Topamax, 60 pcs., 25 mg, capsules
The effect of Topamax® on the concentrations of other antiepileptic drugs (AEDs)
Concomitant use of Topamax® with other AEDs (phenytoin, carbamazepine, valproic acid, phenobarbital, primidone) does not affect their plasma Css values, with the exception of certain patients in whom the addition of Topamax® to phenytoin may cause an increase in plasma phenytoin concentrations . This may be due to inhibition of a specific polymorphic isoform of the enzyme of the cytochrome P450 system (CYP2C19 isoenzyme). Therefore, phenytoin plasma concentrations should be monitored in any patient taking phenytoin who develops clinical signs or symptoms of toxicity.
In a pharmacokinetic study in patients with epilepsy, the addition of topiramate to lamotrigine did not affect the latter's Css at doses of topiramate 100–400 mg/day. During therapy and after discontinuation of lamotrigine (average dose 327 mg/day), Css of topiramate did not change.
Impact of other AEDs on the concentration of Topamax®
Phenytoin and carbamazepine reduce plasma concentrations of Topamax®. The addition or removal of phenytoin or carbamazepine during treatment with Topamax may require a change in the dose of the latter. The dose should be selected based on achieving the desired clinical effect. The addition or removal of valproic acid does not cause clinically significant changes in the plasma concentration of Topamax® and therefore does not require a change in the dose of Topamax®. The results of these interactions are summarized in Table 1.
Table 1
| Added PEP | PEP concentration | Topamax® drug concentration |
| Phenytoin | 1 ** | $ (48%) |
| Carbamazepine | 1 | $ (40%) |
| Valproic acid | 1 | 1 |
| Phenobarbital | 1 | NI |
| Primidon | 1 | NI |
1 - No effect.
** - Increased concentration in isolated patients.
$ - Decreased plasma concentration.
NI - Not studied.
Other drug interactions
Digoxin.
In a single-dose study, the plasma AUC of digoxin decreased by 12% when coadministered with Topamax®. The clinical significance of this observation is unclear. When prescribing or discontinuing Topamax® in patients taking digoxin, special attention should be paid to monitoring the concentration of digoxin in the serum.
CNS depressants.
In clinical studies, the consequences of simultaneous use of Topamax® with alcohol or other substances that depress the functions of the central nervous system have not been studied. It is not recommended to take Topamax® together with alcohol or other drugs that cause depression of central nervous system function.
St. John's wort.
When taking Topamax together with drugs based on St. John's wort
(Hypericum perforatum),
the plasma concentration of topiramate may decrease and, as a result, the effectiveness of the drug may also decrease. Clinical studies of the interaction of Topamax® and drugs based on St. John's wort have not been conducted.
Oral contraceptives.
In a drug interaction study with oral contraceptives using a combination product containing norethisterone (1 mg) and ethinyl estradiol (35 mcg), Topamax® at doses of 50–800 mg per day did not have a significant effect on the effectiveness of norethisterone and at doses of 50–200 mg per day - on the effectiveness of ethinyl estradiol. A significant dose-dependent decrease in the effectiveness of ethinyl estradiol was observed at doses of Topamax® 200–800 mg per day. The clinical significance of the described changes is unclear. The risk of decreased contraceptive effectiveness and increased breakthrough bleeding should be considered in patients taking oral contraceptives in combination with Topamax®. Patients taking estrogen-containing contraceptives should be informed of any changes in the timing and pattern of menstruation. The effectiveness of contraceptives may be reduced even in the absence of breakthrough bleeding.
Lithium.
In healthy volunteers, a decrease in lithium AUC by 18% was observed while taking topiramate at a dose of 200 mg/day. In patients with manic-depressive psychosis, the use of topiramate in doses up to 200 mg/day did not affect the pharmacokinetics of lithium, however, at higher doses (up to 600 mg/day), the AUC of lithium was increased by 26%. When using topiramate and lithium simultaneously, the concentration of the latter in the blood plasma should be monitored.
Risperidone.
Drug interaction studies with single and multiple doses of topiramate in healthy volunteers and patients with bipolar disorder yielded similar results. When coadministered with topiramate at doses of 250 or 400 mg/day, the AUC of risperidone taken in doses of 1–6 mg/day is reduced by 16 and 33%, respectively. At the same time, the pharmacokinetics of 9-hydroxyrisperidone did not change, and the total pharmacokinetics of the active substances (risperidone and 9-hydroxyrisperidone) changed slightly. The change in systemic exposure of risperidone/9-hydroxyrisperidone and topiramate was not clinically significant and this interaction is unlikely to be of clinical significance.
