (besides BS). The duration of treatment with disease-modifying antirheumatic drugs was at least 3 years.
The diagnosis of BS was made using European-American criteria (American-European Consensus Group Criteria for Sjögren's Syndrome, 2002) [18]. To assess the degree of activity and course of BS, the classification developed by the Institute of Rheumatology of the Russian Academy of Medical Sciences (2011) [1] and the ESSDAI activity index (EULAR Sjogren Syndrome Disease Activity Index, 2009) [11] were used.
In order to analyze the quality of life of patients with BS, based on an assessment of their physical, mental and social well-being, the 36-Item Short Form Health Survey (SF-36) questionnaire was used as a diagnostic tool [28]. The Russian-language version of the SF-36 questionnaire has been validated by the International Center for Quality of Life Research (ICSQL). In the process of studying the psychometric properties, the reliability, validity and sensitivity of the questionnaire were confirmed, and population data on indicators of the quality of life of residents of St. Petersburg were obtained. This technique reflects the general well-being of a person and his level of satisfaction with those aspects of life that are influenced by his health status. The questionnaire consists of 36 questions combined into eight scales: physical functioning (PF), role physical functioning (RPF), bodily pain (TB), general health (GO), vitality (V), social functioning (SF), role emotional functioning (REF) and mental health (MH). The scales are grouped into two health components – physical (PHC), which includes the first four scales, and mental (MCH), which combines the next four scales, which were calculated using special formulas [30].
Statistical data processing was carried out using nonparametric tests (Mann-Whitney test, χ2). Data that do not have a normal distribution are expressed through the median (Me), the range of values is expressed through the lower and upper quartiles (LQ–UQ). Differences were considered statistically significant at p < 0.05. The Statistica 7.0 program was used.
Results and discussion
All patients with BS included in the described study were female. The median age of patients in the study group was 56 (LQ–UQ: 51–61) years. In accordance with the severity of clinical and laboratory activity at the time of the study, all patients were divided into subgroups corresponding to minimal (I) - 51 (54.84%), moderate (II) - 28 (30.11%) and high (III) – 14 (15.05%) degrees of BS activity.
The majority of patients, 52 (55.91%), were diagnosed with a subacute variant of the course of BS, and 41 (44.09%) patients were diagnosed with a chronic variant. The median disease duration was 6 (LQ–UQ: 3–8) years. The median age of onset of the disease is 50 (LQ–UQ: 44–56) years. Most patients, in addition to basic disease-modifying drugs, took GCs at a dose of 5–10 mg per day in terms of prednisolone, without statistically significant differences between the groups.
An assessment of the structure of basic antirheumatic therapy showed a predominance in the study group of patients receiving methotrexate (about 52.7%), and an almost equal number of patients taking hydroxychloroquine (23.0%) and chlorambucil (24.3%) (Table 1). There were no patients taking cyclophosphamide, leflunomide and rituximab in the described study group.
Table 1
Distribution of patients with Sjögren's disease, taking into account the basic antirheumatic therapy performed
| Basic antirheumatic therapy | Number of patients ( n = 74) | |
| Absolute | Relative, % | |
| Receive chlorambucil (2–4 mg per day) | 18 | 24,3 |
| Receive methotrexate (7.5–15 mg per week) | 39 | 52,7 |
| Receive hydroxychloroquine (400 mg per day) | 17 | 23,0 |
When analyzing the duration of basic therapy, it was found that all patients using hydroxychloroquine and chlorambucil received it from 3 to 5 years. Among the patients taking methotrexate, 33 patients used it for 3 to 5 years, 6 patients for more than 5 years.
All three groups of patients did not differ significantly from each other in age (p = 0.655) and duration of BS (p = 0.183), as well as in the nature of the course (p = 0.216) and degree of activity (p = 0.716) of the pathological process at the time of the study.
