HOLLOW VEINS
[
venae cavae;
vena cava superior (PNA, BNA),
vena cava cranialis
(JNA);
vena cava inferior
(PNA, BNA),
vena cava caudalis
(JNA)] - the main venous trunks (superior and inferior vena cava), collecting blood from the entire body and flowing into the heart.
Upper P. v. collects blood from the head, neck, chest and upper extremities and flows into the right atrium. The lower venous vein is the largest venous trunk of the human body; it collects blood from the lower extremities, organs and walls of the pelvis and abdominal cavity and also flows into the right atrium.
Ancient anatomists mentioned only one P. v. Thus, K. Galen described the beginning of the vena cava from the liver, and the vein is divided into ascending and descending parts. Ibn Sina was of the same opinion, and only A. Vesalius pointed out the connection between the vein and the heart.
Comparative anatomy
For the first time, the posterior (lower) P. v. in phylogeny it appears in lobe-finned ganoids and double-breeding fishes in the form of an unpaired venous trunk flowing into the right atrium. In mammals, the renal portal system completely disappears, and the posterior (lower) renal v. acquires predominant importance compared to the posterior cardinal veins. The common cardinal veins (ducts of Cuvier) therefore carry blood from the anterior half of the body, head, neck and forelimbs. The large trunk, formed as a result of the fusion of the veins of the head, neck and forelimbs and flowing into the heart, is called the anterior (upper) P. v.
Embryology
Rice.
1. Schematic representation of some stages of the development of the vena cava in humans (a - embryo 4 weeks, b - embryo 8 weeks, c - fetus before birth): 1 - anterior cardinal vein, 2 - common cardinal vein, 3 - umbilical vein, 4 - vitelline-mesenteric vein, 5 - subcardinal vein, 6 - posterior cardinal vein, 7 - venous plexus of the primary kidney, 8 - liver, 9 - heart, 10 - external jugular vein, 11 - superior vena cava, 12 - azygos vein, 13 - hemizygos vein, 14 - inferior vena cava, 15 - adrenal vein, 16 - disappearing (reducing) vessels of the primary kidney, 17 - intercostal veins, 18 - right subclavian vein, 19 - right internal jugular vein, 20 - left brachiocephalic vein, 21 - left subclavian vein, 22 - coronary sinus of the heart, 23 - accessory hemizygos vein, 24 - adrenal gland, 25 - renal vein, 26 - left testicular (ovarian) vein, 27 - common iliac vein, 28 - external iliac vein, 29 - right brachiocephalic vein vein. In the early stages of ontogenetic development (4 weeks), bilateral symmetry of the systemic veins is characteristic. The main change during the development of the venous system is a change in the direction of blood flow from the left half of the body to the cardinal veins lying on the right, and the formation of unpaired venous trunks. As a result of complex transformations associated with changes in the direction of blood flow, the upper P. v. formed from the proximal part of the anterior right cardinal vein and the common right cardinal vein. Development of the lower P. v. associated with the expansion and lengthening of initially small veins of the abdominal cavity as a result of reduction of the posterior cardinal veins. Depending on from which veins or groups of veins the section of the lower P. v. is formed, it is divided into mesenteric, hepatic and postrenal parts, merging by the end of the 8th week. embryonic development into a single trunk (Fig. 1).
Inferior vena cava syndrome
Inferior vena cava syndrome is considered a rather rare pathology, and it is usually associated with blockage of the lumen of the vessel by a thrombus.
clamping of the inferior vena cava in pregnant women
A special group of patients with impaired blood flow through the vena cava consists of pregnant women, in whom the prerequisites are created for the vessel to be compressed by the enlarging uterus, and changes in blood clotting towards hypercoagulation are also common.
According to the course, nature of complications and outcomes, thrombosis of the vena cava is considered one of the most severe types of venous circulation disorders, because one of the largest veins of the human body is involved. Difficulties in diagnosis and treatment may be associated not only with the limited use of many research methods in pregnant women, but also with the rarity of the syndrome itself, about which not much has been written even in the specialized literature.
The causes of inferior vena cava syndrome can be thrombosis, which is especially often combined with blockage of the deep vessels of the legs, femoral and iliac veins. Almost half of the patients have an ascending path of thrombosis.
