Compound
In one tablet: pantoprazole sodium sesquihydrate - 45.1 mg (40 mg in terms of pantoprazole ) + calcium carbonate , sodium lauryl sulfate, colloidal silicon dioxide, magnesium oxide, crospovidone, calcium stearate, triethyl citrate, titanium dioxide, macrogol 6000, methacrylic acid and ethyl acrylate in the form of a copolymer, copovidone, talc, yellow iron oxide.
One bottle contains: 40 mg of pantoprazole sodium chloride solution .
Release form and composition
Sanpraz is available in the following forms:
- Enteric-coated tablets: biconvex, round, yellow (10 pieces in aluminum strips, 1, 2 or 3 strips in a cardboard box);
- Lyophilisate for the preparation of solution for intravenous administration: white or almost white; solvent – colorless, transparent (40 mg in colorless glass bottles, 1 bottle in a cardboard box complete with 1 ampoule (10 ml) of solvent).
Composition of 1 tablet:
- Active ingredient: pantoprazole (in the form of pantoprazole sodium sesquihydrate) – 40 mg;
- Auxiliary components: calcium carbonate, calcium stearate, magnesium oxide, sodium lauryl sulfate, colloidal silicon dioxide, crospovidone;
- Shell composition: copovidone, macrogol 6000, triethyl citrate, copolymer of ethyl acrylate and methacrylic acid (1:1), talc, yellow iron oxide, titanium dioxide.
Lyophilisate for the preparation of solution for intravenous administration (set):
- 1 bottle with lyophilisate, active ingredient: pantoprazole (in the form of pantoprazole sodium sesquihydrate) – 40 mg;
- 1 ampoule with solvent: 0.9% sodium chloride solution – 10 ml.
Pharmacodynamics and pharmacokinetics
The drug inhibits the process of proton transfer of H+-K+-ATPase .
hydrochloric acid is blocked at the last stage of the process, basal and stimulated secretion decreases, regardless of the origin of the specific stimulus.
If a patient has a stomach ulcer caused by the bacteria Helicobacter pylori , then by reducing the production of hydrochloric acid the sensitivity of the bacteria to antibiotics . It is noteworthy that the drug does not affect normal gastrointestinal .
After taking the tablets, the therapeutic effect occurs within an hour, reaching a maximum after 2-4 hours. 3-4 days after finishing taking the drug, the secretion of hydrochloric acid and other substances returns to normal. The substance is stable, at neutral pH it does not affect the oxidase system of the liver, which depends on the enzyme cytochrome P450 .
When taken orally, the active substance is quickly absorbed into the gastrointestinal tract . Cmax in blood plasma equal to 2-3 mcg per ml is achieved after 3 hours. About 98% of Pantoprazole is bound to plasma proteins. Parallel food intake does not affect pharmacokinetic parameters. With frequent and long-term use, this indicator remains constant.
The half-life is one hour. The drug undergoes metabolic reactions in the liver. Metabolites are excreted through the kidneys and with feces.
The drug does not accumulate in the body. For persons suffering from renal failure undergoing hemodialysis , no dose adjustment is required.
With liver cirrhosis, the half-life increases to 7-9 hours, the maximum concentration is 1.5 times higher. In elderly people, the maximum blood concentration and AUC of the drug slightly increase. However, no adjustment of the daily dose is required.
When administered intravenously, the indicators are similar to taking tablets.
special instructions
Before starting therapy and after its completion, endoscopic monitoring is required to exclude malignant tumors of the esophagus or stomach, since treatment with Sanpraz can mask the symptoms of the disease and delay the correct diagnosis.
For the treatment of dyspepsia of neurogenic origin, the use of the drug is not effective.
If any of the alarming symptoms are present (recurrent vomiting, melena, significant weight loss, anemia, dysphagia) and if an ulcer is suspected, or in the case of an existing gastric ulcer, it is necessary to exclude the presence of a malignant neoplasm. If symptoms persist with adequate treatment, further diagnosis should be performed.
Sanpraz does not affect the ability to drive vehicles or perform other work that requires increased concentration and quick reaction.
