Neutrophils are the largest group of granulocytes, the main function of which is phagocytosis (destruction) of pathogenic flora in the human body.
Neutropenia is a decrease in the number of neutrophils in the blood, which leads to the risk of bacterial and fungal infections.
In a healthy state, protection of the immune “borders” is provided by mature segmented neutrophils. Younger band neutrophils also help to “control” the situation, but there are much fewer of them (this is clearly visible from the results of a blood test, where normally mature neutrophils number from 42-72%, and young ones only from 1-6%).
But, in the event of certain diseases, the ratio of all types of neutrophils (segmented, band, myelocytes, metamyelocytes) is sharply disrupted, which leads to a pathological increase in neutrophils or their decrease (neutropenia).
At the same time, the number of neutrophils in human blood is not stable, unlike other cell groups of the leukocyte formula. The maturation, development and normal ratio of these granulocytes are affected not only by congenital and acquired pathologies, but also by severe emotional shock, stress, dietary disturbances, etc.
Neutropenia in children should be carefully investigated to prevent both severe pathologies and radical treatment without serious reasons.
Classification of severity of neutropenia:
- Light (1-1.5 x 109/l);
- Moderate (0.5-1 x109/l);
- Heavy (less than 0.5 x109/l).
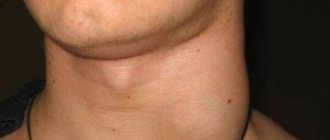
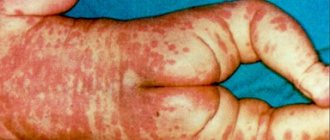
If the level of neutrophils decreases critically, this leads to the development of infection in the intestines, mouth, thereby causing digestive system disorders, gingivitis, stomatitis, fungal skin lesions, etc.
Severe neutropenia in the presence of cancer pathology has an extremely adverse effect on the functioning of the immune system, leading to progressive inflammation and infections, including death.
Etiology
Neutrophils develop in the bone marrow within 15 days, remain in the bloodstream for about 8 hours, constitute the overwhelming cellular part of the leukocyte formula, and when “pests” are detected, they are immediately sent to eliminate them.
The main causes of neutropenia that disrupt this physiological process:
- Development of pathogenic flora in the body (bacteria, viruses, fungi).
- Exposure to radiation, chemotherapy.
- Pathologies in the functioning of the bone marrow.
- Folic acid deficiency.
- Congenital pathologies of internal organs, glands and systems.
Neutropenia in children can also develop due to disruption of the pancreas and retarded mental development (Shwachman-Diamond-Oski syndrome).
Neutropenia is divided into:
- Spicy;
- Chronic.
Acute neutropenia develops rapidly due to the rapid consumption/destruction of neutrophils, chronic - due to a decrease in the production of granulocytes or excessive splenic secretion.
- Primary;
- Secondary.
Primary neutropenia is associated with internal disorders (congenital pathologies, idiopathic neutropenia, cyclic neutropenia, benign ethnic), secondary - due to the influence of external factors on the maturation and development of bone marrow cells.
Care
Recombinant granulocyte-colony-stimulating factor drugs such as filgrastim[21] may be effective in people with congenital forms of neutropenia, including severe congenital neutropenia and cyclic neutropenia;[22] the amount (dosage) needed to stabilize neutrophil counts varies significantly (depending on depending on the human condition).[23] Dietary recommendations for neutropenia are currently being studied.[24]
Most cases of neonatal neutropenia are temporary. Antibiotic prophylaxis is not recommended due to the potential for promoting the development of multidrug-resistant bacterial strains.[1]
Neutropenia can be treated with hematopoietic growth factors, granulocyte-colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF). These are cytokines (chemicals that cause inflammation) that are naturally present in the body. These factors are regularly used in the treatment of cancer in adults and children. These factors promote neutrophil recovery after anticancer therapy.[1]
The administration of intravenous immunoglobulins (IVIG) has had some success in the treatment of neutropenia of alloimmune and autoimmune origin, with a response rate of approximately 50%. Blood transfusions were ineffective.[1]
Antifungal drugs may be given to patients with neutropenia caused by cancer treatment. A Cochrane review [25] found that lipid formulations of amphotericin B had fewer side effects than conventional amphotericin B, although it is unclear whether there are specific advantages over conventional amphotericin B when used under optimal conditions. Another Cochrane review [26] failed to find a difference in effect between amphotericin B and fluconazole because the available trial data analyzed the results in a way that did not favor amphotericin B.