Disruption of blood flow through the vena cava can be caused by targeted ligation of the vein in order to avoid embolism of the pulmonary arteries when the veins of the lower extremities are affected. Malignant neoplasms of the retroperitoneum and abdominal organs provoke blockage of the IVC in approximately 40% of cases.
During pregnancy, conditions are created for compression of the IVC by the constantly enlarging uterus, which is especially noticeable when there are two or more fetuses, polyhydramnios is diagnosed, or the fetus is quite large. According to some data, signs of impaired venous outflow in the inferior vena cava system can be detected in half of expectant mothers, but symptoms occur only in 10% of cases, and pronounced forms occur in one woman out of 100, while a combination of pregnancy with pathology of hemostasis and somatic diseases.
The pathogenesis of NVC syndrome consists of a disorder in the return of blood to the right side of the heart and its stagnation in the lower half of the body or legs. Against the background of overflow of the venous lines of the legs and pelvis with blood, the heart experiences a lack of it and is not able to transport the required volume to the lungs, resulting in hypoxia and a decrease in the release of arterial blood into the arterial bed. The formation of bypass pathways for the outflow of venous blood helps to reduce the symptoms of both thrombotic lesions and compression.
Clinical signs of inferior vena cava thrombosis are determined by its degree, the rate of luminal occlusion and the level where the occlusion occurred. Depending on the level of blockage, thrombosis can be distal, when a fragment of the vein is affected below the point where the renal veins flow into it; in other cases, the renal and hepatic segments are involved.
The main signs of thrombosis of the inferior vena cava are:
- Pain in the abdomen and lower back, the muscles of the abdominal wall may be tense;
- Swelling of the legs, groin, pubic area, abdomen;
- Cyanosis below the occlusion zone (legs, lower back, abdomen);
- Expansion of the saphenous veins is possible, which is often combined with a gradual decrease in edema as a result of the establishment of collateral circulation.
With renal thrombosis, there is a high probability of acute kidney failure due to severe venous congestion. At the same time, the impairment of the filtration capacity of organs rapidly progresses, the amount of urine produced sharply decreases until it is completely absent (anuria), and the concentration of nitrogenous metabolic products (creatinine, urea) increases in the blood. Patients with acute renal failure due to venous thrombosis complain of lower back pain, their condition progressively worsens, intoxication increases, and possible impairment of consciousness similar to uremic coma.
Thrombosis of the inferior vena cava at the point where the hepatic tributaries flow into it is manifested by severe abdominal pain - in the epigastrium, under the right costal arch, characterized by jaundice, rapid development of ascites, symptoms of intoxication, nausea, vomiting, fever. In case of acute blockage of a vessel, symptoms appear very quickly, there is a high risk of acute liver or hepatorenal failure with high mortality.
Disturbances of blood flow in the vena cava at the level of the hepatic and renal tributaries are among the most severe types of pathology with high mortality, even with the capabilities of modern medicine. Occlusion of the inferior vena cava below the branching site of the renal veins proceeds more favorably, since vital organs continue to perform their functions.
When the lumen of the inferior vena cava is closed, the damage to the legs is always bilateral. Typical symptoms of the pathology include pain, affecting not only the limbs, but also the groin area, abdomen, buttocks, as well as swelling, evenly spreading throughout the entire leg, the anterior wall of the abdomen, groin and pubis. Dilated venous trunks become noticeable under the skin, taking on the role of bypass routes for blood flow.
More than 70% of patients with thrombosis of the inferior vena cava suffer from trophic disorders in the soft tissues of the legs. Against the background of severe swelling, non-healing ulcers appear, often multiple, and conservative treatment does not bring any results. In most male patients with lesions of the inferior vena cava, blood stagnation in the pelvic organs and scrotum causes impotence and infertility.
In pregnant women, when the vena cava is compressed from outside the growing uterus, symptoms may be little noticeable or even absent with adequate collateral blood flow. Signs of pathology appear by the third trimester and may consist of swelling of the legs, severe weakness, dizziness and fainting in the supine position, when the uterus actually lies on the inferior vena cava.