Indications for use
The drug is prescribed:
- with Zollinger-Ellison syndrome ;
- for the treatment and prevention of ulcers caused by stress;
- for erosive gastritis , including those associated with taking non-steroidal anti-inflammatory drugs ;
- for the treatment of GERD ;
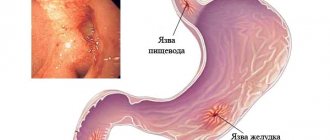
- for the treatment of gastric and duodenal , usually in acute form;
- to eliminate the bacterium Helicobacter pylori in combination with antibiotics ;
- for the treatment of bleeding, perforations and penetrations that occur as complications of a gastric ulcer .
Contraindications
The medicine is not prescribed:
- for indigestion caused by neurotic disorders;
- children;
- if you are allergic to the components of the product;
- for oncological diseases of the stomach and intestines.
In the presence of liver disease, liver cirrhosis and during pregnancy, the medicine is prescribed with caution.
Side effects
When taking the medicine you may experience:
- depression , headache , disorientation , hallucinations and confusion ;
- thrombocytopenia , leukopenia ;
- thrombophlebitis and phlebitis ;
- epigastric pain, constipation or diarrhea , nausea, flatulence , dry mucous membranes, jaundice , increased levels of liver enzymes;
- arthralgia , myalgia ;
- urticaria , skin itching , anaphylactic shock , erythema , angioedema , photosensitivity , Stevens-Johnson syndrome ;
- triglyceride levels , nephritis .
Side effects
- Digestive system: sometimes – diarrhea, flatulence, constipation, pain in the upper abdomen; rarely – dry mouth, vomiting and nausea; very rarely - increased activity of liver enzymes, hepatocellular failure with jaundice and liver failure;
- Musculoskeletal system: rarely – arthralgia; in isolated cases – myalgia;
- Central nervous system: sometimes – headache; rarely - weakness, confusion, disorientation, hallucinations, depression; very rarely - blurred vision, dizziness;
- Hematopoietic system: very rarely - thrombocytopenia, leukopenia;
- Dermatological reactions: very rarely - skin rash and itching, severe skin reactions (erythema multiforme, Lyell's syndrome, photosensitivity, Stevens-Johnson syndrome);
- Allergic reactions: very rarely - urticaria, anaphylactic shock, angioedema;
- Local reactions (with intravenous administration): phlebitis and thrombophlebitis;
- Other reactions: very rarely - increased triglyceride levels, peripheral edema, interstitial nephritis, painful tension of the mammary glands; in isolated cases - hyperthermia.
When taking the drug Sanpraz according to indications and in recommended doses, side effects occur extremely rarely.
Instructions for use of Sanpraz (method and dosage)
Depending on the patient’s condition, the doctor must prescribe one or another dosage form.
Sanpraz tablets, instructions for use
The tablets are taken orally with liquid, without biting or crushing them. It is best to take the medicine in the morning, on an empty stomach, an hour before the first meal.
For stomach ulcers, 40-80 mg per day is prescribed for treatment and 20 mg per day for prevention. Duration of treatment is 4-8 weeks.
For duodenal ulcers and erosive gastritis , the daily dosage is 40-80 mg; for prevention, 20 mg of the drug per day is also taken. The course of treatment is half a month.
To eradicate Helicobacter bacteria, 80 mg per day is prescribed in 2 doses. Duration of treatment is 7-14 days. In parallel, antibiotics are usually prescribed.
For gastrointestinal erosion caused by taking NSAIDs , the daily dosage is 40-80 mg, 20 mg for prevention. The course of treatment is up to 2 months.
For the treatment of reflux esophagitis, 20-40 mg of the drug per day is used. For prevention – 20 mg. Duration of treatment is 4-8 weeks.
Instructions for solution for intravenous administration
This form of medication is prescribed when it is impossible to use tablets. As soon as this becomes possible, switch to tablet form.
To prepare the solution, you must mix the contents of the bottles.
The solution is administered in a stream or through a dropper. The injection time is from 2 to 15 minutes, the pH of the solution is 9-10. After 3 hours, the prepared solution becomes unsuitable for administration.
As a rule, use 40 mg (one bottle) per day. It is permissible to make intravenous injections for 7-10 days. The maximum amount of the drug per day is 80-160 mg. The dose should be divided into 2 injections.
If the patient has severe liver dysfunction, the maximum daily dosage is 20 mg. Liver enzyme levels should be closely monitored during treatment.