Secondary neutropenia
Secondary neutropenia most often develops due to:
- Taking certain medications (antibiotics, antihistamines, antipyretics, barbiturates, analgesics);
- Development of infections;
- Immune reactions;
- Bone marrow infiltration.
Secondary neutropenia provokes pathologies:
- Liver, spleen, kidneys;
- Pancreas, thyroid gland;
- Aplastic anemia;
- Megaloblastic anemia;
- Myelodysplastic disorders;
- Myelofibrosis, leukemia;
- Sepsis, HIV, Felty's syndrome;
- Rh-conflicting neutropenia;
- Lymphoproliferative diseases, etc.
In the case of secondary neutropenia, it is not the blood “test” that needs to be treated, but the patient with specific symptomatic manifestations.
Primary and secondary neutropenia should be distinguished, since in the first case, a decrease in the level of neutrophils is the only manifestation, while secondary ones develop on the basis of infections, systemic autoimmune pathologies and onco-neoplasia.
Severe congenital neutropenia (SCN)
SCN is a heterogeneous group of diseases based on impaired granulocytopoiesis at the level of promyelocytes with a decrease in the number of neutrophils below 0.2*109/l in the peripheral blood. The disease manifests itself in the first days of life. SCN is divided into several forms based on the type of inheritance and the genes in which mutations lead to the disease:
| Form SCN | Inheritance type | Gene |
| SCN1 (OMIM 202700) | HELL | ELANE |
| SCN2 (OMIM 600871) | HELL | GFI1 |
| SCN3 (OMIM 610738) | AR | HAX1 |
| SCN4 (OMIM 612541) | AR | G6PC3 |
*Genes are highlighted in blue, the analysis of which is carried out at the Center for Molecular Genetics LLC.
These forms of SCN are not clinically distinguishable.
According to the literature, 60% of SCN cases in Europe have an autosomal dominant mode of inheritance.
Other hereditary forms of severe hereditary neutropenia have also been described, such as X-linked severe hereditary neutropenia OMIM300299 (mutations in the WAS ).
Clinical manifestations
In most cases, neutropenia occurs without any symptoms. Symptoms of a decrease in neutrophils usually occur only if secondary neutropenia has developed against the background of certain pathologies.
General symptoms of neutropenia:
- Increased body temperature;
- Recurrent stomatitis, gingivitis, glossitis;
- Marked weakness;
- Increased sweating;
- Chills;
- Difficulty urinating (burning);
- Cough (against the background of pneumonia, inflammation in the lungs);
- Fungal skin lesions.
As a result of a sharp decrease in granulocytes, febrile neutropenia may also develop. This is a febrile state aggravated by tachycardia, tremor, chills, increased blood pressure, up to the development of cardiovascular collapse.
Febrile neutropenia usually develops as a result of oncopathologies, exposure to radiation or chemotherapy, in the form of a hyperergic reaction of the body to toxic influences (cytostatics, decay of healthy and cancer cells).
It can also manifest itself against the background of a severe infectious process, which is provoked by staphylococci, streptococci, clostridia, and, less commonly, fungi and viruses. In more rare cases, febrile neutropenia may develop from primary autoimmune neutropenia.
When should you contact your healthcare provider?
Contact your healthcare provider immediately if you have any of the following signs of infection:
- temperature 100.4°F (38°C) or higher;
- tremendous chills;
- persistent nausea and vomiting;
- facial redness;
- sweating;
- cough;
- lightheadedness or dizziness;
- diarrhea (loose or watery stools);
- constipation (bowel movements occur less frequently than usual);
- Mouth ulcers
- Headache
- pain in a new place;
- Irritability
- pain or burning when urinating (when you go to the toilet in small quantities);
- feeling weak, especially when combined with flu-like symptoms (such as fever, sore throat, and chills).
to come back to the beginning
Diagnostics
Diagnosis can be planned, unscheduled (in the presence of specific symptoms), and can also be carried out if the attending physician has any suspicions (unusual or frequently recurring infections).
A routine blood test in children under 1 year of age is carried out once every 3 months, after a year - once every 6 months (including adults).
If the diagnosis indicates a decrease in neutrophils in the blood, the following should be additionally prescribed:
- Screening study for neutropenia;
- Differentiation of neutropenia from other pathologies;
- Clarification of the form of neutropenia;
- Study of the mechanisms of development of this condition in a patient.