A comparative assessment of the level of quality of life of patients with BS was carried out in three groups depending on the basic antirheumatic drug taken (chlorambucil, methotrexate or hydroxychloroquine). The obtained data are presented in table. 2.
table 2
Differences in quality of life indicators in patients with Sjögren's disease depending on the basic antirheumatic drug taken
| QoL scales | Chlorambucil (1), Me ( LQ– UQ) | Methotrexate (2), Me ( LQ– UQ) | Hydroxychloroquine (3), Me ( LQ– UQ) | Significance, p |
| FF | 70,00 (60,00–85,00) | 50,00 (35,00–75,00) | 50,00 (35,00–70,00) | p1–2 = 0.166 p1–3 = 0.316 p2–3 = 1 |
| RFF | 50,00 (25,00–100,00) | 0,00 (0,00–50,00) | 0,00 (0,00–75,00) | p1–2 = 0.153 p1–3 = 0.207 p2–3 = 1 |
| TB | 45,00 (40,00–50,00) | 40,00 (20,00–60,00) | 40,00 (30,00–60,00) | p1–2 = 0.541 p1–3 = 1 p2–3 = 1 |
| OZ | 45,00 (40,00–65,00) | 45,00 (30,00–52,00) | 40,00 (25,00–57,00) | p1–2 = 0.532 p1–3 = 0.546 p2–3 = 1 |
| AND | 50,00 (45,00–65,00) | 40,00 (30,00–55,00) | 35,00 (30,00–55,00) | p1–2 = 0.679 p1–3 = 0.612 p2–3 = 1 |
| SF | 62,50 (62,50–87,50) | 62,50 (37,00–75,00) | 62,50 (50,00–87,50) | p1–2 = 0.185 p1–3 = 1 p2–3 = 1 |
| REF | 50,00 (33,33–100,00) | 33,33 (0,00–100,00) | 33,33 (0,00–100,00) | p1–2 = 0.735 p1–3 = 0.935 p2–3 = 1 |
| PZ | 60,00 (52,00–72,00) | 52,00 (32,00–60,00) | 48,00 (44,00–64,00) | p1–2 = 0.205 p1–3 = 0.415 p2–3 = 1 |
| FKZ | 39,08 (35,01–43,27) | 34,31 (26,74–42,03) | 33,65 (27,56–43,78) | p1–2 = 0.182 p1–3 = 0.568 p2–3 = 1 |
| PKZ | 41,09 (37,72–55,02) | 39,43 (30,43–47,71) | 38,95 (33,59–46,72) | p1–2 = 0.560 p1–3 = 1 p2–3 = 1 |
Note: QOL - quality of life, FF - physical functioning, RFF - role physical functioning, TB - bodily pain, OH - general health, F - vitality, SF - social functioning, REF - role emotional functioning, MH - mental health, FKZ - physical component of health, PKZ – mental component of health.
As can be seen from table. 2, the indicators of patients' quality of life fluctuated over a wide range, especially when assessing role-emotional functioning. Role-emotional functioning and role-physical functioning were the lowest. In the group of patients taking chlorambucil, the quality of life in a number of domains was higher compared to patients taking methotrexate and hydroxychloroquine, but the differences did not reach statistical significance. Both health components (physical health component and mental health component) were almost equally reduced in all patients; their values did not differ significantly between groups of patients taking various disease-modifying antirheumatic drugs.
In general, for eight scales (FF, RFF, OZ, TB, F, SF, Z, REF) and for both health components (FCZ and PKZ) of the SF-36 questionnaire, no statistically significant differences were revealed (p > 0.05) between the groups patients taking chlorambucil, methotrexate and hydroxychloroquine. The data obtained may indicate an equivalent association of the level of quality of life of patients with BS with the use of disease-modifying antirheumatic therapy with methotrexate, chlorambucil and hydroxychloroquine.
Treatment of Sjögren's disease is challenging. It should be noted that there are difficulties in treating patients with BS due to the fact that approaches to systemic therapy for this pathology are currently insufficiently developed. Drugs that have proven their effectiveness in a number of systemic connective tissue diseases and systemic vasculitis do not find unambiguous confirmation in BS [13]. In addition, none of the synthetic disease-modifying and biological drugs in the Russian Federation have registered indications for Sjögren's disease. Taking into account the fact that when treating patients, not only the prognosis in terms of the risk of developing LPZ is important, but also the quality of life at the current moment in time, in a number of patients, along with other disease-modifying antirheumatic drugs, hydroxychloroquine can be used, which has shown equivalent indicators of quality of life scales when comparison with methotrexate and chlorambucil. The good safety profile of the drug is also important: in clinical studies, no serious adverse events or retinopathy were reported in the treatment of BS [13].