Hydrochlorothiazide.
Drug interactions were assessed in healthy volunteers with the separate and combined use of hydrochlorothiazide (25 mg) and topiramate (96 mg). Study results showed that when topiramate and hydrochlorothiazide were taken concomitantly, the topiramate Cmax increased by 27% and the topiramate AUC increased by 29%. The clinical significance of these studies has not been established. Prescribing hydrochlorothiazide to patients taking topiramate may require a dose adjustment of topiramate. The pharmacokinetic parameters of hydrochlorothiazide were not significantly altered by concomitant therapy with topiramate.
Metformin.
Drug interactions were assessed in healthy volunteers receiving metformin or a combination of metformin and topiramate. Study results showed that when topiramate and metformin were taken concomitantly, the Cmax and AUC of metformin increased by 18 and 25%, respectively, while the clearance of metformin when used concomitantly with topiramate decreased by 20%. Topiramate had no effect on metformin plasma Tmax. The clearance of topiramate is reduced when used concomitantly with metformin. The extent of the observed changes in clearance has not been studied. The clinical significance of the effect of metformin on the pharmacokinetics of topiramate is unclear. If Topamax® is added or discontinued in patients receiving metformin, the patient's condition should be carefully monitored to assess the course of diabetes mellitus.
Pioglitazone.
Drug interactions were assessed in healthy volunteers with the separate and simultaneous use of pioglitazone and topiramate. A decrease in the AUC of pioglitazone by 15% was detected, without changing the Cmax of the drug. These changes were not statistically significant. For the active hydroxymetabolite pioglitazone, a decrease in Cmax and AUC was also detected by 13 and 16%, respectively, and for the active ketometabolite, a decrease in both Cmax and AUC was detected by 60%. The clinical significance of these data is unclear. When patients use Topamax® and pioglitazone simultaneously, the patient's condition should be carefully monitored to assess the course of diabetes mellitus.
Glibenclamide.
A drug interaction study was conducted to examine the pharmacokinetics of glibenclamide (5 mg/day) at steady state, administered alone or concomitantly with topiramate (150 mg/day) in patients with type 2 diabetes mellitus. When topiramate was used, the AUC of glibenclamide was reduced by 25%. Systemic exposure to 4-trans-hydroxy-glibenclamide and 3-cis-hydroxy-glibenclamide was also reduced (by 13 and 15%, respectively). Glibenclamide did not affect the pharmacokinetics of topiramate at steady state. A statistically insignificant decrease in the AUC of pioglitazone by 15% was found with no change in Cmax. When prescribing topiramate to patients receiving glibenclamide (or prescribing glibenclamide to patients receiving topiramate), the patient's condition should be carefully monitored to assess the course of diabetes mellitus.
Other drugs.
Concomitant use of Topamax® with drugs that predispose to nephrolithiasis may increase the risk of kidney stones. During treatment with Topamax®, the use of drugs that predispose to nephrolithiasis should be avoided, as they may cause physiological changes that contribute to nephrolithiasis.
Valproic acid.
The combined use of topiramate and valproic acid in patients who tolerate each drug separately is accompanied by hyperammonemia with or without encephalopathy. In most cases, symptoms and signs disappear after stopping one of the medications. This adverse event is not due to a pharmacokinetic interaction. The relationship between hyperammonemia and the use of topiramate alone or in combination with other drugs has not been established. When topiramate and valproic acid are taken together, hypothermia (an unintentional decrease in body temperature below 35 °C) may occur in combination with hyperammonemia or independently. This phenomenon can occur both after the start of co-administration of valproic acid and topiramate, and with an increase in the daily dose of topiramate.
Additional drug interaction studies: A number of clinical studies have been conducted to evaluate potential drug interactions between topiramate and other drugs. The results of these interactions are summarized in Table 2.
table 2
| Added drug | Concentration of added drug* | Topiramate concentration* |
| Amitriptyline | Increase in Cmax and AUC of nortriptyline metabolite by 20% | NI |
| Dihydroergotamine (oral and s.c.) | 1 | 1 |
| Haloperidol | Increase in metabolite AUC by 31% | NI |
| Propranolol | Increase in Cmax for 4-OH propranolol by 17% (topiramate 50 mg) | Increase in Cmax by 9 and 16%; AUC of 9 and 17% for propranolol 40 and 80 mg every 12 hours, respectively |
| Sumatriptan (oral and subcutaneous) | 1 | NI |
| Pizotifen | 1 | 1 |
| Diltiazem | Reduced AUC of diltiazem by 25% and desacetyldiltiazem by 18%; 1 for N-demethyldilthiazem | Increase AUC by 20% |
| Venlafaxine | 1 | 1 |
| Flunarizine | Increase in AUC by 16% (50 mg every 12 hours)** | 1 |
* — Expressed as % of Cmax values in blood plasma and AUC during monotherapy.
