Pharmacodynamics
Pimecrolimus is a derivative of the macrolactam ascomycin and has an anti-inflammatory effect. Pimecrolimus selectively inhibits the production and release of cytokines and inflammatory mediators from T lymphocytes and mast cells.
Pimecrolimus specifically binds to the cytosolic receptor macrophilin-12 and inhibits the calcium-dependent phosphatase calcineurin. Inhibition of calcineurin leads to suppression of T-lymphocyte proliferation and prevents the transcription and production of early cytokines in T-helper types 1 and 2, such as IL-2, interferon-γ, IL-4, IL-5, IL-10, tumor necrosis factor (TNFα) and granulocyte-macrophage colony-stimulating factor. Pimecrolimus and tacrolimus equally suppress the secondary immune response in isolated skin T helper cell colonies obtained from patients with atopic dermatitis.
In addition, in vitro, after interaction with the antigen/IgE complex, pimecrolimus prevents the antigen/IgE-mediated release of cytokines and inflammatory mediators from mast cells. Pimecrolimus does not affect the growth of keratinocytes, fibroblasts and endothelial cells and, unlike corticosteroids, has a selective effect on cells of the immune system and does not cause dysfunction, viability, differentiation processes, maturation of Langerhans cells in mice and dendritic cells of monocytic origin in humans. The drug does not affect the differentiation of “naive” T-lymphocytes into T-effector cells under the influence of Langerhans cells and dendritic cells, which is one of the main mechanisms of a specific immune response.
In experimental models of skin inflammation, the high anti-inflammatory activity of pimecrolimus was demonstrated after its local and systemic application. When applied topically in experimental models of allergic contact dermatitis (ACD), pimecrolimus is comparable in effectiveness to highly active corticosteroids: clobetasol-17-propionate and fluticasone, inhibits the inflammatory response in response to skin irritants, without causing changes in skin consistency and atrophy.
In addition, when administered topically and orally, pimecrolimus effectively reduces skin inflammation, itching, and the severity of histopathological changes in experimental models of ACD. When applied topically, the penetration of tacrolimus and pimecrolimus into the skin is equally good. However, the ability of pimecrolimus to penetrate the skin is less than that of tacrolimus and GCS. Thus, pimecrolimus has a selective effect on the skin.
The unique mechanism of action of pimecrolimus is the combination of a selective anti-inflammatory effect on the skin with a slight effect on the systemic immune response.
When used for 6 weeks in children aged 3 months to 17 years, pimecrolimus effectively reduces itching and skin inflammation (erythema, infiltration, excoriation and lichenification). With long-term use for 12 months, pimecrolimus effectively reduces the incidence of sudden exacerbations of ACD without causing atrophy, irritation or increased sensitivity of the skin, and without phototoxic or photosensitizing effects.
Experience of using Elidel cream in the treatment of atopic dermatitis in children and adults
A
topical dermatitis (AD) remains an important medical and social problem, the significance of which is determined by the steady increase in the incidence of dermatosis, its chronic, recurrent course and the complexity of therapy.
According to domestic and foreign dermatologists, the incidence of AD among the adult population is up to 1.5–2.0% of the general population
, varying in different countries depending on the level of urbanization and the state of ecological cleanliness of the living space (Toropova N.P. et al. , 1997; Kungurov N.V. et al., 2000; Schultz-Larsen F., Hanifin JM, 2002).
Clinically, AD is characterized by intense itching, the occurrence of inflammation, infiltration, lichenification in typical locations of the skin process, as well as general increased dryness of the skin.
The first symptoms of dermatosis, as a rule, occur in infancy and early childhood; relapses are often associated with eating disorders, stress, and exacerbations of somatic diseases. In more than a third of patients, the process with periodic exacerbations continues into adulthood, which leads to a significant decrease in the quality of life of patients (Emerson RM et al., 1998; Lewis-Jones MS et al., 2002).
A key role in the formation of AD belongs to a hereditary predisposition, which is realized by dysfunction of the immune system: hyperproduction of IgE, disruption of cytokine regulation and the ratio of Th1/|Th2 lymphocytes, deterministic deficiency of suppressor T lymphocytes, and disruption of apoptosis processes. In the pathogenesis of the disease, an imbalance of intracellular regulatory mechanisms (cAMP/cGMP ratio), disruption of membrane reception, activation of non-immune mechanisms for the release of allergic mediators, disruption of neurovegetative and peripheral circulation with vascular instability and disruption of endothelial reception are of significant importance); psychophysiological and psychosomatic abnormalities (Skripkin Yu.K. et al., 1997, Hanifin JM, Rajka G., 1980, Bos JD, Sillevis J H., 1996).
Treatment of patients with atopic dermatitis
Treatment of patients with AD poses a complex problem for a specialist due to the peculiarities of the pathogenesis of the disease, the diversity of phenotypic manifestations and the staged course of the process and, in the general opinion of researchers, should be etiopathogenetic in nature (Balabolkin I.I., Grebenyuk V.N., 1999; Sergeev Yu.V. et al., 2001; Fedenko E.S., 2001; Smolkin Yu.S., Cheburkin A.A., 2002).
External therapy for blood pressure
is included in the basic standard of treatment of the disease and is an integral part of the therapeutic complex. The practice of recent years indicates a prevailing trend in the use of a wide range of external agents containing various glucocorticosteroid hormones (GCSH) as active ingredients in the treatment of AD in adults and children. Currently, dermatologists have at their disposal a significant number of local treatment agents with GCSG, which have a universal antiallergic effect: reducing the release of mediators of allergic inflammation, the migration of cells into the affected area, and the proliferation of immunocompetent cells in the affected area. Modern external agents with moderate and strong action of GCSG, which do not contain fluorine atoms, have proven themselves in clinical practice to be effective and quite safe. Tactics and technologies of therapy using various dosage forms of methylprednisolone aceponate, mometasone furoate, alklometasone are described in a significant number of publications (Emelyanov A.V., Monakhov K.N., 2002; Smirnova G.I., 2003; Tofte SJ, Hanifin JM, 2001).