Based on the results of the study, we believe that hydroxychloroquine can be used as a basic therapy in patients with BS who have certain restrictions and contraindications to the use of methotrexate and chlorambucil, taking into account the similar indicators of the level of quality of life of patients obtained in the comparison groups, which is an integrative criterion of human health and well-being .
Conclusion
The data obtained as a result of the study indicate the existence of an alternative possibility of prescribing hydroxychloroquine as a basic therapy for patients with BS, in terms of its similar association with the quality of life of patients when compared with methotrexate and chlorambucil. This is an important aspect due to the fact that, in general, hydroxychloroquine has a lower risk of adverse events and side effects, and has fewer contraindications for use than chlorambucil and methotrexate, which often limits the possibility of using the latter in patients with BS.
Based on the information obtained, we believe that hydroxychloroquine can occupy its niche as a basic therapy for patients with BS with low activity of the pathological process, the presence of contraindications and the development of side effects during therapy with chlorambucil and methotrexate.
The results obtained seem interesting to us and indicate the need for further prospective studies to assess the effect of disease-modifying antirheumatic drugs on the quality of life of patients with BS, as well as on the dynamics of clinical, immunological and histological manifestations of the pathological process.
Limitations of the study
A limitation of the study is the small sample size, which could have contributed to the lack of statistically significant differences between groups. It should also be noted that the SF-36 questionnaire is not specific and its indicators are influenced by other components of the health assessment, including concomitant diseases and conditions. In addition, the lack of randomization in retrospective studies cannot ensure comparability of groups for all characteristics influencing the outcome. New prospective randomized studies are needed to confirm the hypothesis that different disease-modifying antirheumatic drugs have equivalent effects.
Bibliography
- Safonova T.N., Vasiliev V.I., Likhvantseva V.G. Sjögren's syndrome: A guide for doctors / T.N. Safonova, V.I. Vasiliev, V.G. Likhvantseva; edited by V.G. Likhvantseva. M.: Moscow University Publishing House, 2013. 600 p.
- Van der Heijden E, Kruize A, Radstake T, van Roon J. Optimizing conventional DMARD therapy for Sjögren's syndrome. // Autoimmunity Reviews. 2021. Vol. 17. P. 480–492. doi.org/10.1016/j.autrev.2018.03.003.
- Del Papa N, Vitali C. Management of primary Sjögren's syndrome: recent developments and new classification criteria. // Ther Adv Musculoskel Dis. 2021. Vol. 10 (2). P. 39–54. doi.org/10.1177/1759720×17746319.
- Tzioufas AG, Vlachoyiannopoulos PG. Sjögren's syndrome: an update on clinical, basic and diagnostic therapeutic aspects. //J Autoimmun. 2012. Vol. 39 (1–2). P. 1–3.
- Maldini C, Seror R, Fain O, et al. Epidemiology of primary Sjögren's syndrome in a French multiracial/multiethnic area. // Arthritis Care Res. 2014. doi.org/10.1002/acr.22115.
- Shelomkova O.A., Veltishchev D.Yu., Vasiliev V.I., Lisitsyna T.A. Stress factors and mental disorders in Sjögren's disease: current directions of research. // Scientific and practical rheumatology. 2012. – No. 54 (5). pp. 85–89.
- Alunno A, Leone MC, Giacomelli R, et al. Lymphoma and Lymphomagenesis in Primary Sjögren's Syndrome. //Front. Med. 2021. DOI: 10.3389/fmed.2018.00102.
- Nocturne G, Mariette X. Advances in understanding the pathogenesis of primary Sjögren's syndrome. // Nat Publ Gr. 2013. Vol. 9. DOI: 10.1038/nrrheum.2013.110.
- Singh AG, Singh S, Matteson EL. Rate, risk factors and causes of mortality in patients with Sjogren's syndrome: a systematic review and meta-analysis of cohort studies. // Rheumatology (Oxford). 2021. Vol. 55. P. 450–460. DOI: 10.1093/rheumatology/kev354.
