Pharmacological properties of the drug Tamiflu
Mechanism of action. Antiviral drug. Oseltamivir phosphate is a prodrug, its active metabolite (oseltamivir carboxylate) is an effective and selective inhibitor of neuraminidase of influenza viruses type A and B - an enzyme that catalyzes the process of release of newly formed viral particles from infected cells, their penetration into respiratory epithelial cells and further spread of the virus in the body . Inhibits the growth of the influenza virus in vitro and suppresses the replication of the virus and its pathogenicity in vivo, reduces the release of influenza A and B viruses from the body. Tamiflu significantly shortens the period of clinical manifestations of influenza infection, reduces their severity and reduces the incidence of influenza complications requiring the use of antibiotics (bronchitis, pneumonia, sinusitis, otitis media), shortens the time of virus isolation from the body and reduces the area under the “viral titer-time” curve . In children aged 1 to 12 years, Tamiflu significantly reduces the duration of the disease (by 35.8 hours) and the incidence of acute otitis media. Recovery and return to normal activity occurs almost 2 days earlier. When taken for the purpose of prevention, Tamiflu significantly (by 92%) reduces the incidence of influenza among contacted persons, by 76% - the frequency of clinically established influenza during an outbreak of the disease, reduces the frequency of virus isolation and prevents the transmission of the virus from one family member to another. In children aged 1 to 12 years, preventive use of Tamiflu reduces the incidence of laboratory-confirmed influenza from 24 to 4%. Tamiflu does not affect the formation of anti-influenza antibodies, incl. to produce antibodies in response to the administration of an inactivated influenza vaccine. Resistance. When taking Tamiflu for the purpose of prophylaxis (7 days), prophylaxis of family contacts (10 days) and seasonal prophylaxis (42 days), no cases of drug resistance were observed. In adult patients/adolescents, oseltamivir resistance was detected in 0.32% of cases (4/1245) using phenotyping and in 0.4% of cases (5/1245) using phenotyping and genotyping, and in children from 1 year to 12 years in 4.1% (19/464) and 5.4% (25/464) of cases, respectively. All patients had temporary carriage of an OS-resistant virus. This did not affect virus clearance. Several different subtype-specific viral neuraminidase mutations have been discovered. The degree of sensitivity reduction depended on the type of mutation; for example, with the 1222V mutation in N1, sensitivity decreased by 2 times, and with R292K in N2, by 30,000 times. No mutations were found to reduce the sensitivity of influenza B virus neuraminidase in vitro. In patients treated with oseltamivir, reported neuraminidase N1 mutations (including H5N1 viruses) leading to resistance/reduced susceptibility to OS were H274Y, N294S (1 case), E119V (1 case), R292K (1 case), and neuraminidase mutations N2 - N294S (1 case) and SASG245-248del (1 case). In one case, the G402S mutation of the influenza B virus was detected, which resulted in a 4-fold decrease in sensitivity, and in 1 case, the D198N mutation was detected with a 10-fold decrease in sensitivity in a child with immunodeficiency. Viruses with a resistant neuraminidase genotype differ in resistance to varying degrees from the natural strain. Viruses with the R292K mutation in N2 in animals (mice and ferrets) are significantly inferior in infectivity, pathogenicity and contagiousness to viruses with the E119V mutation in N2 and D198N in B and differ slightly from the natural strain. Viruses with the H274Y mutation in N1 and N294S in N2 occupy an intermediate position. Pharmacokinetics Oseltamivir phosphate is easily absorbed from the gastrointestinal tract and is highly converted into an active metabolite under the action of hepatic and intestinal esterases. Concentrations of the active metabolite in plasma are determined within 30 minutes, the time to reach Cmax is 2–3 hours, and are more than 20 times higher than the concentrations of the prodrug. At least 75% of the dose taken orally enters the systemic circulation in the form of an active metabolite, less than 5% in the form of the parent drug. Plasma concentrations of both the prodrug and the active metabolite are dose proportional and independent of food intake. The volume of distribution (Vss) of the active metabolite is 23 l. After oral administration of oseltamivir phosphate, its active metabolite was detected in the lungs, bronchial lavage waters, nasal mucosa, middle ear and trachea in concentrations providing an antiviral effect. Binding of the active metabolite to plasma proteins is 3%. Prodrug binding to plasma proteins is 42%, which is not enough to cause significant drug interactions. Oseltamivir phosphate is highly converted into an active metabolite under the action of esterases located mainly in the liver and intestines. Neither oseltamivir phosphate nor the active metabolite are substrates or inhibitors of cytochrome P450 isoenzymes. It is excreted (>90%) as an active metabolite mainly by the kidneys. The active metabolite does not undergo further transformation and is excreted in the urine (>99%) by glomerular filtration and tubular secretion. Renal clearance (18.8 l/h) exceeds the glomerular filtration rate (7.5 l/h), indicating that the drug is also eliminated by tubular secretion. Less than 20% of the drug taken is excreted in feces. T1/2 of the active metabolite - 6-10 hours. Pharmacokinetics in special groups Patients with kidney damage. When Tamiflu is prescribed to patients with varying degrees of kidney damage, AUC values are inversely proportional to the decrease in renal function. Patients with liver damage. In vitro, in patients with hepatic pathology, neither a significant increase in the AUC of oseltamivir phosphate nor a decrease in the AUC of the active metabolite was observed. Elderly patients. In elderly patients (65–78 years), the exposure to the active metabolite at steady state is 25–35% higher than in younger patients when similar doses of Tamiflu are prescribed. T1/2 of the drug in elderly patients did not differ significantly from that in younger patients. Elderly patients do not require dose adjustment for the treatment and prevention of influenza. Children. In young children, clearance of the prodrug and active metabolite occurs more rapidly than in adults, resulting in lower AUCs relative to a given dose. Taking the drug at a dose of 2 mg/kg provides the same AUC of oseltamivir carboxylate as is achieved in adults after a single dose of a capsule with 75 mg of the drug (equivalent to approximately 1 mg/kg). The pharmacokinetics of oseltamivir in children over 12 years of age is the same as in adults.
Instructions for use of TAMIFLU® (TAMIFLU®)
Antiviral drug. Oseltamivir phosphate is a prodrug of the active metabolite (oseltamivir carboxylate). The active metabolite is a selective inhibitor of the viral neuraminidase enzyme, glycoprotein enzymes located on the surface of the virion. The activity of the viral enzyme neuraminidase is important for the release of formed viral particles from infected cells and the further spread of the virus in the body.
Oseltamivir carboxylate in vitro inhibits neuraminidases of influenza A and B viruses. Oseltamivir phosphate inhibits the growth of influenza virus and suppresses its replication in vitro. Oral oseltamivir inhibits the replication and in vivo pathogenicity of influenza A and B viruses in animal models of influenza at doses similar to human doses of 75 mg twice daily.
The antiviral activity of oseltamivir against influenza A and B was confirmed by experimental studies with challenge tests in healthy volunteers.