At the same time, in practical terms, there remains a danger of the formation of local adverse events and complications with prolonged and uncontrolled use of GCSG drugs. And this danger increases significantly when used in children, as well as with long-term applications of GCSG drugs on highly sensitive skin - face, neck, skin folds.
Modern conceptual approach
to the strategy and tactics of treating AD was presented in the materials of the II International Consensus Conference on Atopic Dermatitis II, 2003, held in the USA in February 2002.
The Consensus Statement adopted at the end of the conference identified the main goals of therapy for patients with AD:
– reducing the activity of manifestations and symptoms of AD;
– prevention or minimization of exacerbations;
– ensuring long-term control of the disease and measures to prevent exacerbations;
- alleviation of the course of the disease.
For local treatment of patients with AD, the main types of topical therapy for the disease were presented: moisturizers, external corticosteroids, non-steroidal drugs, and additional therapy. A new strategic class of topical agents were drugs containing calcineurin inhibitors and meeting the specified needs in the treatment of AD (Ellis C, Luger T., 2003).
One of these drugs, recently available in domestic clinical practice, is pimecrolimus cream 1% (Elidel®).
Elidel (E)
is a non-steroidal, cell-selective inhibitor of inflammatory cytokines, specially designed for the treatment of AD and other inflammatory skin diseases.
1 g of Elidel cream contains 10 mg of pimecrolimus, as well as excipients: triglycerides, oleyl alcohol, propylene glycol, stearyl alcohol, cetyl alcohol, mono- and diglycerides, sodium cetostearyl sulfate, benzyl alcohol, citric acid, sodium hydroxide, purified water.
Pimecrolimus is a derivative of the macrolactam ascomycin. Mechanism of action and pharmacological properties
pimecrolimus have been studied
in vitro
and
in vivo
. Studies have established that the pimecrolimus molecule binds with high affinity to its receptor located in the cytosol of the T lymphocyte, macrophilin-1. The pimecrolimus–macrophilin complex inhibits calcineurin, which is a calcium-dependent phosphatase (Grassberger M. et al., 1999). As a result of this inhibition, the transcription of messenger RNA, the corresponding genes of inflammatory cytokines, the synthesis and subsequent release of these activators of allergic inflammation from T lymphocytes (IL-2, IL-4, IL-10 and INF-g) are inhibited, the severity of proliferation of T lymphocytes of the dermal skin infiltrate, which is stimulated by these inflammatory cytokines (Winiski A et al, 2002).
Pimecrolimus also inhibits the synthesis of TNF-a and the release of inflammatory mediators such as histamine from mast cells (Hultsch T et al., 1998; Zuberbier T. et al., 2001). Experimental studies on animals and clinical studies of the effect of pimecrolimus in healthy volunteers using morphometry and local ultrasound have established that the drug does not have a pathological effect on keratinocytes, fibroblasts, endothelial cells or Langerhans cells, and does not cause skin atrophy (Meingassner JG et al., 1997 ; Zuberbier T. et al., 2001). Pimecrolimus has been found to have highly effective suppression of skin inflammation and a weak systemic immunosuppressive effect in animal models (Meingassner JG et al, 1997; Stuetz A. et al., 2001; Queille-Roussel C. et al., 2001; Billich A. et al., 2002).
Clinical studies of E in the treatment of patients with AD demonstrated a rapid onset of action of the drug, in which the intensity of itching decreased after 2-3 days of using E cream, and all symptoms of AD regressed in most patients during the course of therapy (Eichenfield L. et al., 2002) . The beneficial effect of treatment with pimecrolimus was observed throughout the study (up to 43 days) in infants 3–23 months and children 2–17 years old, and E was especially effective when the process was localized on the face and neck, and during subsequent clinical observation there was no significant AD symptoms persisted for up to 6 months (Eichenfield L et al., 2002; Ho V et al., 2003). Subsequent clinical studies confirmed the effectiveness of the use of E in the treatment of AD in children and adults, and established that long-term (up to 6 months) use of E contributes to disease control and prevents the development of severe exacerbations of AD, while at the same time does not lead to the development of significant side effects, such as as pyogenic or viral superinfection (Kapp A. et al., 2002; Wahn U. et al., 2002; Meurer M. et al., 2002).
The appearance on the Russian pharmaceutical market of a new external agent for the treatment of blood pressure and these provisions determined the relevance of accumulating and analyzing our own clinical experience in the use of Elidel cream (1% pimecrolimus).
Purpose
An open non-comparative clinical study conducted at the Ural Research Institute of Dermatovenereology and Immunopathology of the Ministry of Health of the Russian Federation, as well as on the basis of the KVU in Tyumen, Magnitogorsk and Samara, studied the effectiveness of the use of the drug Elidel (cream) in the treatment of patients with AD in children, adolescents and adults.
A total of 49 patients with AD were included in the study:
– children aged from 6 months to 15 years – 16;
– adolescents aged 16 to 18 years – 9 patients;
– adults aged 19 to 30 years and from 31 to 50 years – 24.
Systemic therapy for AD was determined according to the severity of dermatosis and included the use of enteral sorbents, antihistamines and sedatives, and symptomatic treatment. During the study period, the administration of systemic and topical glucocorticosteroid drugs was excluded. In patients of all groups, Elidel cream was used as topical monotherapy with application of the drug to the skin twice a day in the area of clinical manifestations of AD. The duration of the main course was 2–4 weeks.
Monitoring and control methods
included clinical observation of patients with fixation and description of the initial condition of the skin (before the start of treatment), the severity of subjective symptoms, the dynamics of changes in these parameters under the influence of therapy after 7, 14 and 28 days of treatment. The results of the observation were recorded in a standardized observation chart, which also contained a comprehensive assessment of the severity of blood pressure using the SCORAD index in the dynamics of therapy. The SCORAD index was determined using the formula:
S=A/5+7B/2+C
.