- Chiu YH, Chung CH, Lin KT, Lin CS, Chen JH, Chen HC, et al. Predictable biomarkers of developing lymphoma in patients with Sjögren syndrome: a national population-based cohort study. // Oncotarget. 2021. Vol. 8 (30). P. 50098–500108. DOI: 10.18632/oncotarget.15100.
- Seror R, Bowman SJ, Brito-Zeron P, et al. EULAR Sjögren's syndrome disease activity index (ESSDAI): a user guide. // RMD Open. 2015. DOI: 10.1136/rmdopen-2014-000022.
- Ramos-Casals M, Tzioufas AG, Stone JH, et al. Treatment of primary Sjögren syndrome: a systematic review. // JAMA. 2010. Vol. 304(4). P. 452–460.
- Ramos-Casals M., Brito-Zeron P., Bombardieri S., et al. // EULAR-Sjogren Syndrome Task Force Group. EULAR recommendations for the management of Sjogren's syndrome with topical and systemic therapies. // RMD Open. 2021. Vol. 5(2):e001064.
- Vasilyev V.I. Disease (Sjögren's syndrome). Russian clinical recommendations / edited by E. L. Nasonov. M.: GEOTAR-Media. 2017. pp. 228–239.
- Skopouli F, Jagiello P, Moutsopoulos H. Methotrexate in primary Sjögren's syndrome. // Clin Exp Rheumatol. 1996. Vol. 14. P. 555–558.
- Carsons SE, Vivino FB, Parke A, et al. Treatment Guidelines for Rheumatologic Manifestations of Sjögren's: Use of Biologics, Management of Fatigue and Inflammatory Musculoskeletal Pain. // Arthritis Care and Research. 2021. DOI: 10.1002/acr.22968.
- Bowman S, Everett CC, O'Dweyer, et al. Randomized controlled trial and cost-effectiveness analysis in treating fatigue and oral dryness in primary Sjögren's syndrome. // Arthritis Rheumatol. 2017. Vol. 69.
- Leverenz DL, St Clair EW. Recent advances in the search for a targeted immunomodulatory therapy for primary Sjögren's syndrome. // F1000 Res. 2021. Vol. 29. P. 8. DOI: 10.12688/f1000research.19842.1.
- ???
- Vivino FB, Carsons SE, Foulks G, et al. New Treatment Guidelines for Sjogren's Disease. // Rheum Dis Clin N Am. 2021. Vol. 42. P. 531–551. doi.org/10.1016/j.rdc.2016.03.010.
- Gottenberg JE, Ravaud P, Puéchal X, et al. Effects of Hydroxychloroquine on symptomatic improvement in primary Sjögren syndrome the JOQUER randomized clinical trial. // JAMA. 2014. Vol. 312. P. 249–258. DOI: 10.1001/jama. 2014.7682.
- Yoon CH, Lee HJ, Lee EY, et al. Effect of hydroxychloroquine treatment on dry eyes in subjects with primary Sjögren's syndrome: a double-blind randomized control study. // J Korean Med Sci. 2021. Vol. 31. P. 1127–1135.
- Kruize A, Hene R, Kallenberg C, et al. Hydroxychloroquine treatment for primary Sjögren's syndrome: a two year double blind cross over trial. // Ann Rheum Dis. 1993. Vol. 52. P. 360–364.
- Mumcu G, Biçakçigil M, Yilmaz N, et al. Salivary and Serum B-cell Activating Factor (BAF) Levels after Hydroxychloroquine treatment in Primary Sjögren's Syndrome. // Oral Health Prev Dent. 2013. Vol. 11(3). P. 229–234.
- Van Woerkom JM, Kruize AA, Geenen R, et al. Safety and efficacy of leflunomide in primary Sjögren's syndrome: a phase II pilot study. //Ann Rheum Dis. 2007. Vol. 66 (1026). P. 32. doi.org/10.1136/ard.2006.060905.