1 - No changes in plasma Cmax and AUC (<15% of baseline data).
** With multiple doses of flunarizine alone, a 14% increase in AUC was observed, which may be due to drug accumulation during the process of reaching steady state.
NI - not studied.
Nosological classification (ICD-10)
- G40 Epilepsy
- G40.1 Localized (focal) (partial) symptomatic epilepsy and epileptic syndromes with simple partial seizures
- G40.2 Localized (focal) (partial) symptomatic epilepsy and epileptic syndromes with complex partial seizures
- G40.4 Other types of generalized epilepsy and epileptic syndromes
- G40.6 Grand mal seizures, unspecified [with or without petit mal seizures]
- G43 Migraine
Description of the dosage form
Capsules, 15 mg: hard gelatin No. 2, consisting of a white body and a transparent colorless cap. The cap of the capsule bears the inscription “TOP”. The capsule body bears the inscription “15 mg”.
Capsules, 25 mg: hard gelatin No. 1, consisting of a white body and a transparent colorless cap. The cap of the capsule bears the inscription “TOP”. On the body of the capsule there is the inscription “25 mg”.
Capsules, 50 mg: hard gelatin capsules No. 0, consisting of a white body and a transparent colorless cap. On the cap of the capsule there is an inscription in black ink “TOP”. On the body of the capsule there is an inscription in black ink “50 mg”.
Capsule contents: white or almost white granules.
Pharmacodynamics
Topiramate is an antiepileptic drug belonging to the class of sulfamate-substituted monosaccharides. Topiramate blocks sodium channels and suppresses the occurrence of repeated action potentials against the background of prolonged depolarization of the neuron membrane. Topiramate increases the activity of GABA in relation to certain subtypes of GABA receptors (including GABAA receptors), and also modulates the activity of GABAA receptors themselves, and prevents the activation by kainate of the sensitivity of the kainate/AMPK subtype (α-amino-3-hydroxy-5- Methylisoxazole-4-propionic acid) glutamate receptors do not affect the activity of N-methyl-D-aspartate (NMDA) at the NMDA receptor subtype. These effects of topiramate are dose-dependent at drug plasma concentrations ranging from 1 to 200 µM, with trough activity ranging from 1 to 10 µM. In addition, topiramate inhibits the activity of some carbonic anhydrase isoenzymes. In terms of the severity of this pharmacological effect, topiramate is significantly inferior to acetazolamide, a known inhibitor of carbonic anhydrase, therefore this activity of topiramate is not considered the main component of its antiepileptic activity.
Use during pregnancy and breastfeeding
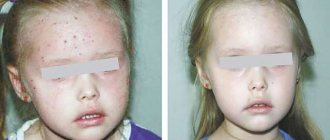
There have been no special controlled studies in which Topamax® was used to treat pregnant women. Topiramate may cause fetal harm when used in pregnant women. Pregnancy data indicate that infants exposed to topiramate in utero have an increased risk of developing congenital malformations (eg, craniofacial defects such as cleft lip or palate, hypospadias, and developmental anomalies of various body systems). These malformations were recorded both during monotherapy with topiramate and when it was used as part of polytherapy.
Compared with the group of patients not taking antiepileptic drugs, data from pregnancies during monotherapy with Topamax® indicate an increased likelihood of having children with low body weight (less than 2500 g). The connection between the observed phenomena and the use of the drug has not been established.
In addition, pregnancy records and the results of other studies indicate that the risk of developing teratogenic effects with combination treatment with antiepileptic drugs is higher than with monotherapy.
The use of Topamax® during pregnancy is justified only if the potential benefit of the drug for the mother outweighs the possible risk to the fetus. When treating and counseling women of childbearing potential, the treating physician must weigh the benefits and risks of treatment and consider alternative treatment options. If Topamax® is used during pregnancy or the patient becomes pregnant while taking this drug, she should be warned of the potential risk to the fetus.