Research results:
In total, 49 patients with AD were treated, including 21 men and 28 women. In most patients, the disease debuted in the first years of life and was recurrent in nature with seasonal exacerbations.
Table 1 presents the clinical characteristics of patients with AD included in the study (components of the SCORAD index and its value) before treatment with Elidel cream. From the data in Table 1 it is clear that the study included patients with significant severity of AD, in the stage of exacerbation of the skin process, as evidenced by high scores of the SCORAD index and its components.
The process was especially pronounced in the groups of young children (6 months - 3 years) and adolescents, where the average SCORAD scores were 65.3 ± 6.9 and 61.1 ± 7.1 units, respectively, both due to the prevalence of skin manifestations and and by the severity of objective and subjective symptoms. All patients were treated in a specialized hospital (18 patients) or outpatient (31 patients). Clinical improvement after the end of therapy was recorded in 47 of 49 treated patients with AD (95.9%), with 26 (53.0%) achieving clinical remission of the process, and 42.9% of patients achieving significant clinical improvement. Clinical observations indicated a generally uniform regression of blood pressure manifestations in typical locations: face, neck, limbs, where treatment with Elidel cream was carried out. However, we noted that hyperemia, swelling, and inflammatory infiltration of the skin in the facial area were resolved somewhat more actively, the itching disappeared in these localizations earlier than in other localizations (limbs, torso). A series of photographs illustrates the regression of blood pressure manifestations after 2 weeks of using Elidel cream.
Photo 1,2. B-ya A., 5 years old, before and after treatment with 2-week use of Elidel cream
Photo 3,4. B-ya N., 4 years old, before and after treatment with 2-week use of Elidel cream
All 49 patients completed the study. Only 2 patients (4.1%) did not experience any significant positive dynamics during therapy.
Table 2 shows the group average data on changes in the components of the SCORAD index in the dynamics of therapy in patients of different ages.
The actual data presented in Table 2 demonstrate a unidirectional decrease during therapy in all manifestations of blood pressure in groups of patients of different ages. Favorable dynamics of regression of blood pressure manifestations during therapy and after its completion, a decrease in the severity of objective symptoms (edema, hyperemia, the presence of papular rashes, crusts), as well as a decrease in the intensity of itching and sleep disturbances were also reflected in a significant decrease in the SCORAD integrative index. To analyze the data, we attempted to calculate the ratio (or coefficient - K) of each of the components of the SCORAD index and its total value obtained before the start of treatment to the same indicator after 1 week of therapy and after the end of therapy (1/2; 2/3; 1 /3).
Prevalence (area) of rashes
decreased proportionally during the course of therapy (weeks 1 and 2) in all age groups, and the reduction coefficients were 1.1–1.5 after the first week; 1.2–2.2 after the second week of treatment. The regression coefficient for the area of distribution of rashes was maximum in the group of sick children under 3 years of age (3.1) and in adolescents 16–18 years of age (2.4). In groups of older patients (19–50 years), the reduction in the area of affected skin was 1.4–1.5 times.
Intensity of objective symptoms of blood pressure
(B) in children aged 6 months - 3 years, adolescents and people in the older age group, it decreased more significantly after the 2nd week of treatment. After the end of treatment, the maximum regression of symptoms of the disease was recorded in children under 3 years of age (5.6) and in adolescents (3.9). Good results were also obtained in a group of patients aged 31–50 years (3.2).
Studying the dynamics of itching regression
and sleep disturbances showed that after 1 week of treatment the severity of these subjective symptoms decreased significantly (2.3–2.6 times), and at week 2 the reduction in itching intensity also continued (2.0–1.3). The final ratio of indicator C to the end of therapy was 4.4–2.3 times, which confirmed the clinical effect of the course of treatment. An interesting fact was the significant reduction in the intensity of itching and sleep disturbances in patients aged 31–50 years (4.4), where this indicator was the maximum among all age groups studied.
Analysis of data on changes in the SCORAD integrative index
showed a decrease comparable in weeks 1 and 2 of treatment. The greatest clinical effect was achieved in children under 3 years of age (a 4.8-fold decrease in the S value) and in the group of adolescents (3.3-fold).
Tolerability of Elidel cream
was rated as “good” in 31 of 49 patients (63.3%); as “satisfactory” – in 16 patients (32.6%). The use of Elidel cream caused a significant increase in itching and skin hyperemia in 2 patients (4.1%). During the use of Elidel cream and during 4 weeks of follow-up, no pyogenic, viral, or fungal infections occurred; no adverse events or complications were recorded.
Thus, clinical studies of the treatment of patients with AD of various age groups using the new topical drug Elidel cream demonstrated effectiveness in 95.9% of patients. The effect of the drug was noted already in the first week of use, when the area of skin lesions and the severity of AD symptoms decreased by 1.4–1.8 times, and the intensity of itching and sleep disturbances decreased by almost 2 times. A 2-week course of treatment contributed to the achievement of clinical remission in 53% of patients and a significant improvement in the process in 42.9% of patients, with a decrease in the SCORAD index compared to that before treatment by 4.8–3.3 times. Elidel cream was especially clinically effective in the treatment of patients with AD in children under 3 years of age and adolescents, which was confirmed by a significant decrease in the SCORAD index and its components. In adult patients, a significant decrease in the intensity of itching was noted after completion of therapy.
During the study, there were no adverse events or complications; Elidel cream was well tolerated in most patients.
Clinical studies have shown that Elidel cream is effective in the treatment of children, adolescents, and adults with AD, and its use in widespread clinical practice by dermatologists allows optimizing the treatment of patients with AD.
Literature:
1. Balabolkin I.I., Grebenyuk V.N. Atopic dermatitis in children, - M.: Medicine, 1999. -238 p.
2. Emelyanov A.V., Monakhov K.N. Topical corticosteroids in the treatment of allergic dermatoses: the significance of the extragenomic effect // Bulletin of Dermatology and Venereology. –2002. -No. 3. –P.59–61.