- Van der Heijden E, Hartgring S, Kruize A, et al. Additive inhibition of interferons, B and T cell activation and Tfh related cytokine CXCL13 by leflunomide and hydroxychloroquine supports rationale for combination therapy in pSS patients. // ARD EULAR J. 2017. Vol. 76(2). P. 1206.
- Benevolenskaya S.S., Korolkova A.A., Myachikova V.Yu. et al. Combined biological therapy with belimumab and rituximab in a patient with Sjögren’s disease. // Therapy. 2021. No. 8. pp. 140–150.
- Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. // Med Care. 1992. Vol. 30 (6). P. 473–483.
- Segal B, Bowman SJ, Fox PC, et al. Primary Sjogren's Syndrome: health experiences and predictors of health quality among patients in the United States. // Health Qualified Life Outcomes. 2009. Vol. 7. P. 46.
- Bagirova G.G. Assessing the quality of life in rheumatology / G.G. Bagirova, T.V. Chernysheva, L.V. Sizova. M.: Binom, 2011. 248 p.
Reasons for development
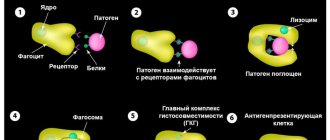
The causes of Sjögren's syndrome have not yet been fully established. Among the most likely is the theory of a pathological reaction of the body's immune system. This reaction develops in response to damage to the cells of the external glands by a retrovirus, in particular the Epstein-Barr virus, herpes virus VI, cytomegalovirus, and human immunodeficiency virus. Despite the significant similarity of immunological disorders with changes in the body affected by the virus, direct evidence of the role of the virus as the cause of the development of pathology has not been obtained.
The viruses themselves and the epithelial cells of the glands changed by their influence are perceived by the immune system as antigens (foreign agents). The immune system produces antibodies against such cells and gradually causes the destruction of gland tissue. The disease often occurs as a hereditary or familial disorder, particularly among twins, suggesting that there is a genetic predisposition.
Thus, it is assumed that in the mechanism of development and occurrence of pathology, a combination of many factors is important:
- stress reaction of the body, which occurs as a result of the immune response;
- immune regulation with the participation of sex hormones, as evidenced by the rare incidence among people under 20 years of age, while girls are most often affected among children;
- immune control using T lymphocytes;
- viral;
- genetic.
There are two types of Sjögren's syndrome: primary, where symptoms of the disease are the first manifestations of it, and secondary, when symptoms appear in patients suffering from other rheumatic diseases, such as scleroderma, rheumatoid arthritis or systemic lupus erythematosus. Primary and secondary variants of the syndrome occur with approximately the same frequency. This sometimes makes it difficult to make an accurate diagnosis. Sjögren's syndrome is quite common: in the UK, for example, there are about half a million sufferers. Women aged 40 to 60 years are most often affected, while only one patient in 13 is male.
Etiology
Etiology
unknown. A number of researchers consider Sjogren's disease as a consequence of the development of immunopathological reactions to various microbial, viral and other antigens. There is a well-known viral hypothesis for the development of Sjogren's disease, based on the detection of virus-like tubuloreticular structures in the epithelium of the salivary glands and the detection of increased levels of antibodies to cytomegalovirus in the blood of patients. As suggested by EJ Shillitoe and co-workers (1982), cytomegalovirus may be an etiological factor in the development of Sjögren's disease, but direct evidence of the viral etiology of the disease has not yet been obtained.
Symptoms of Sjögren's syndrome
Symptoms of the disease are divided into two groups:
- Glandular manifestations in which epithelial glands are affected and their functions are disrupted.
- Extraglandular manifestations. The symptoms of this group vary greatly, due to damage to various human organs.
Glandular symptoms include the following:
- Pathology of the lacrimal glands. In this case, unpleasant and painful sensations occur in the eyes (burning, as if the eyes are starting to sting, a sensation of sand in them), and characteristic itching and redness appear near the eyes. As a result, vision decreases, pinpoint hemorrhages, swelling, sensitivity to light, and eye pain occur.
- Pathology of the salivary glands. The manifestation of inflammation is mainly of the parotid glands, which are increased in size, sometimes with painful sensations. Dryness of the oral mucosa also occurs, swallowing food is difficult, often patients have to wash down food with water due to the problem of swallowing. In this case, the mucous membrane of the mouth takes on a bright pinkish tint.