If the patient is on hemodialysis or is elderly, the dosage should not exceed 40 mg per day.
Sanpraz
Sanpraz
(lat.
Sunpras
) - antiulcer, proton pump inhibitor, reducing gastric acidity drug.
Dosage forms and composition of Sanpraz
Active substance Sanpraz
: pantoprazole (lat.
pantoprazole
).
Sanpraz is available in the form of round, biconvex tablets coated with a
yellow enteric coating. One tablet of Sanpraz contains 45.1 mg of pantoprazole sodium sesquihydrate, which corresponds to 40 mg of pantoprazole. Excipients of Sanpraza tablets: magnesium oxide, calcium carbonate, crospovidone, sodium lauryl sulfate, calcium stearate, colloidal silicon dioxide, polymethacrylate type C, triethyl citrate, copovidone, purified talc, titanium dioxide, iron oxide yellow, macrogol 6000, isopropyl alcohol, acetone, water purified.
Sanpraz is also available as a lyophilisate for the preparation of a solution for intravenous administration.
. One vial contains 45.1 mg of pantoprazole sodium sesquihydrate, which corresponds to 40 mg of pantoprazole. The bottle is accompanied by an ampoule with a solvent containing 10 ml of 0.9% sodium chloride solution.
Indications for use of Sanpraz
Peptic ulcer of the duodenum or stomach in the acute phase, including those associated with taking non-steroidal anti-inflammatory drugs, or refractory to therapy with H2-blockers; moderate and severe forms of gastroesophageal reflux disease; Zollinger-Ellison syndrome; combined anti-Helicobacter eradication therapy in patients with peptic ulcers to reduce the frequency of relapses.
How to use Sanpraz and dosage
tablets
are swallowed whole without crushing or dissolving, washed down with liquid. Sanpraz is recommended to be taken an hour before breakfast, but if it is prescribed to be taken twice a day, then the second tablet is taken an hour before dinner.
For stomach and duodenal ulcers and erosive gastritis, Sanpraz is prescribed one or two tablets per day. The course of treatment for exacerbation of duodenal ulcers is 2 weeks, and for gastric ulcers - 4–8 weeks.
For eradication of Helicobacter pylori
take a Sanpraz tablet twice a day only in combination with antibiotics for one to two weeks.
Helicobacter pylori
monotherapy with Sanpraz is not used.
For erosive and ulcerative lesions of the stomach and duodenum associated with taking NSAIDs, one or two Sanpraz tablets are prescribed per day for 4–8 weeks.
For reflux esophagitis, one Sanpraz tablet is prescribed per day for 4–8 weeks.
In patients with severe liver dysfunction, take one Sanpraz tablet every two days, and biochemical blood parameters should be monitored. If the activity of liver enzymes increases, Sanpraz should be discontinued.
Injection form
Sanpraza is indicated only for intravenous administration and only in cases where taking Sanpraza tablets orally is not indicated. When oral administration becomes possible, the injection form of Sanpraz is replaced with tablets
The recommended dose for intravenous administration is the contents of one bottle per day. The duration of therapy depends on the patient's condition.
Professional medical publications concerning the use of Sanpraz
- Kurilovich S.A., Chernosheikina L.E. Antisecretory potential of pantoprazole (Sanpraz) // Experimental and clinical gastroenterology. - 2008. - N 7. — P. 119-122.
- Pakhomova I.G. Clinical capabilities of the drug Sanpraz // Effective pharmacotherapy in gastroenterology. – 2008. – No. 1. – p. 4–5.
- Sharova E.P. The drug Sanpraz (pantoprazole): experience of use in the treatment of gastroesophageal reflux disease and other acid-dependent diseases // Experimental and clinical gastroenterology. – 2008. – No. 6. – p. 74–76.
- Pakhomova I.G. The use of pantoprazole (sanpraz) for the treatment of gastroesophageal reflux disease and NSAID-induced gastropathy // Experimental and clinical gastroenterology. – 2007. – No. 6. – p. 110–115.
- Lazebnik L.B., Vasiliev Yu.V. The effectiveness of pantoprazole in the treatment of gastroesophageal reflux disease // Experimental and clinical gastroenterology. – 2007. – No. 2. – pp. 1–3.