A limited number of patient observations suggests that topiramate is excreted in breast milk in women, so the physician should decide whether to breastfeed or discontinue the drug.
Pharmacokinetics
Topiramate is absorbed quickly and efficiently. Its bioavailability is 81%. Food intake does not have a clinically significant effect on the bioavailability of topiramate. 13–17% of topiramate is bound to plasma proteins. After a single dose of up to 1200 mg, the average Vd is 0.55–0.8 l/kg. The value of Vd depends on gender: in women it is approximately 50% of the values observed in men, which is associated with a higher content of adipose tissue in the body of women.
After oral administration, about 20% of the dose taken is metabolized. However, in patients receiving concomitant therapy with antiepileptic drugs that induce enzymes responsible for drug metabolism, the metabolism of topiramate increases up to 50%. Six essentially inactive metabolites have been isolated and identified from human plasma, urine, and feces. The main route of elimination of unchanged topiramate (70%) and its metabolites is the kidneys. After oral administration, plasma clearance of topiramate is 20–30 ml/min. The pharmacokinetics of topiramate is linear, plasma clearance remains constant, and AUC in the dose range from 100 to 400 mg increases in proportion to the dose. In patients with normal renal function, it may take 4 to 8 days to achieve steady-state plasma concentrations. The Cmax value after repeated oral administration of 100 mg of the drug twice a day averaged 6.76 mcg/ml. After multiple doses of 50 and 100 mg twice daily, T1/2 of topiramate from plasma averaged 21 hours.
In patients with moderate to severe renal impairment, the plasma and renal clearance of topiramate is reduced (Cl creatinine <70 ml/min), as a result, the Css of topiramate in the blood plasma may increase compared to patients with normal renal function. In addition, patients with impaired renal function take longer to reach Css of topiramate in the blood. In patients with moderate or severe renal impairment, half the recommended initial and maintenance dose is recommended. Topiramate is effectively eliminated from plasma by hemodialysis. Long-term hemodialysis may result in a decrease in blood concentrations of topiramate below the amount required to maintain anticonvulsant activity. To avoid a rapid fall in plasma concentrations of topiramate during hemodialysis, an additional dose of Topamax may be required. When adjusting the dose, you should take into account:
- duration of hemodialysis;
— the clearance value of the hemodialysis system used;
- effective renal clearance of topiramate in a patient on dialysis.
Plasma clearance of topiramate is reduced by an average of 26% in patients with moderate or severe hepatic impairment. Therefore, patients with hepatic impairment should use topiramate with caution.
In elderly patients without renal disease, the plasma clearance of topiramate does not change.
Pharmacokinetics of topiramate in children under 12 years of age. The pharmacokinetic parameters of topiramate in children, as in adults receiving this drug as adjuvant therapy, are linear, while its clearance does not depend on the dose, and Css in plasma increases in proportion to the dose. One should take into account the fact that in children the clearance of topiramate is increased and its T1/2 is shorter. Therefore, at the same dose per 1 kg of body weight, plasma concentrations of topiramate in children may be lower than in adults. In children, as in adults, antiepileptic drugs that induce liver microsomal enzymes cause a decrease in plasma concentrations of topiramate.
Compound
| Capsules | 1 caps. |
| active substance: | |
| topiramate | 15 mg |
| 25 mg | |
| 50 mg | |
| excipients (for capsules 15 mg): granulated sugar (sucrose, starch syrup) - 45 mg; povidone - 10.4199 mg; cellulose acetate - 5.423 mg | |
| hard gelatin capsule (for 15 mg capsules): gelatin - 50.8–52.7 mg; water - 9.3–11.2 mg; sorbitan laurate - 0.0252 mg; sodium lauryl sulfate - 0.0252 mg; titanium dioxide (E171) - 0.63 mg; ink Opacode Black S-1-17822/23 black (shellac glaze solution in ethanol, black iron oxide, n-butyl alcohol, isopropyl alcohol, propylene glycol, ammonium hydroxide) - 5–10 mcg | |
| excipients (for capsules 25 mg): granulated sugar (sucrose, starch syrup) - 75 mg; povidone - 17.3665 mg; cellulose acetate - 9.038 mg | |
| hard gelatin capsule (for 25 mg capsules): gelatin - 64.7–67 mg; water - 10–12.3 mg; sorbitan laurate - 0.0312 mg; sodium lauryl sulfate - 0.0312 mg; titanium dioxide (E171) - 0.78 mg, Opacode Black ink S-1-17822/23 black (shellac glaze solution in ethanol, black iron oxide, n-butyl alcohol, isopropyl alcohol, propylene glycol, ammonium hydroxide) - 5–10 mcg | |
| excipients (for capsules 50 mg): granulated sugar (sucrose, starch syrup) - 150 mg; povidone - 34.733 mg; cellulose acetate - 18.076 mg | |
| hard gelatin capsule (for 50 mg capsules): gelatin - 80.6–83.5 mg; water - 12.5–15.4 mg; sorbitan laurate - 0.0397 mg; sodium lauryl sulfate - 0.0397 mg; titanium dioxide (E171) - 0.99 mg; ink Opacode Black S-1-17822/23 black (shellac glaze solution in ethanol, black iron oxide, n-butyl alcohol, isopropyl alcohol, propylene glycol, ammonium hydroxide) - 5–10 mcg |
Indications for Topamax®
epilepsy:
- as a monotherapy in adults and children over 2 years of age with epilepsy (including patients with newly diagnosed epilepsy);
- as part of complex therapy: in adults and children over 2 years of age with partial or generalized tonic-clonic seizures, as well as for the treatment of seizures associated with Lennox-Gastaut syndrome.