The median inhibitory concentration (IC50) of oseltamivir against the neuraminidase enzyme for clinically isolated influenza A ranged from 0.1 nM to 1.3 nM, for influenza B it was 2.6 nM. Published studies have reported higher IC50 values for influenza B, up to 8.5 nM.
Clinical researches
Flu treatment
Oseltamivir is effective only against diseases caused by the influenza virus. Therefore, analysis statistics are presented only for influenza-infected patients. In a pooled study of influenza virus-positive and influenza virus-negative patients (ITT), primary efficacy decreased proportionally to the number of influenza virus-negative patients. In the entire treatment population, influenza infection was confirmed in 67% (range, 46% to 74%) of recruited patients. Of all elderly patients, 64% were positive for influenza virus, and of all elderly patients with chronic heart failure and/or respiratory disease, 62% were positive for influenza virus. In all phase III clinical trials, patients were recruited only during an influenza epidemic in the area.
Adults and teenagers aged 13 years and older.
We selected those patients who reported onset of symptoms within 36 hours and who had a fever ≥37.8°C accompanied by at least one respiratory symptom (cough, nasal symptoms, sore throat) and at least one systemic symptom (muscle pain , chills/sweating, malaise, weakness or headache). In a pooled analysis of all influenza virus-positive adults and adolescents (n=2413) enrolled in treatment studies, oseltamivir 75 mg twice daily for 5 days reduced the mean duration of influenza by one day compared with placebo, with 5.2 days (95% confidence interval 4.9-5.5 days) to 4.2 days (95% confidence interval 4.0-4.4 days; p ≤ 0.0001).
The proportion of patients who experienced lower respiratory tract complications requiring antibiotic treatment (mainly bronchitis) decreased from 12.7% (135/1063) in the placebo group to 8.6% (116/1350) in patients treated with oseltamivir (p =0.0012).
Treatment of influenza in high-risk populations.
The mean duration of influenza was not significantly reduced in older adults (≥65 years) or in patients with chronic heart and/or respiratory disease who received oseltamivir 75 mg twice daily for 5 days. In the oseltamivir treatment group, the duration of the fever period decreased by one day. In elderly patients positive for influenza virus, oseltamivir significantly reduced the incidence of lower respiratory tract complications requiring antibiotic treatment (mainly bronchitis), from 19% (52/268) in the placebo group to 12% (29). /250) in patients treated with oseltamivir (p = 0.0156).
In influenza virus-positive patients with chronic heart and/or respiratory disease, the combined incidence of lower respiratory tract infections requiring antibiotic treatment (mainly bronchitis) was 17% (22/133) in the placebo group and 17% (22/133) in the placebo group. those receiving oseltamivir - 14% (16/118) (p = 0.5976).
Treatment of influenza in children.
In a study of otherwise healthy children (65% positive for influenza infection) aged 1–12 years (mean age 5.3 years) who had fever (≥ 37.8°C) along with cough or runny nose, 67% of influenza-infected patients were infected with influenza A and 33% with influenza B. Treatment with oseltamivir, started within 48 hours of the onset of symptoms, significantly reduced the time required to cure the disease (defined as simultaneous restoration of health and activity and freedom from fever, cough and runny nose ) by 1.5 days (95% confidence interval 0.6-2.2 days; p < 0.0001) compared with placebo. Taking oseltamivir reduced the incidence of acute otitis media from 26.5% (53/200) in the placebo group to 16% (29/183) in the oseltamivir group (p=0.013).
Another study included 334 children with bronchial asthma aged 6-12 years, of whom 53.6% were positive for the influenza virus. In the group treated with oseltamivir, the average duration of illness did not decrease significantly. At day 6 (the last day of treatment) in this population, FEV1 increased by 10.8% in the oseltamivir group compared with 4.7% in the placebo group (p=0.0148).
The European Medicines Agency (EMA) has deferred the requirement to submit results from studies of Tamiflu in one or more subgroups of the pediatric influenza population.
Treatment of influenza B infection.
Overall, 15% of the influenza virus-positive population became infected with influenza B; According to various studies, this proportion ranges from 1 to 33%. The mean duration of illness in patients with influenza B did not differ significantly between treatment groups across studies. Data from all 504 influenza B patients included in all studies were pooled for analysis. Oseltamivir reduced the time required for resolution of all symptoms by 0.7 days (95% confidence interval 0.1-1.6 days; p=0.022) and the duration of fever (≥37.8°C), cough and runny nose by one day (95% confidence interval 0.4- 1.7 days; p < 0.001), compared with placebo.
Flu prevention
The effectiveness of oseltamivir for the prevention of naturally acquired influenza was demonstrated in a household post-exposure prophylaxis study and two seasonal prophylaxis studies. In all of these studies, the first outcome measure was the incidence of laboratory-diagnosed influenza. The virulence of the influenza virus is unpredictable and varies within a region and from season to season, so the number of people needing treatment to prevent influenza (NNT) also varies.
Post-exposure prophylaxis.
In one study, individuals who had been in contact with influenza patients (12.6% had been vaccinated against influenza) were started on oseltamivir 75 mg once daily within two days of the patient's symptoms developing and continued for 7 days. The diagnosis of influenza in patients was confirmed in 163 cases out of 377. Oseltamivir significantly reduced the incidence of clinically significant influenza in persons in contact with cases of confirmed influenza, from 24/200 (12%) in the placebo group to 2/205 (1%) in the group oseltamivir (92% reduction [95% confidence interval (CI) 6-16], p≤0.0001]). In contacts of actual influenza cases, the NNT was 10 (95% CI 9-12) and in all study participants (ITT) 16 (95% CI 15-19), regardless of infection in the first case.
The effectiveness of oseltamivir in the prevention of naturally acquired influenza was demonstrated in a post-exposure prophylaxis study in households that included adults, adolescents, and children aged 1 to 12 years as both sources and contacts. The primary outcome measure for this study was the incidence of laboratory and clinically diagnosed influenza in families. Prophylaxis with oseltamivir lasted 10 days. In the entire population, the incidence of laboratory and clinically diagnosed influenza in families decreased from 20% (27/136) in the group not receiving prophylaxis to 7% (10/135) in the group receiving prophylaxis (62.7% reduction [95% CI 26- 81.2; p=0.0042]). In households with sources of influenza infection, the incidence of influenza decreased from 26% (23/89) in the group not receiving prophylaxis to 11% (9/84) in the group receiving prophylaxis (58.5% reduction [95% CI15.6-79.6; p = 0.0114]).
In accordance with the analysis of the subgroup of 1-12 year old children, the incidence of laboratory and clinically diagnosed cases of influenza decreased markedly from 19% (21/111) in the group not receiving prophylaxis to 7% (7/104) in the group receiving prophylaxis (64.4% reduction (95% CI 15.8-85; p=0.0188). Among children who were not yet infected at baseline, the incidence of laboratory and clinically diagnosed influenza decreased from 21% (15/70) in the group not exposed to prophylaxis, up to 4% (2/47) in the group receiving prophylaxis (80.1% reduction (95% CI 22-94.9; p=0.0206)). In the entire pediatric population, NNT was 9 (95% CI 7-24) and 8 (95% CI 6, upper limit undetectable) respectively in the entire population (ITT) and among pediatric contacts of infectious sources (ITTII).