3. Kungurov N.V., Gerasimova N.M., Kokhan M.M. Atopic dermatitis (types of course, principles of therapy), – Ekaterinburg: Ural Publishing House. Univ., 2000.–266 p.
4. Sergeev Yu.V., Ivanov O.L., Novikov D.K. Atopic dermatitis: modern diagnosis and treatment // Immunopathology, allergology, infectology. –2001. –No. 4. -WITH. 28–48
5. Skripkin Yu.K., Samsonov V.A., Selissky G.D., Gomberg M.A. Modern problems of dermatovenerology // Bulletin of dermatology and venereology. –1997. –N6. –P.4–8.
6. Smirnova G.I. Modern technologies for local treatment of atopic dermatitis in children // Immunopathology, allergology, infectology. –2003. -No. 3. -WITH. 75–82.
7. Smolkin Yu.S., Cheburkin A.A. Atopic dermatitis in children: principles of diagnosis and rational therapy // Attending physician. –2002. – No. 9.
8. Toropova N.P., Sinyavskaya O.A., Gradinarov A.M. Severe (disabling) forms of atopic dermatitis in children. Methods of medical and social rehabilitation // Russian. honey. magazine. –1997. –Vol. 5. –No. 11. -WITH. 713–720
9. Fedenko E. S. Atopic dermatitis: rationale for a step-by-step approach to therapy // Consilium medicum. –2001. –No. 3 (4). -WITH. 176–184.
10. Billich A. et al. Pimecrolimus permeates less through skin than corticosteroids and tacrolimus // J. Invest. Dermatol. – 2002. –Vol.119. –P.346
11. Bos JD, Sillevis JH Atopic dermatitis // JEADV. – 1996. – Vol.7.– P.101–114.
12. Ellis C., Luger T. International Consensus Conference on Atopic Dermatitis II (ICCAD II) Clinical update and current treatment strategies // Br. J. Dermatol. –2003. –Vol.148. –P.3(10.
13. Emerson RM, Williams HC, Allen BR Severity distribution of atopic dermatitis in the community and its relationship to secondary referral // Br. J. Dermatol. –1998. –Vol. 139(1). –P.73–76.
14. Grassberger M. et al. A novel anti–inflammatory drug, SDZ ASM 981, for the treatment of skin diseases: in vitro pharmacology // Br. J. Dermatol. – 1999. –Vol.141. –P. 264–273.
15. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis // Acta Dermatol. Venereol., 1980.– Vol.92. –P.44.
16. Hultsch T. et al. Ascomycin macrolactam derivative SDZ ASM 981 inhibits the release of granule–associated mediators and of newly synthesized cytokines in RBL 2H3 mast cells in an immunophilin–dependent manner // Arch. Dermatol. Res. – 1998. –Vol.290. –P.501–507.
17. Kapp A. et al. Long-term management of atopic dermatitis in infants with topical pimecrolimus, a nonsteroid anti-inflammatory drug // J. Allergy Clin. Immunol. – 2002. –Vol.110. –P.277–284.
18. Lewis–Jones MS, Finlay AY, Dykes PJ The Infants' Dermatitis Quality of Life Index // Br. J. Dermatol. – 2001. –Vol. 144. –P. 104–110.
19. Meingassner JG et al. A novel anti–inflammatory drug, SDZ ASM 981, for the topical and oral treatment of skin diseases: in vivo pharmacology // Br. J. Dermatol. – 1997. –Vol.137. –P.568–576.
20. Meurer M. et al. Pimecrolimus cream in the long-term management of atopic dermatitis in adults: A six-month study // Dermatology. – 2002. –Vol. 205. –P.271–277.
21. Queille–Roussel C. et al. The new topical ascomycin derivative SDZ ASM 981 does not induce skin atrophy when applied to normal skin for four weeks: a randomized, double–blind controlled study // Br. J. Dermatol. – 2001. –Vol.144. –P.507–513.
22. Schultz–Larsen F, Hanifin JM. Epidemiology of atopic dermatitis // Immunol. Allergy Clin. North Am. –2002. –Vol.22. –P.1–24.
23. Stuetz A et al. Pimecrolimus does not affect Langerhans' cells in murine epidermis, in contract to corticosteroids // J. Invest. Dermatol. – 2002. –Vol.119. P. 347
24. Tofte SJ, Hanifin J..M. Current management and therapy of atopic dermatitis // J. American Acad. Dermatol. –2001. –Vol. 44(1). -R. 13–16.
25. Wahn U et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children // Pediatrics. – 2002. – Vol. 110. –P.158–159.
26. Winiski A et al. Inhibitory activity of pimecrolimus and tacrolimus on induced cytokine mRNA and protein expression in a human T cell line (Jurkat) measured via RT PCR and ELISA // J. Invest. Dermatol. – 2002. –Vol.119. –P. 347
27. Zuberbier T et al. The ascomycin macrolactam pimecrolimus (Elidel(r), SDZ ASM 981) is a potent inhibitor of mediator release from human dermal mast cells and peripheral blood basophils // J. Allergy Clin. Immunol. – 2001. –Vol.108. –P.275–280.
Pharmacokinetics
Adults. The concentration of pimecrolimus in the blood was determined in 12 adult patients with atopic dermatitis (eczema) affecting 15–59% of the body surface area, treated with Elidel cream 2 times a day for 3 weeks. In 77.5% of observations, the concentration of pimecrolimus in the blood was below 0.5 ng/ml (minimum detectable concentration), and in 99.8% it was below 1 ng/ml. Cmax of pimecrolimus in the blood, recorded in 1 patient, was 1.4 ng/ml.
In 98% of 40 adult patients with initial lesions of 14–62% of the body surface area, after 1 year of treatment with Elidel cream, pimecrolimus blood concentrations remained low and in most cases were below the minimum detectable concentration.