In severe cases during pregnancy, inferior vena cava syndrome can manifest itself as episodes of loss of consciousness and severe hypotension, which affects the development of the fetus in the uterus, which experiences hypoxia.
To identify occlusions or compression of the inferior vena cava, venography is used as one of the most informative diagnostic methods. It is possible to use ultrasound, MRI, blood tests for coagulation and urine tests are required to exclude renal pathology.
Anatomy
Rice.
2. Schematic representation of some variants of the formation of the superior vena cava and its sources: a - symmetrically developed internal jugular veins (short trunk of the right brachiocephalic vein), b - asymmetry in the development of the internal jugular veins (long trunk of the right brachiocephalic vein); 1 - internal jugular veins, 2 - right brachiocephalic vein, 3 - left brachiocephalic vein, 4 - superior vena cava. Superior vena cava
- a short trunk located in the thoracic cavity, in the upper mediastinum (see). It begins at the level of the cartilage of the first rib at the right edge of the sternum from the confluence of the right and left brachiocephalic veins (vv. brachiocephalicae dext, et sin.). Heading down, it flows into the right atrium at the level of the cartilage of the right third rib. To the left of it is the ascending part of the aorta, to the right it is partially covered by the mediastinal pleura and is adjacent to the right lung. The right phrenic nerve passes through this location. Behind the upper P. v. the root of the right lung is located. At the level of the cartilage of the right second rib, it is covered by the pericardium. Before entering the pericardial cavity in the upper P. v. The azygos vein (v. azygos) flows into it. Some options for the formation of the upper P. v. and its origins are presented in Fig. 2.
Inferior vena cava
begins in the abdominal cavity from the confluence of the right and left common iliac veins (vv. iliacae communes dext, et sin.) at the level of LIV-V and goes up to the right of the aorta, deviating from it to the right to the diaphragm.
At this point it lies in the groove of the inferior vena cava of the liver, and then through an opening in the tendon center of the diaphragm it passes into the chest cavity and flows into the right atrium. Rice.
3. Schematic representation of the trunk and tributaries of the inferior vena cava, the abdominal part of the aorta and its branches: 1 - inferior phrenic artery, 2 - left adrenal gland, 3 - left adrenal vein, 4 - left renal artery, 5 - kidney, 6 - left renal vein , 7 - superior mesenteric artery, 8 - aorta (abdominal part), 9 - inferior mesenteric artery, 10 - left ascending lumbar vein, 11 - left common iliac artery and vein, 12 - median sacral arteries and vein, 13 - right common iliac artery and vein, 14 - right ascending lumbar vein, 15 - right lumbar arteries and veins, 16 - right testicular (ovarian) artery, 17 - right testicular (ovarian) vein, 18 - right renal vein, 19 - right renal artery, 20 - celiac trunk, 21 - inferior vena cava, 22 - hepatic veins. In the lower P. century. drain (Fig. 3) lumbar veins (vv. lumbales), right testicular or ovarian vein (v. testicularis dext. s. ovarica dext.), renal veins (vv. renales), right adrenal vein (v. Suprarenalis dext.) , inferior phrenic veins (vv. phrenicae inf.) and hepatic veins (vv. hepaticae). At the confluence with the lower P. v. of the left hepatic vein lies the venous ligament (lig. venosum), the remnant of the ductus venosus (see).
In a wedge, in practice, it is customary to distinguish the following sections of the lower P. v.: infrarenal, renal (or renal), hepatic.
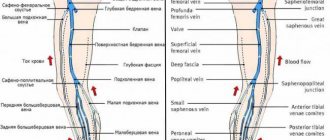
Rice. 1. Human veins (the portal vein and its tributaries are indicated by thick black stripes). Connections of tributaries of the portal vein with tributaries of the superior and inferior vena cava (cavo-portal anastomoses - at the junction of the black and light elements of the picture): 1 - v. subclavia; 2 - v. jugularis int.; 3—v. brachiocephalica dext.; 4 - v. brachiocephalica sin.; 5 - v. cava sup.; 6 - vv. intercostales post.; 7 - v. hemiazygos accessoria; 8 - v. hemiazygos; 9—v. lienalis; 10 - v. lumbalis ascendens; 11—v. renalis; 12 - v. cava inf.; 13 - v. mesenterica inf.; 14 - v. testicularis; 15 - v. rectalis sup.; 16 - v. iliaca communis; 17 - v. iliaca ext.; 18 - v. iliaca int.; 19 - v. epigastrica superficialis; 20 - v. epigastrica inf.; 21 - vv. paraumbilicales; 22 - v. mesenterica sup.; 23 - v. portae; 24 - v. epigastrica sup.; 25 - vv. esophagae; 26 - v. thoracoepigastrica; 27 - v. thoracica int.; 28 - v. azygos.