- Bordin D.S., Masharova A.A., Silvestrova S.Yu. and others. Pharmacokinetics of a proton pump inhibitor and the mental status of the patient as factors, ow. on ef. treatment of GERD with pantoprazole // Experimental and class. gastroenterology. 2010. No. 9. pp. 90–96.
- Shapovalyants S.G., Chernyakevich S.A., Mikhalev A.I. and others. The effectiveness of pantoprazole when administered parenterally in patients with acute ulcerative gastroduodenal bleeding with a high risk of relapse // RZHGGK. 2012. T.21. No. 2. P.22-28.
- Shapovalyants S.G., Mikhalev A.I., Babkova I.V. Modern pharmacotherapy of ulcerative gastroduodenal bleeding // Gastroenterology. 2021. Special issue No. 4.
On the website GastroScan.ru in the “Literature” section there is a subsection “Pantoprazole”, containing articles for healthcare professionals regarding the use of pantoprazole.
Pharmacological properties of Sanpraz, interactions with other drugs
The pharmacological properties of Sanpraz and its interaction with other drugs are determined by its active substance - pantoprazole and are described in the article "pantoprazole".
Contraindications to the use of Sanpraz
Hypersensitivity to pantoprazole, hepatitis and cirrhosis of the liver, accompanied by severe liver failure.
Restrictions on the use of Sanpraz
Liver dysfunction, childhood, pregnancy, lactation.
Use of Sanpraz during pregnancy and breastfeeding
Therapy with Sanpraz during pregnancy is possible only under strict indications, when the benefit to the mother outweighs the potential risk to the fetus.
Due to the fact that the active substance Sanpraza pantoprazole is secreted into breast milk, breastfeeding should be stopped during treatment. FDA category of possible risk to the fetus is B.
Comparison of Sanpraz with other proton pump inhibitors
Among gastroenterologists, there are different points of view on the comparative effectiveness of specific types of proton pump inhibitors.
Some of them argue that, despite some differences that exist between PPIs, today there is no convincing data to suggest that any PPI is more effective than others (Vasiliev Yu.V. et al.), including in schemes used in the eradication of HP type PPI (Nikonov E.K., Alekseenko S.A.). Others write that, for example, esomeprazole is fundamentally different from the other four PPIs: omeprazole, pantoprazole, lansoprazole and rabeprazole (Lapina T.L., Demyanenko D., etc.). Still others believe that rabeprazole is the most effective (Ivashkin V.T. et al., Maev I.V. et al.). According to Bordin D.S., the effectiveness of all PPIs for long-term treatment of GERD is similar. In the early stages of therapy, lansoprazole has some advantages in the speed of onset of effect, which potentially increases patient adherence to treatment. If it is necessary to take several drugs for the simultaneous treatment of other diseases, pantoprazole (i.e. Sanpraz) is the safest.
There are many generic PPIs on the Russian and other CIS markets. Due to possible differences in the quality of drugs, an objective assessment of their clinical effectiveness is important. Currently, 24-hour monitoring of intragastric pH levels is an objective and accessible method for testing antisecretory agents in clinical practice (Alekseenko S.A.).
Sapraz belongs to high-quality generics and is in the middle of the price range between cheap generics of omeprazole, pantoprazole and lansoprazole and expensive original Nexium and Pariet.
Side effects of Sanpraz
- From the gastrointestinal tract: diarrhea, rarely - dry mouth, increased appetite, nausea, belching, vomiting, flatulence, abdominal pain, constipation, increased transaminase activity, gastrointestinal carcinoma (isolated case).
- From the nervous system and sensory organs: headache; rarely - asthenia, dizziness, drowsiness, insomnia; in some cases - nervousness, depression, tremor, paresthesia, photophobia, visual impairment, tinnitus.
- From the genitourinary system: in isolated cases - hematuria, edema, impotence.
- On the part of the skin: in isolated cases - alopecia, acne, exfoliative dermatitis.
- Allergic reactions: rarely - rash, urticaria, itching, angioedema.
- Other: rarely - hyperglycemia, myalgia; in isolated cases - fever, eosinophilia, hyperlipoproteinemia, hypercholesterolemia.