A Cmax value of 0.8 ng/ml was recorded after 6 weeks of treatment in only 2 patients. None of the patients showed an increase in concentration over 12 months of treatment. During a 3-week period of treatment with Elidel cream twice daily in 13 adult patients with hand dermatitis (using the cream on the palms and dorsum of the hands and bandaging at night), the maximum recorded blood concentration of pimecrolimus was 0.91 ng/ml.
In 8 patients with pimecrolimus blood levels above the minimum detectable concentration, the AUC value was 2.5–11.4 ng/ml.
Children. Pharmacokinetic studies of pimecrolimus were conducted in 58 children aged 3 months to 14 years with atopic dermatitis (eczema) affecting 10–92% of the body surface area, treated with Elidel cream 2 times a day for 3 weeks. Five children received treatment for 1 year as needed.
Pimecrolimus blood concentrations were consistently low, regardless of the area of skin lesions and duration of therapy, and were in the same range as in adult patients receiving Elidel cream therapy at the same doses. In 97% of cases, pimecrolimus blood concentrations were below 2 ng/ml, and in 60% they were below 0.5 ng/ml (minimum detectable concentration). The Cmax of pimecrolimus recorded in 2 patients aged 8 months and 14 years was 2 ng/ml.
Among the youngest children (from 3 to 23 months), the Cmax of pimecrolimus was 2.6 ng/ml and was recorded in 1 patient.
In 5 children treated with Elidel cream for 1 year, pimecrolimus concentrations were at consistently low levels. The maximum value recorded in 1 child was 1.94 ng/ml. Throughout the entire treatment period, an increase in drug concentrations was not observed in any of the patients.
In 8 children aged 2 to 14 years with blood levels of pimecrolimus above the minimum detectable concentration when measured three times, the AUC value ranged from 5.4–18.8 ng/ml. AUC values in patients with skin lesions less than or greater than 40% were comparable.
In in vitro studies, the binding of pimecrolimus to plasma proteins (mainly various lipoproteins) was 99.6%.
Since the concentrations of pimecrolimus in the blood are very low when applied topically, determination of metabolic parameters is not possible.
Pharmacokinetics in special clinical situations
Atopic dermatitis (eczema) is rarely observed in patients aged 65 years and older. The number of patients of this age in clinical studies of Elidel 15% cream was insufficient to detect any difference in treatment effectiveness compared with younger patients.
Dosing recommendations for infants (3–23 months), children (2–11 years), and adolescents (12–17 years) are the same as for adult patients.
Elidel and corticosteroids: allies or rivals?
N
Topical corticosteroids (CS) are the basis for the treatment of atopic dermatitis (AD), as they have anti-inflammatory, immunosuppressive and antiproliferative properties. These drugs act quickly and effectively, which satisfies both the doctor and the patient. However, steroids have a number of serious side effects. Systemic complications are especially dangerous, developing, as a rule, as a result of the absorption of drugs from large-area lesions with prolonged use. The No. 1 risk group in this regard is children under 2 years of age, whose skin permeability is much higher than that of adults [1]. The most serious systemic complications include inhibition of the hypothalamic-pituitary-adrenal system and associated growth retardation, Cushing's syndrome, hypertension, and diabetes [2–6]. Long-term use of topical steroids carries a risk of immunosuppression, manifested by bacterial, viral, and fungal infections. These effects are predominantly characteristic of early generations of CS, especially fluorinated CS. In recent decades, drugs have been created whose absorption when applied externally does not exceed 1%, however, they also have a number of disadvantages. Among local complications, skin atrophy, striae, telangiectasia, pigmentation disorders, and acneiform rashes predominate [7]. Important disadvantages of external CS include tachyphylaxis - addiction and loss of effectiveness.
All these side effects and complications have led to the fact that 73% of patients with AD suffer from the so-called “steroid phobia” - anxiety of varying degrees, up to a complete refusal to use CS [8]. For this reason, 24% of adult patients and 36% of parents of sick children admit to violating the external therapy regimen. “Steroid phobia” affects not only patients, but also doctors themselves, so treatment with these drugs is often started late, the duration of therapy is insufficient, and the doses, especially in children, are too small. All this leads to inadequate relief of exacerbation, early relapse and the formation of tachyphylaxis.
In this regard, the question arises: how to shorten the period of use of CS during exacerbation of blood pressure and at the same time prolong the remission of the disease?
To solve this problem, a selective inhibitor of the synthesis and release of proinflammatory cytokines, pimecrolimus (SDZ ASM 981), is currently used. It has been established that this substance is in vitro
selectively binds to macrophilin-12, and inhibits calcineurin and thereby the synthesis of inflammatory cytokines in T cells (IL-2, INF-g), as well as the release of inflammatory mediators (for example, histamine) from mast cells [9–12]. At the same time, pimecrolimus does not affect keratinocytes, fibroblasts, endothelial cells and Langerhans cells. In vivo, the drug has high anti-inflammatory and slight immunosuppressive activity [9–12] and does not cause atrophy [13]. Pimecrolimus has a high affinity for the skin, therefore it penetrates well into it and practically does not penetrate the skin [11].
Clinical trials have established the safety and effectiveness of 1% pimecrolimus cream - Elidel® (Novartis, Switzerland) for short-term and long-term use in children and adults with AD.
In order to try to reduce with the help of Elidel the frequency and duration of relapses and the dependence of patients on external CS for blood pressure in adults, a multicenter, double-blind, randomized controlled trial lasting 24 weeks was conducted [14].
We observed 192 patients with AD aged 18 years and older. The diagnosis was made in accordance with the criteria of Hanifin [15] and Rajka [16]. The affected area before treatment was at least 5%, with an average of 17% in both groups. In each group, patients with moderate AD predominated (3 points on the IGA scale).
Based on the results of randomization, 96 patients were included in the main group and 96 in the control group. There were no statistically significant demographic or clinical differences between the groups (Table 1).