Anastomoses
. Anastomoses of the roots of the upper and lower P. are of great practical importance. between themselves and with the roots of the veins, which are tributaries of the portal vein (see Fig. 1). They are observed ch. arr. in the area of the anterior and posterior walls of the chest and abdominal cavities, as well as in a number of organs (for example, in the esophagus, rectum).
Blood supply
. Arteries and veins of the walls of the P. v. are branches and tributaries of nearby large arteries and veins. In the outer shell of P. v. arteries and veins form plexuses, due to which all layers of the walls of the P. v. are supplied with blood. According to V. Ya. Bocharov (1968), in the middle shell of the lower P. v. arterioles and a three-dimensional network of capillaries lie. In this layer, venules are formed that flow into the veins of the outer shell. In the subintimal layer of the wall of the lower P. v. There is a planar network of blood capillaries. Wall of the upper P. v. differs in a smaller number of intramural blood vessels than the wall of the lower P. v. This circumstance is explained by the smaller number of muscle elements in its wall. I.M. Yarovaya (1971) indicates that the network of blood capillaries in the wall of the upper ventricle. thickens towards the heart.
Lymphatic drainage
. Lymph. capillaries and vessels form in the walls of the P. v. networks and plexuses located mainly in the outer and also in the middle shell. The draining lymph vessels flow into nearby lymph, collectors and nodes.
Innervation
complex. Nonidez (J. Nonidez) was the first to show two types of nerve endings in the walls of the venous v., and morphologically substantiated the origin of the Bainbridge reflex (increased heart contractions in response to an increase in venous blood flow). B. A. Dolgo-Saburov described P. v. in all membranes. nerve plexuses, especially well expressed in the middle. In the outer shell of P. v. nerve cells were found. According to V.V. Kupriyanov et al. (1979), in the wall of the lower P. v. they are represented by spinal type afferent neurons and Dogel type II cells, as well as efferent autonomic multipolar neurons. Neurons with high cholinesterase activity (parasympathetic) are found predominantly in areas of the P. v. close to the heart; extensive accumulations of adrenergic (sympathetic) neurons are found throughout its entire length. Adrenergic nerve fibers accompany blood vessels and form plexuses in the outer membrane and among smooth muscle cells. Cholinergic system of conductors in the wall of the lower P. v. It is represented by large nerve bundles and forms plexuses that penetrate all membranes. In the wall of the P. v. Various types of encapsulated and non-encapsulated receptors were found, as well as areas of their predominant accumulation, especially near the heart, and in the lower half of the vein, in addition, in the area of the confluence of the renal and the confluence of the common iliac veins.
Anomaly of the postrenal segment of the IVC
Duplication of the IVC is an anomaly in which two infrarenal segments of the IVC are identified; the left IVC, after the left renal vein flows into it, crosses the aorta in front, connecting with the right renal vein and the right IVC. The detection rate in the population is 0.2–3%. Embryologically, this anomaly is the result of the functioning of both supracardinal veins. There are complete and incomplete doublings. In complete duplication, the left IVC crosses the aorta anteriorly and enters the right IVC, with the left renal vein joining the left IVC until it confluences with the right IVC. There are also three types of complete doubling of the IVC:
- in type I, both the IVC and the trunk crossing the aorta have the same diameter;
- in type II, the trunks of both IVCs are symmetrical, smaller in diameter than the trunk crossing the aorta;
- in type III, the right IVC has a larger diameter than the left IVC and the trunk crossing the aorta.