Next, the dynamics of the leukocyte formula indicators is assessed (after previous infections, between them, after recovery), including ESR (erythrocyte sedimentation rate), color index, etc.
Determining the cause of neutropenia may include the following diagnostic procedures:
- Visual inspection.
- Physical examination (assessment of the condition of all mucous membranes).
- X-ray.
- CT, ECG, ultrasound, MRI.
- Laboratory tests of blood (general analysis, biochemical), urine.
- Cultural seeding (to determine the type of bacteria and fungi).
- Biopsy.
- Bone marrow studies (to determine the cause of specific neutropenia, for leukemia, aplastic anemia, myelofibrosis).
In the case of severe acute neutropenia, a comprehensive diagnosis should be performed as quickly as possible.
If congenital pathology is considered, diagnosis includes molecular genetic testing of neutropenia.
A single blood test, which showed a low level of granulocytes, cannot make a definitive diagnosis. Since these are the only blood cells whose concentration can change daily, due to their short “life” in the bloodstream (6-8 hours).
To accurately confirm the patient’s condition, the blood test must be repeated at a short time interval (the time for testing is set by the doctor).
One of the most common and dangerous side effects of cytostatic therapy is neutropenia. Cytostatics, acting on rapidly dividing cells, also affect the hematopoietic system. Circulating neutrophils live on average 6–9 hours, and the hematopoietic system constantly produces about 50 million of these cells per minute to replace those that have retired. A decrease in neutrophil production after exposure to chemotherapy leads to a rapid decrease in the content of these cells in the peripheral blood, which increases the risk of developing infectious complications.
Prolonged neutropenia often does not allow chemotherapy (CT) to be carried out as planned and forces the dose of cytostatics to be reduced, which in turn reduces the effectiveness of antitumor treatment. In addition, infectious complications due to neutropenia in the absence of adequate therapy are characterized by a rapid course and high mortality. In this regard, there is a need to carefully assess the myelotoxic potential of current chemotherapy regimens and identify groups of patients at high risk of developing neutropenia who require prophylactic administration of colony-stimulating factors (CSF).
The increased risk of developing infection against the background of neutropenia led to the identification of the symptom complex “febrile neutropenia” (FN). According to the NCCN (National Comprehensive Cancer Network) guidelines [1], FN is characterized by an increase in oral temperature above 38.5 °C in a single measurement or above 38.0 °C in two consecutive measurements within 2 hours for the absolute neutrophil count (ANC). ) in the blood less than 0.5 × 109/L or an expected decrease <0.5 × 109/L.
Before the patient's first cycle of chemotherapy, it is necessary to assess the risk of developing FN. In this case, the following should be taken into account: • chemotherapy regimen (high-dose, dose-intensive, standard); • tumor type; • additional risk factors; • the purpose of chemotherapy (directed towards recovery, palliative).
The risk of developing FN is directly related to the intensity of the chemotherapy regimen. According to NCCN guidelines [1], chemotherapeutic regimens that cause FN in more than 20% of cases should be considered “high risk” regimens for developing FN. In such situations, granulocyte-CSF (G-CSF) is recommended as primary prevention. Similar recommendations are made by the American Society of Clinical Oncology (ASCO - American Society Clinical Oncology) [2] and the European Organization for Research and Treatment of Cancer (EORTC - European Organization Research and Treatment of Cancer) [3].
At “intermediate risk,” the likelihood of developing FN or neutropenic infection is 10–20%. Regardless of the purpose of treatment, the NCCN recommends that in this situation the need for CSF be considered on an individual case basis. In this case, the risk-benefit ratio of the use of CSF, the likelihood of developing FN, the potential consequences of neutropenic infection, as well as long-term results of treatment of cancer patients receiving chemotherapy in reduced doses should be taken into account. When the treatment is not aimed at recovery and it is symptomatic, and the risk is determined
CT regimen, it is recommended to consider alternative methods of preventing FN (use of less myelosuppressive chemotherapy regimens, reduction of chemotherapy doses). In this situation, the use of CSF is inappropriate.
In patients at “low risk” of FN (<10%), routine use of CSF is not recommended. CSF can be used only in cases where the patient receives adjuvant chemotherapy or there is a high risk of developing serious complications of FN, and reducing the doses of chemotherapy leads to a decrease in the effectiveness of antitumor treatment.
Below are chemotherapy regimens with a high (Table 1) and intermediate (Table 2) risk of developing FN.
. CT regimens with a high risk of developing FN.