migraine:
- prevention of migraine attacks in adults. The use of Topamax® for the treatment of acute migraine attacks has not been studied.
Side effects
Side effects are given with a distribution by frequency and organ system. The frequency of side effects was classified as follows: very common (≥1/10); frequent (≥1/100, <1/10); uncommon (≥1/1000 and <1/100); rare (≥1/10000 and <1/1000) and very rare (<1/10000).
The most common adverse reactions (the frequency of which was more than 5% and higher than that in the placebo group for at least one of the indications during controlled clinical trials of topiramate) are: anorexia, decreased appetite, slow thinking, depression, impaired speech fluency, insomnia, disturbances coordination of movements, impaired concentration, dizziness, dysarthria, dysgeusia, hypoesthesia, retardation, memory impairment, nystagmus, paresthesia, drowsiness, tremor, diplopia, blurred vision, diarrhea, nausea, fatigue, irritability and weight loss.
Infections and infestations: very often - nasopharyngitis.
From the blood and lymphatic system: often - anemia; uncommon - leukopenia, lymphadenopathy, thrombocytopenia, eosinophilia; rarely - neutropenia*.
From the immune system: often - hypersensitivity; frequency unknown - allergic edema*, conjunctival edema*.
From the side of metabolism and nutrition: often - anorexia, loss of appetite; uncommon - metabolic acidosis, hypokalemia, increased appetite, polydipsia; rarely - hyperchloremic acidosis.
Mental disorders: very often - depression; often - slow thinking, insomnia, impaired speech, anxiety, confusion, disorientation, aggressive reactions, mood disorders, agitation, emotional lability, depressed mood, anger, behavioral disturbances; uncommon - suicidal thoughts, suicide attempts, hallucinations, psychotic disorders, auditory hallucinations, visual hallucinations, apathy, difficulty speaking, sleep disturbances, affective lability, decreased libido, restlessness, crying, dysphemia, euphoric mood, paranoia, perseveration of thinking, panic attacks , tearfulness, impaired reading skills, difficulty falling asleep, flattening of emotions, pathological thinking, loss of libido, lethargy, intrasomnic disorder, absent-mindedness, early awakenings in the morning, panic reactions, high spirits; rarely - mania, panic disorder, feelings of hopelessness*, hypomania.
From the side of the central nervous system: very often - paresthesia, drowsiness, dizziness; often - impaired concentration, memory impairment, amnesia, cognitive disorders, impaired thinking, psychomotor impairment, convulsions, impaired motor coordination, tremor, lethargy, hypoesthesia, nystagmus, dysgeusia, impaired sense of balance, dysarthria, intention tremor, sedation; uncommon - depressed consciousness, tonic-clonic grand mal seizures, visual field impairment, complex partial seizures, speech impairment, psychomotor hyperactivity, fainting, sensory disturbances, drooling, hypersomnia, aphasia, repetitive speech, hypokinesia, dyskinesia, postural dizziness , low quality of sleep, burning sensation, loss of sensitivity, parosmia, cerebral syndrome, dysesthesia, hypogeusia, stupor, clumsiness, aura, ageusia, dysgraphia, dysphasia, peripheral neuropathy, presyncope, dystonia, pins and needles sensation; rarely: apraxia, circadian sleep rhythm disorder, hyperesthesia, hyposmia, anosmia, essential tremor, akinesia, lack of response to stimuli.