Prevention during an influenza epidemic among the population.
The other two studies involved unvaccinated and otherwise healthy adults. A pooled analysis of these studies showed that oseltamivir 75 mg once daily for 6 weeks significantly reduced the incidence of clinically significant influenza from 25/519 (4.8%) in the placebo group to 6/520 (1.2%) in the oseltamivir group (76% reduction (95% CI 1.6-5.7); p=0.0006) during an influenza outbreak in the population. The NNT in this study was 28 (95% CI 24–50).
In a study of older adults living in nursing homes, where 80% of participants were vaccinated in the appropriate season, oseltamivir 75 mg once daily for 6 weeks significantly reduced the incidence of clinical influenza from 12/272 ( 4.4%) in the placebo group to 1/276 (0.4%) in the oseltamivir group (92% reduction 95% [CI 1.5-6.6]; p=0.0015. NNT in this study was 25 (95% CI 23-62) .
Prevention of influenza in patients with immunodeficiency.
A double-blind, placebo-controlled, randomized trial of seasonal influenza prophylaxis was conducted in 475 immunocompromised patients (388 solid organ transplant patients (195 placebo, 193 oseltamivir), 87 hematopoietic stem cell transplant patients (43 placebo, 44 oseltamivir), patients with other immunosuppressive conditions were not studied), incl. 18 children aged 1-12 years. The primary outcome measure in this study was the incidence of laboratory and clinically diagnosed influenza by viral culture and/or a 4-fold increase in HAI titer. The incidence of laboratory and clinically diagnosed influenza was 2.9% (7/238) in the placebo group and 2.1% (5/237) in the oseltamivir group (95% CI - 2.3% - 4.1%; p = 0.772).
No specific studies have been conducted to evaluate the reduction in the risk of complications.
Resistance to oseltamivir
Clinical researches.
The risk of influenza viruses with reduced susceptibility or apparent resistance to oseltamivir was addressed in clinical studies sponsored by Roche. In all patients carrying oseltamivir-resistant virus, carriage was transient, viral clearance was normal, and no clinical deterioration was observed.
| Patient Population | Patients with susceptibility mutations (%) | |
| Determination of phenotype* | Determination of geno- and phenotype * | |
| Adults and teenagers | 4/1245(0.32%) | 5/1245(0.4%) |
| Children (1-12 years old) | 19/464(4.1%) | 25/464 (5.4%) |
*Full genotyping was not performed in all studies.
There is currently no evidence of drug resistance associated with the use of Tamiflu in clinical trials of post-exposure (7 days), household post-exposure (10 days) and seasonal (42 days) influenza prophylaxis in immunocompetent patients. No resistance was observed in immunocompromised patients during a 12-week prophylaxis study
Clinical and observational data.
Natural mutations associated with reduced sensitivity to oseltamivir in vitro have been found for influenza A and B viruses isolated from patients not exposed to oseltamivir. Resistant strains selected during oseltamivir treatment have been isolated from both immunocompetent and immunocompromised patients. Immunocompromised patients and young children are at higher risk of developing oseltamivir-resistant viruses during treatment.
Oseltamivir-resistant viruses isolated from patients treated with oseltamivir and oseltamivir-resistant laboratory strains of influenza viruses had neuraminidase N1 and N2 mutations. Resistance mutations tended to be specific to viral subtypes. Since 2007, resistance associated with the H275Y mutation in seasonal H1N1 strains has become widespread. Susceptibility to oseltamivir and the distribution of such viruses vary depending on the season and location. In 2008, H275Y was found in >99% of circulating H1N1 influenza strains in Europe. In 2009, H1N1 influenza ("swine flu") was almost uniformly susceptible to oseltamivir, with only sporadic reports of resistance in both treatment and prophylactic regimens.
Results of preclinical studies
Standard non-clinical pharmacological safety, chronic toxicity and genotoxicity studies have demonstrated no adverse effects in humans.
Conventional carcinogenicity studies in rats have demonstrated a dose-dependent trend toward increased incidence of some tumors that are typical of the rodent species studied. When comparing the tolerable concentration limits with the concentrations that would be expected at doses expected in humans, these data do not change the risk-benefit ratio for the use of Tamiflu for its registered indications.
Teratogenicity studies were conducted in rats and rabbits, respectively, at doses up to 1500 mg/kg/day and 500 mg/kg/day. No harmful effects on fetal development were noted. A fertility study has been conducted in rats with doses up to 1500 mg/kg/day, which demonstrated no adverse effects in either sex. In a pre- and postnatal study conducted on rats at a dose of 1500 mg/kg/day, prolongation of labor was noted:
- the safety margin between the concentration achieved in humans and the highest dose (500 mg/kg/day) in rats was 480-fold for oseltamivir and 44-fold for the active metabolite. The concentration of oseltamivir in the blood of fetal rats and rabbits was approximately 15-20% of the maternal level.
In lactating rats, oseltamivir and the active metabolite are excreted in breast milk. Limited data indicate that oseltamivir and its active metabolite are excreted in human breast milk. Based on data obtained in animal experiments, the derived values are 0.01 mg/day and 0.3 mg/day.The occurrence of skin sensitization to oseltamivir was studied in guinea pigs using a “maximization” test. Approximately 50% of animals treated with oseltamivir developed erythema. Transient eye irritation has been reported in rabbits.
Oseltamivir phosphate in very high single oral doses (up to 1310 mg/kg) had no effect on adult rats, but these doses were toxic in young 7-day-old rats, causing, among other things, deaths. These effects were noted at doses of 657 mg/kg or more. When using a dose of 500 mg/kg, no adverse reactions were noted, incl. and with chronic administration at a dose of 500 mg/kg/day from 7 to 21 days of the postnatal period.
Use of the drug Tamiflu
During preclinical studies, oseltamivir and its active metabolite were excreted into the milk of lactating rats. Whether oseltamivir or the active metabolite is excreted in human milk is unknown, but their amounts in breast milk may be 0.01 and 0.3 mg/day, respectively. Since there is insufficient data on the use of the drug in pregnant women, Tamiflu should be prescribed during pregnancy or nursing mothers only if the possible benefits of its use outweigh the potential risk to the fetus or infant. Inside, during meals or regardless of meals. Tolerability of the drug can be improved if taken with food. Preparation of the suspension:
- Gently tap the closed bottle with your finger several times so that the powder is distributed at the bottom of the bottle.
- Measure 52 ml of water using a measuring cup, filling it to the indicated level.
- Add 52 ml of water to the bottle, cap and shake well for 15 seconds.
- Remove the cap and insert the adapter into the neck of the bottle.
- Screw the cap onto the bottle tightly to ensure proper placement of the adapter.