Patients in the main group received external treatment with Elidel cream 2 times a day, while patients in the control group received only the base of the cream. In the first week of the study, this treatment regimen was mandatory. Subsequently, in case of exacerbation, external CS (prednicarbate 0.25% cream) was prescribed twice a day for 7 days and once a day for the next week. After CS therapy, treatment with the study drug was carried out for another 1 week to relieve residual symptoms of exacerbation (Table 2). The main efficacy criterion was the number of days (%) during which CS were used for acute treatment of exacerbations. Additional evaluation of effectiveness was carried out according to the following parameters: number of exacerbations, IGA results, EASI, itching intensity. The last parameter was assessed by the patients themselves, using the following point scale: 0 – no itching, 1 – slight itching, 2 – moderate itching, 3 – severe itching, 4 – very severe itching. Tolerability was assessed based on clinical and laboratory data.
Patients were examined during the initial examination, and then at 1, 3, 6, 12 and 24 weeks of treatment. In addition, additional telephone contact was carried out at 9 and 18 weeks. In cases of severe exacerbation, unscheduled examinations were performed.
results
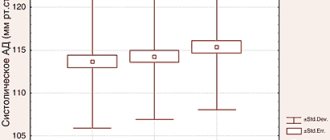
In the main group of patients treated with Elidel cream, a statistically significant (compared to the control group) reduction in the number of days during which patients were forced to use CS was recorded (Fig. 1).
Rice. 1. Number of days patients used corticosteroids (%)
In the main group, patients used CS on average for 14.2% ± 24.2% of 168 days (total study duration), and in the control group - in 37.2% ± 34.6% (p < 0.001). Moreover, half of the patients receiving Elidel did not require the use of CS for the entire 24 weeks (Fig. 2).
Rice. 2. Number of days patients required treatment with topical corticosteroids (%)
The frequency of exacerbations and time to first exacerbation also differed significantly between the two groups of patients. In the main group, on average, 1.1±1.4 cases of exacerbation were registered, in the control group – 2.4±2.3 (p<0.001). Almost half (44.8%) of patients treated with Elidel showed no exacerbations for six months. In persons receiving traditional therapy, this figure was 18.8% (Fig. 3.4).
Rice. 3 Frequency of exacerbations (%)
Rice. 4. Time until first exacerbation
When assessed on the IGA scale, 82.3% of patients in the main group showed an improvement of at least one point versus 51.0% in the control group, and the EASI score decreased by an average of 48.3% versus 15.9% (respectively) (p<0.001) . Among patients treated with Elidel, half as many people stopped treatment due to its ineffectiveness compared to traditional therapy (15.3% and 27.1%, respectively).
The dynamics of itching are presented in Figure 5. Noteworthy is the decrease in itching during the first three days of therapy in the main group and a temporary increase in the control group.
Rice. 5. Dynamics of itching during the first week of treatment
Elidel cream was well tolerated by patients; pathologies in traditional laboratory tests were not detected in any of the patients throughout the study.
Thus, external treatment of patients with blood pressure with Elidel cream allows:
- reduce the use of local CS or completely abandon them,
- reduce the number of exacerbations,
- increase the duration of the period without exacerbations of blood pressure,
- leave the CS as a reserve for short courses of treatment for exacerbation of blood pressure and minimize the risk of complications from long-term steroid therapy,
- improve control of the disease in general, the general well-being of patients, and, consequently, the quality of life of patients.
References:
1. Giusti F, Martella A, Bertoti L, Seidenari S. Skin Barrier, Hydration, and pH of the Skin of infants under 2 years of age. Ped Derm 2001; 18:93–6.
2. Keipert JA, Kelly R. Temporary Cushing's syndrome from percutaneous absorption of betamethasone-17-valerate. Med J Austr 1971; 1:542–4.
3. Pascher F. Systemic reactions to topically applied drugs. Int Dermatol 1978; 17: 768–75.
4. Bode HH. Dwarfish following long long–term topical corticosteroid therapy. JM Med Assoc 1980; 244:813–14.
5. Bartorelli A, Rimondini A. Severe hypertension in childhood due to prolonged skin application of mineralocorticoid ointment. Hypertension 1984; 6:586–8.
6. Walsh P, Aeling JL, Huff L, Weston WL. Hypothalamus–pituitary–adrenal axis suppression by superprotent steroids. J Am Acad Dermatol 1999; 29:501–3.
7. Fisher DA. Adverse effects of topical corticosteroid use. West J Med 1995; 162:123–6.
8. Charman C, Morris A, Willians H. Topical corticosteroid phobia in patients with atopic dermatitis. Br J Dermatol 2000;142:931–6.
9. Meingassner JG, Grassberger M, Fahrngruber H et al. A novel anti–inflammatory drug, SDZ ASM 981, for the topical and oral treatment of skin diseases: in vivo pharmacology. Br J Dermatol 1997; 137:568–76.
10. Grassberger M, Baumruker T, Enz A et al. A novel anti–inflammatory drug, SDZ ASM 981, for the treatment of skin diseases: in vitro pharmacology. Br J Dermatol. 1999 Aug;141(2):264–73
11. Stuetz A, Grassberger M, Meingassner JG. Pimecrolimus (Elidel(, SDZ ASM 981) – Preclinical pharmacological profile and skin selectivity. Seminars Cutan Med Surg 2001; 20(4):233–41.
12. Zuberbier T, Chong SU, Grunow K et al. The ascomycin macrolactam pimecrolimus (Elidel, SDZ ASM 981) is a potent inhibitor of mediator release from human dermal mast cells and peripheral blood basophils. J Allergy Clin Immunol. 2001 Aug; 108(2): 275–80.
13. Queille–Roussel C, Paul C, Duteil L et al. The new topical ascomycin derivative SDZ ASM 981 does not induce skin atrophy when applied to normal skin for 4 weeks: a randomized, double–blind controlled study. Br J Dermatol. Mar 2001; 144(3): 507–13.