. CT regimens with an intermediate risk of developing FN.
In addition to the main risk factors for the development of FN described above, there are additional ones that must be taken into account when determining the overall risk of FN. Additional factors may increase the overall risk to a higher category.
Additional factors include the patient's age over 65 years (higher risk of developing FN, higher rates of complications and mortality); previous chemotherapy; a history of deep neutropenia (ANC < 100/μl), neutropenia lasting >10 days or functional function after chemotherapy; involvement of the bone marrow in the tumor process; history of radiation therapy; recent surgery; the presence of a wound process; poor somatic status of the patient; impaired liver and kidney function; pneumonia; associated fungal infection.
The risk of developing infectious complications in patients with neutropenia is due not only to a decrease in ANC, but also to a violation of the functional properties of neutrophils (weakening of chemotaxis and phagocytosis; disruption of the exogenous production of growth factors, such as interleukin-1, interleukin-3, granulocyte-macrophage CSF, G-CSF; change in enzyme activity; decrease in the formation of reactive oxygen species).
An assessment of the risk of developing FN should be carried out before each course of chemotherapy. If the patient suffered an episode of FN or a dose-limiting neutropenic infection during the previous cycle of chemotherapy and the ongoing chemotherapy is aimed at recovery, subsequent courses of chemotherapy in the same doses entail a “high risk” of developing FN. In such cases, the use of CSF should be considered. If a patient experiences an episode of FN despite the use of CSF, the NCCN recommends reducing chemotherapy doses during subsequent cycles or changing the chemotherapy regimen.
These recommendations are based on the results of large randomized trials showing that the risk of developing FN can be significantly reduced through primary prevention using CSF.
Vogel et al. [4] in a double-blind, randomized, placebo-controlled, multicenter study found that during the first and all subsequent cycles of chemotherapy, prophylactic administration of CSF (pegylated filgrastim) significantly reduces the risk of developing FN in chemotherapy regimens that were previously associated with a “high risk” of this complication. The study included breast cancer patients who received docetaxel 100 mg/m2 every 3 weeks. The patients were divided into 2 groups: group 1 (465 women) received placebo injections; Group 2 (463 women) – pegfilgrastim subcutaneously (administered 24 hours after completion of the chemotherapy cycle). In the placebo group, the incidence of FN was 17%, in the pegfilgrastim group – 1%. The need for hospitalization decreased from 14 to 1%, and the use of intravenous antibiotic therapy (ABT) from 10 to 2% (p < 0.001).
In a study by Timmer-Bonte et al. [5] 175 patients with SCLC were treated with the combination of cyclophosphamide + doxorubicin + etoposide. Subsequently, all patients were randomized into 2 groups: in group 1, prophylactic ABT (ciprofloxacin + roxithromycin) was performed; Group 2 received ABT + G-CSF (on days 4–13 of the cycle). After the first cycle of chemotherapy, the development of FN was noted in 20 (24%) patients in the group receiving only antibiotics, compared to 9 (10%) patients receiving ABT + G-CSF (p = 0.01).
Thus, in patients with a “high risk” of developing FN, primary prevention with CSF in combination with ABT significantly reduces the risk of developing infectious complications [6, 7].
Some studies (Labro M.T., 2000; Nelson S., 2001) found that G-CSF enhances the effect of ABT by increasing the intracellular concentration of the antibiotic in neutrophils, which in turn has a modifying effect on the functional activity of neutrophils. Their antibody-dependent cytotoxicity towards tumor cells increases; phagocytosis of bacteria and fungi is accelerated; neutrophils migrate from the peripheral blood to sites of infection and inflammation.
Currently used G-CSFs include filgrastim, pegfilgrastim and sargramostim. Filgrastim is recommended to be used at a daily dose of 5 mcg/kg/day subcutaneously 24–72 hours after the last dose of anticancer drugs until a stable and sufficient ANC is achieved. Pegfilgrastim (used only for the prevention of FN) is administered subcutaneously once at a total dose of 6 mg 24–72 hours after the last dose of anticancer drugs. It is used in chemotherapy regimens, the course of which is 3 weeks. Sargramostim is administered at a dose of 250 mcg/m²/day subcutaneously 24–72 hours after the last dose of anticancer drugs until a stable and sufficient ANC is achieved. It should be noted that the condition of administering G-CSF 1–3 days after the last dose of cytostatics is associated with the sensitivity of actively proliferating myeloid cells to myelosuppressive chemotherapy.