The expiration date of the prepared suspension should be indicated on the bottle label. Before use, the bottle with the prepared suspension must be shaken. To dose the suspension, a dosing syringe is supplied with labels indicating dose levels of 30, 45 and 60 mg (see "Form and Packaging"). In cases where adults, adolescents ≥12 years of age and children weighing >40 kg or ≥8 years have problems swallowing capsules and Tamiflu powder for the preparation of oral suspension is not available, or if there are signs of “aging” of the capsules, it is necessary open the capsule and pour the contents into a small amount (maximum 1 teaspoon) of a suitable sweetened food (chocolate syrup (normal or sugar-free), honey, light brown sugar or table sugar dissolved in water, sweet dessert , sweetened condensed milk, applesauce or yogurt) to cover up the bitter taste. The mixture must be mixed thoroughly and given to the patient as a whole. The mixture should be swallowed immediately after preparation. Detailed recommendations are given below (see “Extemporaneous preparation of Tamiflu suspension”). Standard dosage regimen Treatment The drug should be started no later than 2 days from the development of symptoms of the disease. Adults and adolescents ≥12 years 75 mg (capsules or suspension) 2 times a day orally for 5 days. Increasing the dose to more than 150 mg/day does not increase the effect. Children >40 kg or ≥8 years Children who can swallow capsules can also be treated by taking 1 capsule. 75 mg 2 times a day, as an alternative to the recommended dose of Tamiflu suspension (see below). Children ≥1 year
Table 1 Recommended dosage regimen for Tamiflu oral suspension
Weight, kg | Recommended dose for 5 days (2 times a day, mg) |
| ≤15 | 30 |
| >15-23 | 45 |
| >23-40 | 60 |
| >40 | 75 |
To dose the suspension, use the supplied syringe marked 30, 45 and 60 mg. Take the required amount of suspension from the bottle with a dosing syringe, transfer it to a measuring cup and take orally.
Prevention Adults and adolescents ≥12 years old 75 mg 1 time per day for at least 10 days after contact with an infected person. The drug should be taken no later than in the first 2 days after contact. During a seasonal flu epidemic - 75 mg 1 time per day for 6 weeks. The preventive effect lasts as long as the drug is taken. Children >40 kg Children who can swallow capsules can also receive preventive therapy by taking 1 capsule. 75 mg once daily as an alternative to the recommended dose of Tamiflu suspension (see below). Children ≥1 year
Table 2 Recommended dosage regimen for Tamiflu suspension for oral administration
Weight, kg | Recommended dose for 10 days (once daily, mg) |
| ≤15 | 30 |
| >15-23 | 45 |
| >23-40 | 60 |
| >40 | 75 |
To dose the suspension, use the supplied syringe marked 30, 45 and 60 mg. Take the required amount of suspension from the bottle with a dosing syringe, transfer it to a measuring cup and take orally.
Dosing in special cases Patients with kidney damage Treatment. Patients with creatinine Cl >30 ml/min do not require dose adjustment. In patients with creatinine Cl from 10 to 30 ml/min, the dose of Tamiflu should be reduced to 75 mg once a day for 5 days. There are no dosing recommendations for patients on chronic hemodialysis or chronic peritoneal dialysis for end-stage chronic renal failure and for patients with creatinine Cl ≤10 ml/min. Prevention. Patients with creatinine Cl >30 ml/min do not require dose adjustment. In patients with creatinine Cl from 10 to 30 ml/min, it is recommended to reduce the dose of Tamiflu to 75 mg every other day or 30 mg suspension daily. There are no dosing recommendations for patients on chronic hemodialysis or chronic peritoneal dialysis for end-stage chronic renal failure and for patients with creatinine Cl ≤10 ml/min. Patients with liver damage. No dose adjustment is required for the treatment and prevention of influenza in patients with mild to moderate liver dysfunction. The safety and pharmacokinetics of Tamiflu in patients with severe hepatic impairment have not been studied. Elderly patients. No dose adjustment is required for the prevention or treatment of influenza. Children. The safety and effectiveness of Tamiflu in children under 1 year of age have not been established. Extemporaneous preparation of Tamiflu suspension In cases where adults, adolescents and children have a problem with swallowing capsules, and the powder for preparing the Tamiflu oral suspension is not available or if there are signs of “aging” of the capsules, it is necessary to open the capsule and pour its contents into a small amount ( maximum 1 teaspoon) of a suitable sweetened food item (see above) to cover the bitter taste. The mixture must be mixed thoroughly and allowed to be taken completely by the patient. The mixture should be taken immediately after preparation. If patients require a dose of 75 mg, the following instructions should be followed:
- Carefully open one 75 mg Tamiflu capsule over a small container and pour the powder into the container.
- Add a small amount (no more than 1 teaspoon) of a suitable sweetened food (to cover the bitter taste) and mix well.
- Mix the mixture thoroughly and give the entire contents of the container to the patient. The mixture should be taken immediately after preparation. If there is a small amount of mixture left in the container, you should rinse the container with a small amount of water and give the patient the remaining mixture to drink.
If patients require doses of 30–60 mg, the following instructions should be followed for proper dosing:
- Carefully open one 75 mg Tamiflu capsule over a small container and pour the powder into the container.
- Add 5 ml of water to the powder using a syringe with marks indicating the amount of liquid collected. Mix thoroughly for 2 minutes.
- Draw the required amount of mixture from the cup into the syringe according to Table 3. There is no need to withdraw undissolved white powder, since it is an inactive filler. By pressing the plunger of the syringe, inject all its contents into the second container. Any remaining unused mixture should be discarded.
- In a second container, add a small amount (no more than 1 teaspoon) of a suitable sweetened food to cover the bitter taste and mix well.
- Mix the mixture thoroughly and give the entire contents of the second container to the patient. The mixture should be taken immediately after preparation. If there is a small amount of mixture left in the container, you should rinse the container with a small amount of water and give the patient the remaining mixture to drink.
Repeat this procedure before each dose of the drug.
Table 3 Recommended dosage regimen for Tamiflu suspension for oral extemporaneous preparation
Weight, kg | Recommended dose, mg | Amount of mixture per dose, ml |
| ≤15 | 30 | 2 |
| >15-23 | 45 | 3 |
| >23-40 | 60 | 4 |
Experience with the use of oseltamivir (Tamiflu) for influenza and ARVI in children
More than half a million people die each year from influenza and its complications [1]. The most effective way to reduce morbidity is specific vaccine prevention. But since influenza viruses are capable of antigenic variability in the surface proteins hemagglutinin and neuraminidase, vaccine developers are forced to annually create new antigenic drugs, the release of which takes at least 9 months, and therefore it is impossible to guarantee a complete match between the vaccine and the circulating strain [2]. (In addition, given the emerging threat of a new pandemic strain of avian influenza (H5N1), which may appear quite suddenly, the question arises of the need to use effective etiotropic chemotherapy drugs.)
Currently, the class of anti-influenza drugs includes the adamantadines amantadine and rimantadine, whose action is aimed at M2 ion channels and the neuraminidase inhibitors oseltamivir and zanamivir [3]. However, adamantadines are currently 30–60% ineffective. Thus, over 10 years of observation, the frequency of cases of drug resistance increased from 0.4% in 1995 to 12.3% in 2004. The development of resistance to both drugs is associated with a mutation of the influenza A virus, as a result of which the function of the M2 protein ion channel was changed protected from the action of adamantadines [4, 5, 6, 7, 8].