14. Meurer M, Folster–Holst R, Brautigam M. Pimecrolimus (SDZ ASM 981) cream reduces the need for corticosteroids in the long–term management of atopic dermatitis in adults. Study, presented at the 60th annual meeting of the American Academy of Dermatology in New Orleans, USA, February 2002
15. Hanifin JM, Thurston M, Omoto M et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. Feb 2001; 10(1): .11–8.
16. Rajka G. Natural history and clinical manifestations of atopic dermatitis. Clin Rev Allergy. 1986 Feb; 4(1): 3–26.
Contraindications
hypersensitivity to timecrolimus or any components of the drug;
children under 3 months of age (the safety and effectiveness of Elidel cream in children under 3 months of age has not been studied);
the presence of acute viral, bacterial or fungal skin infections.
Carefully:
patients with Netherton's syndrome (no safety data available) - there may be a risk of increased systemic absorption of the drug;
severe forms of inflammation or skin damage, incl. generalized erythroderma (no data on safety of use) - there may be a risk of increased systemic absorption of the drug;
weakened immunity - because the effectiveness and safety of use have not been studied.
There are no data on the safety of long-term use of Elidel cream.
Since the effect of long-term use of the drug on the immune defense of the skin and the incidence of malignant neoplasms has not been studied, Elidel cream should not be applied to damaged areas of the skin with possible malignancy or dysplastic changes.
In case of bacterial or fungal infection of the skin, the use of Elidel cream on the affected areas is possible only after the infection has been cured.
Use during pregnancy and breastfeeding
There are no data on the use of the drug in pregnant women. In experimental studies with local use of the drug, no direct or indirect damaging effect of Elidel cream on the course of pregnancy, the development of the embryo/fetus, the course of childbirth and the postnatal development of the offspring was not revealed. Caution should be exercised when prescribing to pregnant women. However, given the minimal absorption of pimecrolimus when administered topically, the potential risk in humans is considered negligible.
The excretion of the drug into breast milk after topical administration has not been studied in experimental models. There are no data on the content of pimecrolimus in the breast milk of lactating women.
Since many drugs are excreted in breast milk, caution should be exercised when prescribing Elidel 1% cream to nursing women. However, given the minimal systemic absorption of pimecrolimus when administered topically, the potential risk to humans is considered negligible.
Breastfeeding women should not apply Elidel 1% cream to the breast area.
The effect of Elidel cream on fertility in men and women has not been established.
Side effects
The use of Elidel cream may cause minor transient reactions at the application site, such as a feeling of warmth and/or burning. If these reactions are severe, patients should consult a doctor.
The most common reactions at the site of application of the drug were observed in 19% of patients treated with Elidel cream and in 16% of patients in the control group. These reactions mainly occurred early in treatment and were minor/moderate and short-lived.
Determination of the frequency of adverse reactions: very often (≥1/10); often (≥1/100, <1/10); sometimes (≥1/1000, <1/100); rare (≥1/10000, <1/1000); very rare (<1/10000), including isolated reports.
Very often - burning at the site of application of the cream.
Often - local reactions (irritation, itching and redness of the skin), skin infections (folliculitis).
Sometimes - suppuration, worsening of the disease, herpes simplex, dermatitis caused by the herpes simplex virus (herpetic eczema), molluscum contagiosum; local reactions such as rash, pain, paresthesia, peeling, dryness, swelling, skin papillomas, boils.
The following adverse reactions were observed during post-marketing use of the drug (frequency estimate based on the number of cases of adverse events in an unspecified population).
From the immune system: very rarely - anaphylactic reactions.
Metabolic disorders (metabolic disorders): rarely - alcohol intolerance.
From the skin and its appendages: rarely - allergic reactions (rash, urticaria, angioedema); changes in skin color (hypopigmentation, hyperpigmentation).
In most cases, facial redness, rash, burning, itching or swelling developed immediately after drinking alcohol.
When using Elidel cream, in rare cases, the development of malignant neoplasms, including skin and other types of lymphomas, and skin cancer was observed. A cause-and-effect relationship between these adverse events and the use of the drug has not been established.
Interaction
The potential interaction of Elidel cream with other drugs has not been studied. Given that the systemic absorption of pimecrolimus is very low, any interaction of Elidel cream with drugs for systemic use is unlikely.
When using Elidel cream in children aged 2 years and older, the drug did not affect the effectiveness of vaccination.
It is not recommended to apply cream to the area where the vaccine was administered until local manifestations of the post-vaccination reaction have completely disappeared.
Incompatibility. Since compatibility studies have not been conducted, it is not recommended to use the drug in combination with other topical agents.
Directions for use and doses
Externally.
Treatment should begin at the first manifestations of the disease to prevent its sudden exacerbation.
The cream is applied in a thin layer to the affected surface 2 times a day and gently rubbed until completely absorbed.
The cream can be applied to the skin of any part of the body, including the head, face, neck, as well as to diaper rash areas. Elidel cream should be used 2 times a day until the symptoms of the disease completely disappear. If the severity of symptoms persists, after 6 weeks of using the drug, it is necessary to re-examine the patient to confirm the diagnosis of atopic dermatitis. After cessation of treatment, in order to avoid subsequent exacerbations, at the first signs of relapse of atopic dermatitis, therapy should be resumed. Emollients can be applied immediately after applying Elidel 1% cream. However, after water procedures, emollients should be used before applying Elidel cream.
Given the very low systemic absorption of pimecrolimus, there are no restrictions on the total daily dose of the drug applied, the area of skin surface treated, or the duration of treatment. If Elidel cream gets into your eyes or mucous membranes (oral or nasal cavity), you should immediately remove the cream and rinse your eyes and mucous membranes with running water.