- Dryness of the respiratory tract, which leads to inflammation of the bronchi, trachea, and lungs.
- Skin disorders resulting in dry skin.
- Damage to the nasal mucosa. The occurrence of dryness in the nose, covering with an intranasal crust, as a result of which inflammation develops.
Extraglandular manifestations are characterized by the following signs:
- The occurrence of tracheobronchitis, accompanied by cough and shortness of breath. It is not uncommon for a patient to be examined for pneumonia or pulmonary fibrosis.
- Enlarged lymph nodes.
- Damage to the peripheral nervous system. At the same time, painful sensations (tingling, burning) begin to appear.
- Fever;
- Pain in joints and muscles.
- Damage to the thyroid gland. It is a rare disorder in which allergic reactions to various foods, medications and other drugs begin to appear.
- Enlarged lymph nodes (submandibular, cervical, occipital). Enlargement of the liver and spleen is often observed.
- Inflammation of blood vessels, which occurs as a result of atherosclerosis of the lower extremities, due to circulatory disorders. When the functioning of blood vessels is disrupted, skin diseases (rashes) appear, which are accompanied by itching, burning and fever.
Often, against the background of the pathology we describe, patients develop increased individual sensitivity to certain drugs, in particular to non-steroidal anti-inflammatory drugs, some antibiotics (penicillin), so-called basic therapy drugs, and cytostatics.
Clinical picture
A distinctive symptom of Sjogren's syndrome is generalized dryness of the mucous membranes, most often including:
- Xerophthalmia (“dry eye”, dry eyes). At the initial stage, patients may not complain. With further progression of the disease, a feeling of burning, stinging, and “sand” appears in the eyes.
- Xerostomia (“dry mouth”, dry mouth). There is a decrease in salivation due to damage to the salivary glands. Chronic mumps, stomatitis, and caries develop. Patients complain of severe dryness in the mouth, “snagging” in the corners of the mouth, difficulty speaking, and in the later stages even difficulty swallowing food (dysphagia).
In addition, Sjögren's syndrome can cause damage to:
- skin - severe dryness;
- nasopharynx - formation of crusts in the nose, development of otitis media with damage to the Eustachian tube, sinusitis;
- vagina - itching, pain;
- respiratory system - tracheobronchitis;
- digestive system - atrophic gastritis with secretory insufficiency, hypokinetic biliary dyskinesia, pancreatitis;
- kidney - glomerulonephritis;
- blood vessels - Raynaud's syndrome;
- peripheral nervous system - polyneuropathy, neuritis of the facial, trigeminal nerve.
A pronounced loss of strength, pain in joints and muscles often develops.
Patients with secondary Sjögren's syndrome have symptoms of a primary rheumatic disease, such as systemic lupus erythematosus, rheumatoid arthritis, or systemic scleroderma.
Diagnostics
Burning eyes and dry mouth may not always mean that a person has this syndrome. Sjögren's syndrome can be diagnosed only in the presence of inflammatory lesions of the glands. However, there are cases when various metabolic diseases can lead to a similar result, in which the secretion of saliva is significantly reduced (most often this is diabetes mellitus).
As a result, the functions of the salivary and lacrimal glands prematurely decline in older people. These forms of dry eyes and dry mouth have nothing to do with Sjögren's syndrome. Tissue examination is also performed to diagnose this syndrome. For this type of study, small fragments of the oral mucosa are taken, which are examined using a microscope. In this way, damage to the mucous glands is established.
Pathogenesis
The autoimmune process leads to apoptosis of secreting cells and the epithelium of the excretory ducts, causing damage to the glandular tissue.
Sjögren's syndrome is associated with increased cerebrospinal fluid levels of IL-1RA, an interleukin-1 (IL-1) antagonist. This suggests that the disease begins with an increase in the activity of the IL-1 system, which entails a compensatory increase in IL-1RA to reduce the binding of IL-1 to receptors. On the other hand, Sjogren's syndrome is characterized by a decrease in the level of IL-1 in saliva, which can lead to inflammation of the oral mucosa and its dryness.