On the contrary, the mutations necessary for the development of resistance to neuraminidase inhibitors occur in a hard-to-reach region of the neuraminidase enzyme molecule, which reduces its activity and the possibility of developing resistance, thereby determining the activity of inhibitors of this enzyme against all subtypes of viruses A and B.
The first drug in this group was zanamivir, intended for local use in the form of inhalation, but does not have a systemic effect against influenza, which led to the development of a new drug - oseltamivir (Tamiflu). According to the literature, the use of Tamiflu for influenza in adult patients reduces the severity of the disease, shortens the period of clinical manifestations, and reduces the frequency of complications requiring the use of antibacterial agents [9, 10, 11, 12, 13]. However, little information has been collected on the use of Tamiflu in children, which served as the purpose of this study to study the clinical effectiveness and safety of this drug for influenza and other respiratory infections in children.
50 children aged 12 to 15 years were observed, 25 of whom received Tamiflu (main group) and 25 received conventional treatment (control group). 34% of children were classified as frequently ill. 9 children had chronic pathology: 1 had recurrent obstructive bronchitis, 1 had bronchial asthma, 7 had chronic tonsillitis, 2 had hay fever.
Based on the totality of clinical data, influenza could be diagnosed in 16 patients of the main and 14 control groups, parainfluenza - in 9 and 11 patients, adenoviral infection - in 8 and 6 patients, respectively.
The drug Tamiflu at a dose of 75 mg was prescribed in the acute period of the disease 2 times a day no later than the first 30 hours of the disease for 5 days against the background of concomitant symptomatic therapy. From the first day of seeing a doctor, children from the control group received antihistamines, antipyretics, and mucolytic drugs, and 13 of them, in addition to symptomatic treatment, received antibiotics due to the occurrence of bacterial complications.
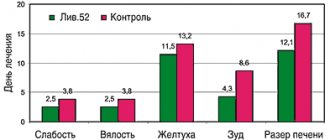
Patients treated with Tamiflu had a significantly shorter duration of clinical symptoms, regardless of the severity of ARVI (Figure a).
As can be seen from the data presented, the duration of fever and other manifestations of intoxication syndrome (malaise, loss of appetite, weakness, drowsiness, decreased physical activity) was reduced by 3 or more times; rhinitis - 2.6 times; During Tamiflu therapy, a productive cough with a sputum thinning effect appeared from the 2nd–3rd day, and in the control group - after the 4th day. The maximum clinical effect among patients with ARVI was observed in 72% of children already on the second day of taking the drug. At the same time, 67% of children showed a change in sputum density towards its dilution, while in the control group these changes were recorded only in 43% of children.
In ARVI patients from the control group with bacterial complications, the duration of fever and other manifestations of intoxication syndrome decreased by 4.4 times; duration of rhinitis - 3.2 times; A productive cough with a sputum thinning effect appeared during Tamiflu treatment on average from the 2nd day, and in the control group - from the 5th day (Figure b).
The drug was well tolerated in all children. There were no adverse reactions in the form of abdominal pain, nausea, vomiting, dyspepsia, sensation of a lump or foreign body, dryness/soreness in the throat associated with receiving Tamiflu. No allergic reactions were detected during Tamiflu therapy. In children suffering from atopic dermatitis, hay fever, and bronchial asthma, there was also no aggravation of the manifestations of the disease.
The study showed that Tamiflu is harmless, well tolerated, and effective in treating not only influenza, but also ARVI. The mechanism of clinical improvement in ARVI during treatment with Tamiflu is not entirely clear. Since during treatment with the drug (75 mg 2 times a day for 5 days), all children, regardless of the etiology of the disease, showed a significant improvement in well-being and a rapid reversal of clinical manifestations, it can be assumed that Tamiflu inhibits not only neuraminidase, but also other factors of viral pathogenicity. Undoubtedly, the clinical effect of the drug is directly related to the suppression of viral aggression, and this, as is known, leads to a decrease in the level of pro-inflammatory cytokines, the number of cells expressing CD95+, a decrease in the adhesive ability of mononuclear blood cells, activation of lymphocytes, targeted polarization of Th-0 lymphocytes in Th-1, expression on the cell membrane of HLA-DR class 11, stimulation of the phagocytic system of macrophages and neutrophils, growth and activation of cytotoxic and NK cells [14, 15, 16].
Such changes in the immune system contribute to the suppression of bacterial and fungal flora, determining the uncomplicated course of the disease.
Our data are consistent with information from other authors. Thus, according to L. Kaiser (2003), when using Tamiflu, the symptoms of the underlying disease not only resolve faster, but also reduce by 55%, compared with placebo, the frequency of secondary complications from the lower respiratory tract, as well as the number of hospitalizations. In high-risk patients, the incidence of bacterial complications requiring antibiotic therapy decreased by 34%, and the number of hospitalizations decreased by 59% [17].
According to CM Machado (2004), in patients with weakened immunity as a result of bone marrow transplantation, Tamiflu was not only highly effective, but also well tolerated [18].
The work of RJ Whitley (2001) showed the effectiveness of Tamiflu in children aged 1 year and older. In children receiving the drug, recovery occurred 36 hours earlier than in the control group. At the same time, the frequency of bacterial complications decreased by 44%, and a significant increase in forced expiratory volume in 1 s was observed in children with bronchial asthma [19].
Based on the results of our study, the following conclusions can be drawn.
- Oseltamivir (Tamiflu) can be considered the drug of choice for the treatment of influenza and other respiratory infections.
- Modern etiotropic therapy of influenza and acute respiratory viral infections with the drug Tamiflu helps reduce the duration of fever, intoxication phenomena, as well as the timing of relief of catarrhal symptoms.
- With systemic therapy with oseltamivir (Tamiflu), the development of airway obstruction and bacterial complications is not observed, which allows one to avoid hospitalization and refuse to prescribe antibacterial therapy.
- No cases of hypersensitivity to Tamiflu have been identified; the drug is well tolerated and has no contraindications.
- Oseltamivir (Tamiflu) for influenza and other respiratory infections should be prescribed 75 mg 2 times a day for 5 days in children over 12 years of age, in the acute period of the disease - no later than 48 hours from the onset of the first symptoms.
For questions regarding literature, please contact the editor.