Duplication of the right IVC is a development option in which the left trunk of the right IVC is located in the middle and behind the aorta, being a continuation of the left common iliac vein, and merges with the right trunk of the IVC at the level of the kidneys. It is formed as a result of the persistence of the right supracardinal and right subcardinal veins, while the left supracardinal vein undergoes involution.
Duplication of the IVC with the retroaortic right renal vein and continuation into the hemizygos vein is a combination of several anomalies of the IVC, in which the lumbar and thoracic sections of the left supracardinal vein and the left suprasubcardinal anastomosis function, while the anastomosis between the right subcardinal and hepatic veins undergoes involution; in addition, the functioning lumen of the dorsal semicircle of the renal ring is preserved, while the lumen of the ventral semicircle regresses. As a result, duplication of the infrarenal segments of the IVC is formed, with the right renal vein communicating with the right IVC, crossing the aorta posteriorly and merging with the left IVC, which continues into the hemizygos vein, crossing the thoracic aorta posteriorly and merging with the rudimentary azygos vein. There are alternative routes of collateral blood flow from the hemizygos vein: in the first case, through the persistent left superior vena cava into the coronary venous system, in the second, into the left brachiocephalic vein. In this anomaly, the hepatic veins usually drain directly into the right atrium. The literature describes a clinical observation of a patient with Budd–Chiari syndrome, when the hepatic veins were drained through the right renal vein into the hemizygos vein, which was a continuation of the left-sided IVC.
Duplication of the IVC with the retroaortic left renal vein and continuation into the azygos vein is another interesting variant of the combination of abnormal development of several sections of the IVC, formed as a result of the functioning of the left supracardinal vein and the dorsal semicircle of the renal ring, involution of the ventral parts of the renal ring and supracardinal-hepatic anastomosis. In this case, the doubling of the infrarenal segments of the IVC is determined, the left renal vein communicates with the left IVC, crosses the aorta from behind and merges with the right IVC, which continues into the azygos vein.
The described anomalies of the IVC - variants of transposition and duplication, usually have an asymptomatic course and are detected during surgical interventions with retroperitoneal access. However, they become of great importance in a number of clinical situations: when installing a vena cava filter in patients with ileofemoral thrombosis and recurrent pulmonary embolism, which requires careful determination of the level of implantation; during laparoscopic left-sided nephrectomy to obtain a donor kidney; when planning surgical interventions on the abdominal aorta, especially for ruptured abdominal aneurysms. In cases of computed tomography of the abdominal cavity and retroperitoneum without intravenous contrast enhancement in the venous phase, the left IVC may be erroneously interpreted as pathologically enlarged para-aortic lymph nodes or an additional retroperitoneal neoplasm.
Histology
Rice.
4. Microscopic specimen of the wall of the human inferior vena cava (cross-section): I - inner membrane, II - middle membrane, III - outer membrane; 1 - smooth muscle cells, 2 - bundles of longitudinally running smooth muscle cells, 3 - connective tissue, 4 - vascular vessels; hematoxylin-eosin staining; x 130. Gistol, structure of the walls of the upper and lower P. v. not the same due to their different functional load. The thickness of the wall of the upper p. in the extrapericardial part of an adult, 300-500 µm. In the wall of the upper P. v. the border between the inner and middle shells is not clearly defined. The middle shell contains a small number of circular bundles of smooth muscle cells, separated by layers of connective tissue, passing into the outer shell, the edges are 3-4 times thicker than the inner and middle shell combined. Bundles of collagen fibers in its composition have a predominantly oblique and circular direction, while elastic fibers have a longitudinal direction. In the middle shell of the lower P. v. Circularly arranged bundles of smooth muscle cells are clearly visible. The outer shell contains a large number of longitudinally arranged bundles of smooth muscle cells, separated by layers of connective tissue and makes up 3/5 of the thickness of the entire wall (Fig. 4). According to V. Ya. Bocharov (1968), the middle shell differs from the outer shell in a smaller number of connective tissue elements and thinner bundles of smooth muscle cells. In the inner shell, a layer of elastic fibers is detected, and at the border of the inner and middle shells there is a thin layer of connective tissue with a predominance of collagen fibers. At the confluence of the upper and lower P. v. in the heart, striated muscle fibers of the myocardium penetrate into their outer shell.