Complications
Common consequences of Sjögren's syndrome:
- lymphomas (neoplasms affecting the blood and lymph nodes);
- vasculitis (an inflammatory process in blood vessels that can occur everywhere);
- addition of a secondary infection;
- development of cancer;
- inhibition of blood formation, reduction in the blood of leukocytes, erythrocytes and/or platelets.
If the patient does not take any measures to treat Sjögren’s disease for a long time or he was prescribed the wrong therapy, the pathology progresses, leading to serious disruptions in the functioning of organs and systems.
Treatment of Sjögren's syndrome
In the presence of Sjögren's syndrome, treatment is carried out depending on the stage of the disease and the presence of systemic manifestations.
In order to stimulate the function of the glands, the following is carried out:
- drip administration of contrical.
- subcutaneous administration of galantamine.
- Vitamin therapy courses are conducted for general strengthening purposes.
- As a symptomatic treatment, “artificial tears” (drops in the eyes) are prescribed - with low viscosity - Lakrisifi (200-250 rubles), Natural Tear (250 rubles), medium viscosity Lakrisin, high viscosity Oftagel 180 rubles, Vidisik 200 rubles, Lacropos 150 rubles .
At the initial stages, in the absence of damage to other systems and unexpressed laboratory changes, long courses of glucocorticosteroids (prednisolone, dexamethasone) in small doses are prescribed.
If symptoms and laboratory parameters are significantly pronounced, but there are no systemic manifestations, cytostatic immunosuppressive drugs - cyclophosphamide, chlorbutin, azathioprine - are added to corticosteroids. Maintenance therapy is carried out with the same drugs for several years.
- If there are symptoms of systemic damage, regardless of the stage of the disease, high doses of corticosteroids and immunosuppressants are immediately prescribed for several days with a gradual transfer to maintenance doses.
- For generalized polyneuritis, vasculitis, kidney damage and other severe manifestations of the disease, the following methods are added to the above treatment, extracorporeal treatment - plasmapheresis, hemosorption, plasma ultrafiltration.
- The remaining drugs are prescribed depending on complications and concomitant diseases - cholecystitis, gastritis, pneumonia, endocervicitis, etc.
In certain cases, it is necessary to follow a dietary diet and limit physical activity.
Forecast
Sjögren's syndrome can damage vital organs with a transition to a stable state, gradual progression, or, conversely, long-term remission. This behavior is also typical for other autoimmune diseases.
- Some patients may have mild symptoms of dry eyes and mouth, while others develop serious complications. Some patients are completely helped by symptomatic treatment, others have to constantly struggle with deterioration of vision, constant discomfort in the eyes, often recurrent infections of the oral cavity, swelling of the parotid salivary gland, difficulty chewing and swallowing. Constant loss of strength and joint pain seriously reduce the quality of life. In some patients, the pathological process involves the kidneys - glomerulonephritis, leading to proteinuria, impaired renal concentrating ability and distal renal tubular acidosis.
- People with Sjögren's syndrome have a higher risk of developing non-Hodgkin's lymphoma compared to healthy people and people with other autoimmune diseases. About 5% of patients develop some form of lymphoma.
In addition, it has been found that children of women with Sjogren's syndrome during pregnancy have a higher risk of developing neonatal lupus erythematosus with congenital heart block.
Prevention
There are no specific preventive methods, since the reasons causing the development of the syndrome have not been fully studied. There are general recommendations that can reduce the likelihood of its occurrence. These include:
- Regular preventive examinations with doctors.
- Timely treatment of infections, especially ENT-related.
- Strict adherence to treatment regimens selected by the doctor.
- Elimination of frequent stress and emotional overload.
- Reducing the load on the organs of vision, digestion and speech.
- Refusal of sunbathing and visiting the solarium.
- Vaccination only if prescribed by a doctor.
Sjögren's syndrome occurs in many patients and is accompanied by a number of unpleasant symptoms. Its treatment includes taking drugs that impair the functioning of the immune system. It is possible to maintain a stable condition only with complex therapy selected by a dentist, gastroenterologist, ophthalmologist and rheumatologist. Treatment and prevention are determined only by doctors.