O. V. Kladova , Doctor of Medical Sciences T. F. Pogodina V. F. Uchaikin , Doctor of Medical Sciences, Professor, Academician of the Russian State Medical University, Moscow
Side effects of Tamiflu
Adults. The most common are nausea and vomiting (usually after taking the first dose; they are transient in nature and in most cases do not require discontinuation of the drug). Side effects (≥1%): diarrhea, bronchitis, abdominal pain, dizziness, headache, cough, sleep disturbances, weakness; pain of various localizations, rhinorrhea, dyspepsia and upper respiratory tract infections. Children. The most common thing is vomiting. Abdominal pain, nosebleeds, hearing impairment, conjunctivitis (occurred suddenly, stopped despite continued treatment, and in the vast majority of cases did not cause cessation of treatment), nausea, diarrhea, asthma (including exacerbation), acute otitis media , pneumonia, sinusitis, bronchitis, dermatitis, lymphadenopathy. Post-marketing surveillance From the skin and subcutaneous tissue: rarely - hypersensitivity reactions: dermatitis, skin rash, eczema, urticaria, very rarely - erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis, anaphylactic and anaphylactoid reactions, Quincke's edema. From the liver: very rarely - hepatitis, increased liver enzymes. Neuropsychiatric: Convulsions and delirium have been reported in patients (mainly children and adolescents) taking Tamiflu to treat influenza (including symptoms such as impaired consciousness, disorientation in time and space, abnormal behavior, delirium, hallucinations, agitation, anxiety, nightmares). These cases rarely involved life-threatening actions. The role of Tamiflu in the development of these phenomena is unknown. Similar neuropsychiatric disorders were also noted in patients with influenza who did not receive Tamiflu. From the gastrointestinal tract: cases of gastrointestinal bleeding were rarely observed during treatment with Tamiflu (in particular, a connection between the phenomena cannot be excluded; they disappeared both after the patient recovered from the flu, as well as after discontinuation of the drug).
Special instructions for the use of Tamiflu
Seizures and delirium-like neuropsychiatric disorders have been reported in patients (mostly children and adolescents) taking Tamiflu to treat influenza. These cases involved life-threatening actions. The role of Tamiflu in the development of these phenomena is unknown. Similar neuropsychiatric disorders were also noted in patients with influenza who did not receive Tamiflu. Careful monitoring of the behavior of patients, especially children and adolescents, is recommended to identify signs of abnormal behavior. There is no data on the effectiveness of Tamiflu for any diseases caused by pathogens other than influenza A and B viruses. When treating and preventing influenza in patients with creatinine Cl from 10 to 30 ml/min, dose adjustment is required. There are no recommendations for dose adjustment in patients receiving hemodialysis or peritoneal dialysis and in patients with creatinine Cl ≤10 ml/min. A 30 g bottle of Tamiflu with powder for preparing a suspension contains 25.713 g of sorbitol. When taking 45 mg of Tamiflu 2 times a day, 2.6 g of sorbitol enters the body. In patients with congenital fructose intolerance, this amount exceeds the daily norm of sorbitol. After preparation, store the suspension at a temperature of 2–8 °C for 17 days or at a temperature not exceeding 25 °C for 10 days. Do not use the suspension after the expiration date.
Tamiflu drug interactions
Clinically significant drug interactions are unlikely. Drug interactions due to competition and binding to the active sites of esterases that convert oseltamivir phosphate into the active substance are not presented. The low degree of binding of oseltamivir and the active metabolite to proteins does not give reason to assume the presence of interactions associated with the displacement of drugs from binding to proteins. In vitro, oseltamivir phosphate and the active metabolite are not the preferred substrate for polyfunctional oxidases of the cytochrome P450 system or for glucuronyltransferases (see “Pharmacokinetics”). There are no reasons for interaction with oral contraceptives. Cimetidine, a nonspecific inhibitor of cytochrome P450 isoenzymes, amoxicillin and paracetamol do not affect plasma concentrations of oseltamivir and its active metabolite. Probenecid leads to an approximately 2-fold increase in the AUC of the active metabolite of oseltamivir, but no dose adjustment is required when used concomitantly with probenecid. When Tamiflu is prescribed together with ACE inhibitors (enalapril, captopril), thiazide diuretics (bendrofluazide), antibiotics (penicillin, cephalosporins, azithromycin, erythromycin and doxycycline), histamine H2 receptor blockers (ranitidine, cimetidine), beta-blockers (propranolol) no changes in the nature or frequency of adverse events were observed.
Tamiflu 75 mg No. 10 caps.
Instructions for medical use of the drug Tamiflu Trade name Tamiflu International nonproprietary name Oseltamivir Dosage form Capsules 75 mg Composition One capsule contains the active substance - oseltamivir phosphate 98.50 (equivalent to oseltamivir) (75.00) excipients: pregelatinized starch, povidone K30, croscarmellose sodium, talc, sodium stearyl fumarate, capsule composition: body – iron (III) black oxide E 172, titanium dioxide E 171, gelatin; cap – iron (III) oxide red E 172, iron (III) oxide yellow E 172, titanium dioxide E 171, gelatin; printing ink. Description Hard gelatin capsules, size No. 2, with an opaque gray body and an opaque light yellow cap, marked in blue “ROCHE” on the body and “75 mg” on the cap. The contents of the capsule are white to yellowish-white powder. Pharmacotherapeutic group: Direct acting antiviral drugs. Neuraminidase inhibitors. ATC code J05AH02 Pharmacological properties Pharmacokinetics After oral administration of oseltamivir, phosphate is easily absorbed from the gastrointestinal tract and is highly converted into an active metabolite under the action of hepatic esterases. Concentrations of the active metabolite in plasma are determined within 30 minutes, reach almost maximum levels 2–3 hours after administration and are significantly (more than 20 times) higher than the concentrations of the prodrug. At least 75% of the dose taken orally enters the systemic circulation in the form of an active metabolite, less than 5% in the form of the parent drug. Plasma concentrations of both the prodrug and the active metabolite are dose proportional and independent of food intake. The average volume of distribution (Vss) of the active metabolite is approximately 23 liters. The binding of the active metabolite to plasma proteins is insignificant (about 3%). Pro-drug binding to plasma proteins is 42%, which is not enough to cause significant drug interactions. Oseltamivir phosphate is highly converted into an active metabolite under the action of esterases located mainly in the liver and intestines. Neither oseltamivir phosphate nor the active metabolite are substrates or inhibitors of cytochrome P450 isoenzymes. Absorbed oseltamivir is eliminated mainly (>90%) by conversion to an active metabolite. The active metabolite does not undergo further transformation and is excreted in the urine (>99%). In most patients, the plasma half-life of the active metabolite is 6–10 hours. The active metabolite is eliminated completely (>99%) by renal excretion. Renal clearance (18.8 L/hour) exceeds the glomerular filtration rate (7.5 L/hour), indicating that the drug is also eliminated by tubular secretion. Less than 20% of the radiolabeled drug taken orally is excreted in the feces. Pharmacokinetics in special groups Patients with kidney damage When prescribing Tamiflu 100 mg 2 times a day for 5 days to patients with varying degrees of kidney damage, the area under the plasma active metabolite concentration-time curve (AUC) is inversely proportional to the