According to Bucciante (L. Bucciante, 1966), in newborns in the walls of the veins of the abdominal cavity, in particular in the lower abdominal cavity, there are only circular bundles of smooth muscle cells. After the birth of improvement in the wall II. V. in humans they are expressed in changes in the number, position and orientation of muscle cells. Longitudinal bundles of smooth muscle cells appear in the wall of the P. v. only after birth. Thus, it was noted that in a 7-year-old child in the wall of the lower P. v. the circular and longitudinal layers of smooth muscle cells are well developed. In the wall of the upper P. v. in a newborn, the muscle elements are very weakly represented, and only by the age of 10 do circular bundles of smooth muscle cells appear. Age-related hypertrophy and hyperplasia of muscle elements in the wall of the P. v. have been established. In old age, a decrease in circularly located smooth muscle cells is observed, and after 70 years, their atrophy. According to Bucciante (1966), elastic membranes in the subendothelial layer also become well defined by the age of 10 years. Elastic elements of the wall of the P. v. in the process of aging they thicken and undergo dystrophic changes. The number of collagen fibers in the subendothelial layer increases, as well as between the muscle bundles in the middle and outer membranes.
Superior vena cava syndrome
Superior vena cava syndrome is usually diagnosed among the male population, both young and old, the average age of patients is about 40-60 years.
The basis of superior vena cava syndrome is compression from outside or thrombus formation due to diseases of the mediastinal organs and lungs:
- Bronchopulmonary cancer;
- Lymphogranulomatosis, enlargement of the mediastinal lymph nodes due to metastases of cancer of other organs;
- Aortic aneurysm;
- Infectious and inflammatory processes (tuberculosis, inflammation of the pericardium with fibrosis);
- Thrombosis due to a catheter or electrode remaining in the vessel for a long time during cardiac stimulation.
compression of the superior vena cava by a lung tumor
When a vessel is compressed or its patency is impaired, the movement of venous blood from the head, neck, arms, shoulder girdle to the heart is sharply hampered, resulting in venous stagnation and serious hemodynamic disorders.
The severity of the symptoms of superior vena cava syndrome is determined by how quickly the blood flow is disrupted and how well developed the bypass blood supply routes are. With a sudden closure of the vascular lumen, the phenomena of venous dysfunction will increase rapidly, causing an acute circulatory disorder in the superior vena cava system, with a relatively slow development of pathology (enlarged lymph nodes, growth of a lung tumor) and the course of the disease will be slowly increasing.
Symptoms accompanying expansion or thrombosis of the SVC “fit” into the classic triad:
- Swelling of the tissues of the face, neck, hands.
- Blueness of the skin.
- Dilation of the saphenous veins of the upper half of the body, arms, face, swelling of the venous trunks of the neck.
Patients complain of difficulty breathing even in the absence of physical activity, the voice may become hoarse, swallowing is impaired, there is a tendency to choking, coughing, and pain in the chest. A sharp increase in pressure in the superior vena cava and its tributaries provokes ruptures of the walls of blood vessels and bleeding from the nose, lungs, and esophagus.
A third of patients experience laryngeal edema due to venous stagnation, which manifests itself as noisy, stridor breathing and is dangerous for asphyxia. Increasing venous insufficiency can lead to cerebral edema, a fatal condition.
To alleviate the symptoms of the pathology, the patient strives to take a sitting or semi-sitting position, in which the outflow of venous blood towards the heart is somewhat facilitated. In the supine position, the described signs of venous stagnation intensify.
Disruption of blood flow from the brain is fraught with symptoms such as:
- Headache;
- Convulsive syndrome;
- Drowsiness;
- Impaired consciousness up to fainting;
- Decreased hearing and vision;
- Puffy eyes (due to swelling of the tissue behind the eyeballs);
- lacrimation;
- Humming in the head or ears.
To diagnose the syndrome of the superior vena cava, radiography of the lungs is used (allows one to identify tumors, changes in the mediastinum, in the heart and pericardium), computed tomography and magnetic resonance imaging (neoplasms, examination of lymph nodes), phlebography is indicated to determine the location and degree of blockage of the vessel.