Application of Elidel cream for atopic dermatitis
Aida
February 18, 2020
Hello. The child is 6 months old. From the age of 3 months, rashes began in the form of redness and peeling with weeping skin on the folds under the knees and neck, rarely on the inside of the elbow folds and on the cheeks. The skin in the area of the rash is very itchy. A diagnosis of Atopic Dermatitis was made. Child on breastfeeding. We are just about to introduce complementary foods. I follow a diet, I don’t eat eggs, milk and dairy products, I don’t eat meat at all, tomatoes, mushrooms, citrus fruits, fish and seafood, nuts, red fruits and vegetables, berries, and I periodically exclude gluten-containing foods. I don’t see any particular effect from the diet (except that when I introduce fermented milk products and cheese into my diet, white lumps appear in the child’s stool, which disappear immediately after eliminating these products from the diet... I’ve experimented several times already, the result is always the same). Based on the results of the last visit to the allergist, some tests were taken, the results: Vitamin D (total): 16 (normal from 30), allergen egg, IgE: 15.7, allergen Corrvier milk, IgE: 0.71. The doctor prescribed treatment: taking vitamin D for a long time, Advantan for two weeks, then Elidel for 14 days, 2 times a day, then 14 days, 1 time a day. Eliminate milk, dairy products and eggs from your diet. But here's the thing - Elidel helps us so well (even better than Advantan) that two days after starting use, not a trace remains of the rashes and on the third day I actually apply it to healthy skin. But the instructions for the drug indicate that it is applied only to damaged skin. It turns out that the scheme proposed by the doctor does not suit us. But at the same time, I note that after achieving “clean” skin on the third day, I tried to stop the drug, as a rule, after a couple of days the rashes appeared again. Taking into account the above, I ask you to recommend a skin treatment regimen with Elidel, which will suit us, taking into account the very fast, but short-term effect of using the drug. Or he can use it according to the scheme previously proposed to us, despite the recommendations in the annotation for the drug. And I also ask you to tell me, is it possible at this stage of medical development to identify the cause of this disease in order to eliminate the “root” of the problem, and not its manifestations in the form of rashes? Which direction should we go? What tests should I take? Which doctors should I contact? After all, Elidel can be used in general for no more than 6 months (that’s what the allergist said), but what should we do next?? The diet, as I indicated earlier, has no effect. I’ll also add that I suffered from this illness as a child. For up to a year, I had rashes on my cheeks, then they went away, and from 2 to 5 years old, the inside of my elbow folds rashed out. As I understand it, atopic dermatitis is my genetic “gift” to my son. But maybe something can still be done? Thank you for your opinion.
The question is closed
allergy
atopic dermatitis
elidel
special instructions
When treated with topical calcineurin inhibitors, including Elidel, the development of malignant neoplasms (for example, skin tumors and lymphomas) has been observed in rare cases. A cause-and-effect relationship between these adverse events and the use of the drug has not been established.
In clinical studies when using Elidel cream, 0.9% of patients (14 out of 1544) experienced the development of lymphadenopathy. Typically, lymphadenopathy was caused by infectious diseases and disappeared after a course of appropriate antibiotic therapy. In all patients, it was either possible to identify the cause of the development of lymphadenopathy, or the disappearance of this undesirable phenomenon was noted. In patients receiving treatment with Elidel, if lymphadenopathy develops, it is necessary to establish the etiology of the process and monitor patients until this adverse event completely disappears. If the etiology of lymphadenopathy is unknown or if the patient has acute mononuclear inflammation, the drug should be discontinued.
When treated with Elidel cream, patients are advised to reduce artificial or natural insolation of the skin to a minimum or completely eliminate UV irradiation. The possible effect of using the drug on skin lesions caused by UV radiation is unknown.
Influence on the ability to drive vehicles and operate machinery. The effect of using Elidel cream on the ability to drive vehicles or operate machinery has not been established.
Instructions for use ELIDEL®
Elidel® should not be applied to skin affected by an acute viral infection.
In case of bacterial or fungal skin infection, the use of appropriate antimicrobial agents is necessary. If the infection does not improve, use of Elidel® should be discontinued until the infection has been adequately treated.
Since the effect on the local immune response of the skin and the manifestations of malignant neoplasms with long-term treatment with Elidel® is not known, its use in cases of potentially malignant neoplasms of the skin or the possibility of the occurrence of such diseases is not recommended. Although a causal relationship has not been established, rare cases of malignancies (eg, skin) and lymphomas have been reported in patients treated with topical calcineurin inhibitors, including. Elidel®.
This drug is not recommended for use in patients with Netherton's syndrome or generalized erythroderma, in which increased absorption occurs, because The safety of this drug in this category of patients has not been clearly established.
It is not recommended to use Elidel® in patients with immunocompromised patients, since its safety and effectiveness have not been studied in such patients. Clinical studies of Elidel® cream revealed 0.9% of cases of lymphadenopathy. As a rule, they were associated with various infections and resolved after adequate antibiotic therapy. However, most of them were of clear etiology or disappeared on their own. If signs of lymphadenopathy appear in patients using Elidel® cream, it is necessary to establish the cause of the process. If there is no obvious cause of lymphadenopathy or if acute infectious mononucleosis occurs, treatment with this drug should be suspended. It is also necessary to monitor patients with lymphadenopathy that occurs during treatment with Elidel to confirm that it has disappeared.
It is advisable that patients avoid or limit their exposure to the sun or artificial ultraviolet rays as much as possible during treatment with this drug, even if the drug is not applied to the affected areas of the skin, since the potential effects of Elidel® Cream on affected skin under the influence of ultraviolet irradiation have not been studied.
Atopic dermatitis (eczema) is rarely observed in patients 65 years of age and older. The number of patients of this age in clinical studies of Elidel® cream was insufficient to detect any differences in treatment effectiveness compared with younger patients.
The use of Elidel® may cause minor transient reactions at the application site, such as a feeling of warmth and/or burning. Patients should be warned to consult a doctor if these reactions are severe.
The cream should not be applied to mucous membranes. If the drug accidentally comes into contact with mucous membranes or eyes, rinse them immediately with water.
Impact on the ability to drive vehicles and operate machinery
The effect of using Elidel® cream on the ability to drive a car and operate machinery has not been established.