decrease in renal function. The pharmacokinetics of oseltamivir in patients with end-stage renal disease (creatinine clearance ≤10 ml/min) not on dialysis have not been studied. Patients with liver damage An in vitro study showed that in patients with hepatic pathology, the AUC value of oseltamivir phosphate is not significantly increased, and the AUC of the active metabolite is not reduced. Elderly patients In elderly patients (65–78 years), the AUC of the active metabolite at steady state was 25–35% higher than in younger patients when similar doses of Tamiflu were prescribed. The half-life of the drug in the elderly did not differ significantly from that in younger adult patients. Taking into account the data on the AUC of the drug and tolerability, dose adjustment is not required for elderly patients in the treatment and prevention of influenza. Children The pharmacokinetics of Tamiflu have been studied in children from 1 to 16 years of age in a single-dose pharmacokinetic study and in a clinical study in a small number of children aged 3–12 years. In young children, elimination of the prodrug and active metabolite was faster than in adults, resulting in lower AUCs relative to a given dose. Taking the drug at a dose of 2 mg/kg gives the same AUC of oseltamivir carboxylate as is achieved in adults after a single dose of a capsule with 75 mg of the drug (equivalent to approximately 1 mg/kg). The pharmacokinetics of oseltamivir in children over 12 years of age is the same as in adults. In children 6-12 months of age, administration of oseltamivir at a dosage of 3 mg/kg twice daily provides plasma levels of the active metabolite similar to the level demonstrating clinical effectiveness in older children and adults. Pharmacodynamics Antiviral drug. Oseltamivir phosphate is a prodrug, its active metabolite (oseltamivir carboxylate) competitively and selectively inhibits neuraminidase of influenza viruses type A and B - an enzyme that catalyzes the process of releasing newly formed viral particles from infected cells, their penetration into respiratory epithelial cells and further spread of the virus in the body . Oseltamivir carboxylate acts outside of cells. Inhibits the growth of the influenza virus in vitro and suppresses the replication of the virus and its pathogenicity in vivo, reduces the release of influenza A and B viruses from the body. Its concentrations required to inhibit enzyme activity by 50% (IC50) are at the lower end of the nanomolar range. When taking Tamiflu for the purpose of post-exposure (7 days) and seasonal (42 days) prevention of influenza, resistance to the drug is not observed. The frequency of transient isolation of influenza virus with reduced sensitivity of neuraminidase to oseltamivir carboxylate in adult patients with influenza is 0.4%. Elimination of the resistant virus from the body of patients receiving Tamiflu occurs without worsening the clinical condition of the patients. Indications for use: Treatment of influenza in adults and children, including full-term neonates, who exhibit flu-like symptoms when influenza virus is circulating in the population. Efficacy has been demonstrated when treatment is started within 2 days of the first onset of flu symptoms. - prevention of influenza in adults and children: - prevention of influenza in adults and children over 1 year of age after cases of contact with persons with clinically confirmed influenza when the influenza virus is circulating among the population - prevention of influenza in children under 1 year of age during an influenza pandemic. Method of administration and dosage Tamiflu is taken orally, with meals or regardless of meals. Some patients tolerate the drug better if it is taken with food. Treatment should begin on the first or second day of flu symptoms. In cases where adults, adolescents ≥12 years of age and children weighing >40 kg or ≥8 years of age have difficulty swallowing capsules, open the capsule and pour its contents into a small amount (maximum 1 teaspoon) of a suitable sweetened food ( chocolate syrup (sweetened or unsweetened), honey dissolved in water, sweet dessert, sweetened condensed milk, applesauce or yogurt) to cover up the bitter taste. The mixture must be mixed thoroughly and given to the patient as a whole. The mixture should be swallowed immediately after preparation. Standard dosage regimen Adults and adolescents over 13 years of age (with body weight more than 40 kg): Treatment of influenza: Recommended dosage regimen of Tamiflu - one capsule 75 mg 2 times a day orally for 5 days or 75 mg suspension 2 times a day orally within 5 days. Prevention of influenza: The recommended dose of Tamiflu for the prevention of influenza after contact with an infected person is 75 mg 1 time per day orally for 10 days. The drug should be taken no later than in the first 2 days after contact. The recommended dose for prevention during a seasonal influenza epidemic is 75 mg once a day; the effectiveness and safety of the drug when taken for 6 weeks is shown. The preventive effect lasts as long as the drug is taken. Children from 1 year to 12 years: Treatment of influenza: Body weight Recommended dosage for 5 days: 10 – 15 kg 30 mg twice a day 15 – 23 kg 45 mg twice a day 23 – 40 kg 60 mg twice a day > 40 kg 75 mg twice daily Flu prevention: Body weight Recommended dosage for 10 days: 10 – 15 kg 30 mg once daily 15 – 23 kg 45 mg once daily 23 – 40 kg 60 mg once daily day > 40 kg 75 mg once daily The effectiveness of Tamiflu for prophylaxis during seasonal influenza epidemics in children under 12 years of age has not been studied. Children under 1 year of age Treatment of influenza: The recommended dose for children under 1 year of age is 3 mg/kg body weight twice a day. Body weight Recommended dosage for 5 days: 3 kg 9 mg twice daily 4 kg 12 mg twice daily 5 kg 15 mg twice daily 6 kg 18 mg twice daily 7 kg 21 mg twice daily 8 kg 24 mg twice daily 9 kg 27 mg twice daily 10 kg 30 mg twice daily This dosing regimen is not applicable to preterm neonates (ie, those born before 36 weeks). There is insufficient data on dosing in this group of patients. Prevention of influenza: The recommended dose of Tamiflu for the prevention of influenza during a pandemic in children under 1 year of age is half the therapeutic dose - 3 mg/kg once a day for 10 days. The effectiveness of Tamiflu for prevention during seasonal influenza epidemics in children under 1 year of age has not been studied. Dosing in special cases Patients with kidney damage Treatment of influenza. Patients with creatinine clearance more than 30 ml/min do not require dose adjustment. In patients with a creatinine clearance of 10 to 30 ml/min, the dose of Tamiflu should be reduced to 75 mg once daily for 5 days. For patients on chronic hemodialysis, Tamiflu can be prescribed at a dose of 30 mg before the dialysis session. To maintain oseltamivir plasma concentrations, Tamiflu 30 mg should be administered after each hemodialysis session. For peritoneal dialysis, Tamiflu is prescribed at a dose of 30 mg before the dialysis session, and then 30 mg per day for 5 days. In patients with end-stage chronic renal failure (creatinine clearance less than 10 ml/min) who are not on hemodialysis, the pharmacokinetics of oseltamivir have not been studied. Flu prevention. Patients with creatinine clearance more than 30 ml/min do not require dose adjustment. In patients with a creatinine clearance of 10 to 30 ml/min, the dose of Tamiflu should be reduced to 75 mg every other day or to 30 mg every day. For patients on chronic hemodialysis, Tamiflu can be prescribed at a dose of 30 mg before the dialysis session. To maintain plasma concentrations of oseltamivir, Tamiflu at a dose of 30 mg should be administered after one hemodialysis session, at the end of the procedure. For peritoneal dialysis, Tamiflu is prescribed at a dose of 30 mg before the dialysis session, and then 30 mg every 7 days. Patients with liver damage No dose adjustment is required for the treatment and prevention of influenza. Elderly patients Dose adjustment is not required for the treatment and prevention of influenza. Extemporaneous preparation of Tamiflu In cases where adults, adolescents and children have a problem with swallowing capsules, and Tamiflu in the dosage form “powder for oral suspension” is not available or if there are signs of “aging” of the capsules, it is necessary to open the capsule and pour out its contents in a small amount (maximum 1 teaspoon) of a suitable sweetened food product to cover the bitter taste. The mixture must be mixed thoroughly and given to the patient as a whole. The mixture should be swallowed immediately after preparation. If patients require a 75 mg dose, the following instructions should be followed: 1. Holding one Tamiflu 75 mg capsule over a small container, carefully open the capsule and pour the powder into the container. 