Other medicines containing the active ingredient pantoprazole
Registered in Russia:
Zipantol, Controloc, Crosacid, Nolpaza, Pantaz, Pantoprazole Canon, Pantoprazole sodium sesquihydrate, Panum, Peptazol, Pigenum-sanovel, Puloref, Ulthera.
Present on the pharmaceutical markets of countries - former republics of the USSR and not registered in Russia:
zogast (BILIM Ilac San. ve Tic., AS, Turkey), zolopent (Kusum Pharm LLC, Ukraine), panocid (Flamingo Pharmaceutical, India), pantasan (Sun Pharmaceutical Industries Ltd, India), pantap (Nobel Almaty Pharmaceutical JSC Factory, Kazakhstan), Proxium (JSC Lubnipharm, Ukraine), Protonex (Abdi Ibrachim, Turkey), Ulthera (Emcure Pharmaceuticals Ltd., India).
Branded pantoprazole approved in the USA (and not registered in Russia): Protonix (Pfizer Inc, USA).
general information
According to the pharmacological index, Sanpraz belongs to the group “Proton pump inhibitors”.
According to ATC, it belongs to the group “Proton pump inhibitors” and has the code A02BC02. Sanpraz is a prescription medicine
.
“Instructions for medical use of the drug Sanpraz”, enteric tablets, film-coated, 40 mg, approved by the Ministry of Health of Russia on November 10, 2017.
Manufacturer: Sanpraza
:
Sun Pharmaceutical Industries Ltd, India.
Sanrpaz has contraindications, side effects and application features; consultation with a specialist is necessary.
Back to section
Interaction
The combination of Sanpraz with ketoconazole , ritonavir and iron salts reduces their absorption due to changes in the pH of gastric juice.
The medicine can be combined with digoxin , metoprolol , clarithromycin , diclofenac , naproxen , glibenclamide , diazepam, phenytoin, tacrolimus, midazolam, theophylline, ethanol, caffeine, Nifedipine, amoxicillin, oral contraceptives, phenazole, piroxicam, levothyroxine sodium, carbamazepine, cyclosporine, cisapride, metronidazole .
When taking the drug and atazanavir , the effectiveness of the latter is reduced.
If you take the medicine and Warfarin the prothrombin index changes , and bleeding may occur. In this regard, PTI must be carefully monitored.
Drug interactions
When used simultaneously with drugs whose bioavailability depends on the pH of gastric juice (for example, ketoconazole, ritonavir, iron salts), their absorption may be reduced.
When taken together with atazanavir, the plasma concentration of atazanavir decreases and its effectiveness decreases.
With the simultaneous use of Sanpraz and warfarin, the prothrombin time increases, which can lead to serious bleeding and even death. Therefore, when prescribing these drugs together, it is recommended to determine the prothrombin time.
No clinically significant interaction of Sanpraz was found with the following drugs: nifedipine, amoxicillin, digoxin, diclofenac, naproxen, glibenclamide, diazepam, phenytoin, tacrolimus, midazolam, theophylline, metoprolol, clarithromycin, phenazone, piroxicam, carbamazepine, levothyroxine sodium, cyclosporine, cisapride, metroni dazole , caffeine, oral contraceptives (ethinyl estradiol/levonorgestrel) and ethanol.
Sanpraz's analogs
Level 4 ATC code matches:
Khairabesol
Noflux
Lancid
Barol
Beret
Ontime
Gastrozol
Omeprazole
Pantoprazole
Proxium
Lansoprazole
Zulbex
Ultop
Epicurus
Pariet
Losek MAPS
Losek
Emanera
Omez Insta
Omez
The analogues of Sanpraz that are closest in action are: Zipantol, Controloc, Peptazol, Panum, Omez, Nolpaza, Proxium .
Sanpraz price
The price of Sanpraz 40 mg is 479 rubles for 30 tablets.
- Online pharmacies in RussiaRussia
ZdravCity
- Sanpraz tab.
ksh/sol. p/o captivity. 40 mg 30pcs SUN Pharmaceutikal Industries Ltd. RUR 478 order - Sanpraz Liof. for solution for intravenous injection. 40mg 10mlSUN Pharmaceutikal Industries Ltd.
RUR 398 order
- Sanpraz tab. p/o intestinal 40mg No. 10SUN Pharmaceutikal Industries Ltd.
RUB 261 order