From the organ of vision: often - blurred vision, diplopia, visual impairment; uncommon - decreased visual acuity, scotoma, myopia*, strange sensations in the eyes*, dry eyes, photophobia, blepharospasm, increased lacrimation, photopsia, mydriasis, presbyopia; rarely - one-sided blindness, transient blindness, glaucoma, impaired accommodation, impaired visual spatial perception, atrial scotoma, eyelid edema*, night blindness, amblyopia; frequency unknown: angle-closure glaucoma*, maculopathy*, ocular motility disorders*.
From the organ of hearing and balance: often - vertigo, ringing in the ears, pain in the ear; uncommon: deafness, one-sided deafness, sensorineural deafness, ear discomfort, hearing impairment.
From the cardiovascular system: infrequently - bradycardia, sinus bradycardia, palpitations.
From the vascular system: infrequently - hypotension, orthostatic hypotension, hot flashes, hot flushes; rarely - Raynaud's phenomenon.
From the respiratory system, chest and mediastinal organs: often - shortness of breath, nosebleeds, nasal congestion, rhinorrhea, cough *; infrequently - shortness of breath on exertion, hypersecretion in the paranasal sinuses, dysphonia.
From the gastrointestinal tract: very often - nausea, diarrhea; often - vomiting, constipation, pain in the epigastric region, dyspepsia, abdominal pain, dry mouth, stomach discomfort, impaired sensitivity in the oral cavity, gastritis, abdominal discomfort; uncommon - pancreatitis, flatulence, gastroesophageal reflux, pain in the lower abdomen, decreased sensitivity in the oral cavity, bleeding gums, bloating, discomfort in the epigastric region, tenderness in the abdominal area, hypersalivation, pain in the oral cavity, bad breath, glossodynia .
From the hepatobiliary system: rarely - hepatitis, liver failure.
From the skin and subcutaneous tissues: often - alopecia, rash, itching; uncommon - anhidrosis, impaired sensitivity in the facial area, urticaria, erythema, generalized itching, macular rash, skin pigmentation disorder, allergic dermatitis, facial swelling; uncommon - Stevens-Johnson syndrome*, erythema multiforme*, change in skin odor, periorbital edema*, localized urticaria; frequency unknown - toxic epidermal necrolysis*.
From the musculoskeletal system and connective tissue: often - arthralgia, muscle spasms, myalgia, muscle cramps, muscle weakness, musculoskeletal pain in the chest; uncommon: joint swelling*, muscle stiffness, side pain, muscle fatigue; rarely: discomfort in the limbs*.
From the kidneys and urinary tract: often - nephrolithiasis, pollakiuria, dysuria; uncommon - exacerbation of urolithiasis (kidney stones), stress urinary incontinence, hematuria, urinary incontinence, frequent urge to urinate, renal colic, pain in the kidney area; rarely - exacerbation of urolithiasis (stones in the urethra), renal tubular acidosis*.
From the genital organs and breast: uncommon - erectile dysfunction, sexual dysfunction.
General disorders and disorders caused by the method of application: very often - fatigue; often - increased body temperature, asthenia, irritability, gait disturbances, poor health, anxiety; uncommon - hyperthermia, thirst, flu-like syndrome*, slowness, cold extremities, feeling of intoxication, feeling of anxiety; rarely - facial swelling, calcification.
Changes in laboratory parameters: very often - loss of body weight; often - weight gain*; infrequently - crystalluria, abnormal result of the tandem gait test, leukopenia, increased activity of liver enzymes in the blood serum, rarely - a decrease in the content of bicarbonates in the blood.
Impaired social functioning: uncommon - learning disability.
*adverse reaction was registered in the post-registration period from spontaneous reports. Frequency calculated based on data from clinical studies.
Special groups:
Children
A list of adverse reactions that, during controlled clinical trials, were recorded in children 2 or more times more often than in adults: decreased appetite, increased appetite, hyperchloremic acidosis, hypokalemia, behavioral disorders, aggressive reactions, apathy, difficulty falling asleep, suicidal thoughts, disorder concentration, lethargy, disruption of the circadian rhythm of sleep, poor quality of sleep, increased lacrimation, sinus bradycardia, poor health, gait disturbances.
List of adverse reactions that were recorded only in children during controlled clinical trials: eosinophilia, psychomotor hyperactivity, vertigo, vomiting, hyperthermia, pyrexia, learning disability.