2. Add a small amount (no more than 1 teaspoon) of a suitable sweetened food (to cover the bitter taste) and mix well. 3. Mix the mixture thoroughly and drink it immediately after preparation. If there is a small amount of mixture left in the container, you should rinse the container with a small amount of water and drink the remaining mixture. If patients require doses of 30-60 mg, the following instructions must be followed for proper dosing: 1. Holding one Tamiflu 75 mg capsule over a small container, carefully open the capsule and pour the powder into the container. 2. Add 5 ml of water to the powder using a syringe with marks indicating the amount of liquid collected. Mix thoroughly for 2 minutes. 3. Draw the required amount of the mixture into the syringe from the container according to the table below: Body weight Recommended dose Amount of Tamiflu® mixture per dose £15 kg 30 mg 2 ml >15-23 kg 45 mg 3 ml >23-40 kg 60 mg 4 ml There is no need to collect undissolved white powder as it is an inactive filler. By pressing the plunger of the syringe, inject all its contents into the second container. Any remaining unused mixture should be discarded. 4. In a second container, add a small amount (no more than 1 teaspoon) of a suitable sweetened food to cover the bitter taste and mix well. 5. Mix the mixture thoroughly and drink it immediately after preparation. If there is a small amount of mixture left in the container, you should rinse the container with a small amount of water and drink the remaining mixture. Side effects Very often (>10%) - nausea, vomiting - headache Often (1-10%) - back pain - cough, nosebleeds, nasal congestion, nasopharyngitis, bronchitis, otitis media, sinusitis, bronchospasm, - diarrhea, pain in the epigastric region - herpes - irritability, fatigue Uncommon (1-0.1%) - insomnia - allergic reactions (dermatitis) - conjunctivitis - lymphadenopathy Rarely (<0.1%) - hypersensitivity reactions (including dermatitis, rash, eczema, multimorphic erythema, anaphylactic reactions), Stevens-Johnson syndrome - gastrointestinal bleeding - hepatitis, increased levels of liver enzymes - convulsions, delirious states, overexcitement. Contraindications - hypersensitivity to oseltamivir phosphate or any component of the drug - chronic renal failure (continuous hemodialysis, chronic peritoneal dialysis, creatinine clearance ≤10 ml/min) Drug interactions Information obtained in pharmacological and pharmacokinetic studies of oseltamivir phosphate allows us to consider clinically significant drug interactions unlikely. Drug interactions caused by competition and binding to the active sites of esterases that convert oseltamivir phosphate into the active substance are not covered in detail in the literature. The low degree of binding of oseltamivir and the active metabolite to proteins does not give reason to assume the presence of interactions associated with the displacement of drugs from protein binding. Neither oseltamivir phosphate nor the active metabolite is a preferred substrate for polyfunctional cytochrome P450 oxidases or glucuronyltransferases. There is no formal basis for interaction with oral contraceptives. Cimetidine, a nonspecific inhibitor of cytochrome P450 isoenzymes, does not affect plasma concentrations of oseltamivir and its active metabolite. Clinically significant drug-drug interactions associated with competition for tubular secretion are unlikely, taking into account the safety margin for most of these drugs, the routes of elimination of the active metabolite of oseltamivir (glomerular filtration and anionic tubular secretion), as well as the excretory capacity of each of the routes. Co-administration of probenecid leads to an approximately 2-fold increase in the AUC of the active metabolite. However, no dose adjustment is required when used concomitantly with probenecid. Concomitant use with amoxicillin does not affect the plasma concentrations of both drugs. Pharmacokinetic interactions between oseltamivir and its main metabolite were not detected when taken simultaneously with paracetamol, acetylsalicylic acid, cimetidine or antacids (magnesium and aluminum hydroxide, calcium carbonate). When Tamiflu is prescribed together with commonly used drugs, such as ACE inhibitors (enalapril, captopril), thiazide diuretics, antibiotics (penicillin, cephalosporins, azithromycin, erythromycin and doxycycline), histamine H2 receptor blockers (ranitidine, cimetidine), beta blockers (propranolol), xanthines (theophylline), sympathomimetics (pseudoephedrine), opiates (codeine), corticosteroids, inhaled bronchodilators and analgesics (aspirin, ibuprofen and paracetamol), no changes in the nature or frequency of adverse events were observed. Special instructions There is no data on the effectiveness of Tamiflu for any diseases caused by pathogens other than influenza A and B viruses. Children's age Children under 13 years of age weighing less than 40 kg are recommended to use Tamiflu powder to prepare an oral suspension. Children 6-12 months: The effectiveness of Tamiflu in children under 1 year of age has not been established. However, there are limited pharmacokinetic data in children 6-12 months of age, which suggest that oseltamivir 3 mg/kg twice daily provides plasma levels of the active metabolite similar to those demonstrated for clinical efficacy in older children and adults. Pregnancy and lactation No controlled clinical studies have been conducted to evaluate the safety of Tamiflu in pregnant women. Tamiflu should be prescribed during pregnancy or lactation only if the possible benefits of its use outweigh the potential risk to the fetus or infant and the safety, pathogenicity of the specific influenza virus strain and the health status of the pregnant woman have been assessed. Features of the effect on the ability to drive vehicles or potentially dangerous mechanisms No effect Overdose Currently, no cases of overdose have been described, however, the expected symptoms of an acute overdose will be nausea with or without vomiting. Single doses of Tamiflu up to 1000 mg were well tolerated, with the exception of nausea and vomiting. Release form and packaging 10 capsules are placed in a blister pack made of polyvinyl chloride film/polyvinyl dichloride film and aluminum foil. 1 blister pack together with instructions for medical use in the state and Russian languages are placed in a cardboard box. Storage conditions Store at a temperature not exceeding 25 °C. Keep out of the reach of children! Shelf life: 7 years Do not use after the expiration date indicated on the package. Conditions for the vacation from pharmacies according to the recipe manufacturer Senxi CAS, France The legal address of the manufacturer: 52 Rue M. et J. Gaucher, 94120 fontenay-sous-nowis, France, the owner of the registration certificate F. Hoffmann-la Rosh LTD. La Rosh LTD., Switzerland, the address of the organization receiving in the territory of the Republic of Kazakhstan claims from consumers on the quality of goods and the Rosh Kazakhstan LLP 050,000, Almaty, ul. Kunaeva, 77, business, 15th floor of tel., Fax: + e-mail,