In addition to the studies described, the patient is sent to an ophthalmologist, who will detect congestion in the fundus and swelling, and an ultrasound examination of the vessels of the head and neck to assess the effectiveness of the outflow through them. In case of pathology of the chest organs, a biopsy, thoracoscopy, bronchoscopy and other studies may be necessary.
Before the cause of venous stagnation becomes clear, the patient is prescribed a diet with minimal salt content, diuretics, hormones, and the drinking regime is limited.
If the pathology of the superior vena cava is caused by cancer, then the patient will undergo courses of chemotherapy, radiation, and surgery in an oncology hospital. In case of thrombosis, thrombolytics are prescribed and the option of surgical restoration of blood flow in the vessel is planned.
The absolute indications for surgical treatment for lesions of the superior vena cava are acute obstruction of the vessel by a thrombus or a rapidly increasing tumor due to insufficiency of collateral circulation.
stenting of the superior vena cava
In case of acute thrombosis, they resort to removing the blood clot (thrombectomy); if the cause is a tumor, it is excised. In severe cases, when the wall of the vein is irreversibly changed or has grown into a tumor, resection of a section of the vessel is possible with replacement of the defect with the patient’s own tissues. One of the most promising methods is stenting of a vein in the place of greatest obstruction of blood flow (balloon angioplasty), which is used for tumors and scar deformation of mediastinal tissue. As a palliative treatment, bypass operations are used to ensure blood discharge bypassing the affected area.
Research methods
Rice.
5. Diagram of chest x-ray: a - direct projection, the superior vena cava is adjacent to the ascending aorta (shaded), b - lateral projection, the inferior vena cava in the form of a triangle is visible between the infero-posterior contour of the heart and the diaphragm (shaded). Conventional wedge methods (examination, changes in skin color, measurement of the circumference of the upper limb, etc.) allow one to suspect various pathologies of P. v. The main diagnostic method is x-ray, ch. arr. X-ray contrast study of P. v. - cavography (see). On a direct radiograph, the upper P. v. together with the ascending aorta it forms the right border of the vascular shadow (Fig. 5, a). When the upper P. v. expands, for example, with a defect of the right atrioventricular (tricuspid) valve or when the vein is displaced to the right, the contour of the vascular shadow shifts to the right. In the first oblique position, the shadow of the lower P. v. can be visible in the form of a strip running from the diaphragm to the posterior contour of the heart, and in a lateral position - in the form of a triangle between the shadow of the heart and the contour of the diaphragm (Fig. 5, b). The absence of a triangle indicates an enlargement of the left ventricle of the heart.
Superior cavography can be performed in an antegrade or retrograde manner. In the first case, a radiopaque substance is administered by puncture or catheterization of the veins of the shoulder or subclavian vein on one or both sides (see Puncture catheterization of veins). For retrograde contrasting of the upper P. century. the catheter is passed through the femoral, external and common iliac, and lower p. and the right atrium (see Seldinger method).
Rice. 6. Angiocardiogram (direct projection): 1 - left subclavian vein, 2 - left brachiocephalic vein, 3 - superior vena cava, 4 - right atrium, 5 - inferior vena cava (contrasted due to reflux).
On the angiocardiogram in a direct projection (Fig. 6) the contrasted upper P. v. serves as a continuation of two brachiocephalic veins, merging with each other below the right sternoclavicular joint; it is located to the right of the shadow of the spine and has the appearance of a clearly defined strip with a width of 7 to 22 mm (depending on age). At the level of the 3rd rib, the shadow of the upper P. v. passes into the shadow of the right atrium. In the first oblique position, the upper P. v. occupies the anterior section of the vascular shadow; in the second oblique position, its shadow is located slightly posterior to the anterior contour of the aorta. In direct projection, contrasted lower P. v. lies to the right of the spine, slightly overlapping it; in the lateral projection it is located in front of the lumbar region, and its upper section deviates anteriorly and flows into the right atrium.
Inferior cavography can also be done antegrade or retrograde. In the first case, a radiopaque substance is administered by puncture or catheterization of the femoral vein on one or both sides. For retrograde cavography, the catheter is inserted into the lower P. v. through the subclavian, brachiocephalic, upper P. v. and right atrium.