The first G-CSF preparation available for clinical use was filgrastim, obtained using recombinant technology in Escherichia coli cultures. Currently, there are many filgrastim drugs, one of which is Granogen.
This G-CSF stimulates the proliferation of colony-forming cells - precursors of the neutrophil sprout of the bone marrow, accelerates their differentiation and the release of mature neutrophils from the bone marrow into the peripheral blood, enhances the effector functions of neutrophils (chemotaxis, phagocytosis, oxidative metabolism). Granogen causes a rapid, specific and dose-dependent increase in the number of neutrophils in the peripheral blood, which is explained by a shortening of the maturation time from 5 to 1 day, an increase in the number of cell divisions and an accelerated release of cells into the peripheral blood. Granogen reduces the frequency, severity and duration of neutropenia and FN in patients receiving myelosuppressive therapy, which reduces the incidence of infectious complications and reduces the need for ABT.
Side effects when using G-CSF, including Granogen, are rare; they are insignificant.
For the treatment of neutropenia after a course of cytotoxic therapy, Granogen is administered at a dose of 0.5 million units (5 mcg) per 1 kg of body weight, once a day, daily, subcutaneously or in the form of short (over 30 minutes) intravenous infusions. Granogen is not compatible with 0.9% sodium chloride solution. The preferred route of administration is subcutaneous. An increase in the number of neutrophils is usually observed 1-2 days after the start of treatment. To achieve a stable therapeutic effect, it is necessary to continue therapy until the number of neutrophils passes through the expected minimum (nadir) and returns to the normal range. It is not recommended to discontinue the drug prematurely. Treatment is stopped if the post-nadir ANC reaches 1 × 109/L. After chemotherapy for solid tumors, the duration of treatment can be up to 14 days. If the number of leukocytes increases > 50 × 109/L, Granogen should be discontinued immediately.
The success of FN treatment largely depends on early recognition of a possible infection and early initiation of therapy. Neutropenia significantly weakens the body's immune response, which does not allow the development of characteristic clinical manifestations of infection, complicating its clinical diagnosis in cancer patients. Hyperthermia in patients with neutropenia is often the only sign of an infectious process.
According to ESMO clinical guidelines, it is necessary to conduct a thorough initial examination and examination to identify possible foci of infection [8]: 1. The presence of a long-standing central venous catheter. 2. Symptoms and signs of infection of the respiratory system, cardiovascular system, gastrointestinal tract, skin, genitourinary system, central nervous system. 3. Data on previous positive results of microbiological analysis. 4. Routine laboratory and instrumental studies: • general and biochemical blood tests, coagulogram, C-reactive protein; • blood culture (at least 2 times), including culture from the central venous catheter; microscopic examination and culture of urine; • microscopic examination of sputum and its culture; • microscopic examination of stool and its culture (for diarrhea); • examination of foci of infection on the skin (aspirate, biopsy, smear); • chest x-ray. 5. Further studies (for prolonged and profound neutropenia): computed tomography of the chest (if fever persists, despite adequate antibiotic therapy, for more than 72 hours).
Immediate blood counts to establish the neutrophil count in parallel with the other studies presented above are key to determining the need to initiate early therapy for FN.
The International Association for Supportive Care in Cancer (MASCC) has developed a prognostic index [9, 10]. It allows you to quickly assess the risk of complications - before the level of neutrophils in the patient’s blood becomes known. The effectiveness of this method has been confirmed in many prospective studies.
The MASCC index criteria are presented in table. 3.
An indicator of “low risk” of complications is a score > 21. The frequency of serious complications does not exceed 6%, and mortality is 1%. With a score <15, mortality reaches 36%.
The standard of care for FN is immediate empiric therapy with broad-spectrum antibiotics. Such therapy should be directed against gram-positive and gram-negative microorganisms, provide a rapid bactericidal effect and have low toxicity. When choosing a route of antibiotic administration, the following principle should be followed: oral ABT can be safely used in patients with “low-risk” FN. This category of patients includes those who are hemodynamically stable and who do not have signs of organ failure, a long-standing central venous catheter, or severe infection. Intravenous antibiotic therapy should be given to patients with “high-risk” FN. After 48 hours of intravenous antibiotic therapy, oral antibiotics can be prescribed to afebrile patients [11].
Numerous studies have shown that monotherapy with fluoroquinolones is no less effective than the combined use of fluoroquinolones and amoxicillin/clavulanate. However, this statement does not apply to patients at “high risk” of FN.
Apart from standard therapy with broad-spectrum antibiotics, there are a large number of clinical situations where the use of specific treatment regimens is required. For example, in the case of pneumonia, it is necessary to prescribe antibacterial drugs that cover the spectrum of atypical microorganisms (Legionella, Mycoplasma), for example, macrolides and β-lactam antibiotics. High doses of co-trimoxazole are the treatment of choice for suspected Pneumocystis jerovecii infection [12].
In all cases of catheter-associated infection (CAI) with FN, it is necessary to decide on the choice and route of antibiotic administration, and the need to remove the catheter. If a patient is suspected of having CAI and is stable, the catheter should not be removed without microbiological evidence of infection. Administration of glycopeptides (vancomycin) through a catheter is recommended. In this case, the gram-positive flora overlaps [13]. In case of CAI caused by coagulase-negative staphylococcus, an attempt to preserve the catheter may be justified only if the patient's condition is stable. Maintaining the catheter in most cases does not affect the resolution of bacteremia caused by coagulase-negative staphylococcus, but is a significant risk factor for recurrent infection [14]. Removal of the catheter is indicated in the presence of inflammation at the site of the catheter or port, persistent fever and bacteremia that persist after adequate antibiotic therapy, in the presence of atypical mycobacterial infection and candidemia. For CAI caused by Staphylococcus aureus, catheter removal is recommended [15].
If diarrhea develops, it is necessary to conduct a test for Clostridium dеfficili, and if a clostridial infection is suspected, therapy with metronidazole should be started. For viral infections, acyclovir is indicated. Ganciclovir should be prescribed only when there is a high degree of suspicion for invasive cytomegalovirus infection. In case of development of candidiasis, the first-line drug is fluconazole. Antifungal therapy should be continued until neutropenia resolves, and for 14 days in patients with proven mycotic infection.
Treatment for suspected invasive aspergillosis should include caspofungin, liposomal amphotericin B, or variconazole. The latter two drugs can be combined with echinocandin in case of lack of response to therapy [16–18]. If meningitis is suspected, a lumbar puncture is indicated. For bacterial meningitis, ceftazidime should be given in combination with ampicillin (for cover against Listeria monocytogenes) or meropenem [19].
If there is clinical or microbiological evidence of intra-abdominal or pelvic sepsis, metronidazole therapy should be initiated. The duration of treatment for FN may vary. If the number of neutrophils has increased to 0.5 × 109/L or more, normal body temperature is observed within 48 hours, blood culture does not reveal the pathogen, ABT can be stopped. If the neutrophil count is <0.5 × 109/L and there are no complications or fever within 5–7 days, ABT can also be completed. The exception is situations where there remains a “high risk” of complications. In this case, ABT continues for up to 10 days or until the number of neutrophils is 0.5 × 109/L or more. In patients with persistent fever and a neutrophil count of 0.5 × 109/L or more, it is more appropriate to prescribe antifungal therapy.
Thus, despite significant advances in the prevention and treatment of FN, it remains one of the most dangerous complications of chemotherapy for malignant tumors and leads to a change in the timing of the use of cytostatics and a reduction in doses of chemotherapy, which negatively affects the effectiveness of antitumor treatment. The widespread introduction of G-CSF into clinical practice can significantly reduce the severity and duration of neutropenia, and reduce the risk of developing FN during myelosuppressive chemotherapy. All this allows you to maintain the planned dose intensity of the regimen, and sometimes intensify the regimen by reducing the intervals between courses.
Treatment
Intermittent neutropenia usually occurs without symptoms or severe infectious complications, and therefore does not require therapeutic treatment. Especially if the patient carefully observes the rules of personal hygiene, eats foods that have been heat-treated (raw fish dishes, meat with blood are not allowed in case of neutropenia), uses gloves when cleaning, and protects the skin from prolonged exposure to the sun.
With secondary neutropenia, treatment is aimed primarily at eliminating the underlying disease, which led to a pathological decrease in granulocytes.
At the Spizhenko Clinic, treatment of neutropenia is carried out taking into account the cause and clinical symptoms and may include:
- Antibacterial therapy.
- Antifungal therapy.
- Immunotherapy.
- Use of glucocorticoids.
Including treatment of associated conditions (ulcers, stomatitis, gingivitis) by rinsing the mouth with saline solution, using antiseptics (chlorhexidine), nystatin, clotrimazole, fluconazole (for candidiasis).
In case of acute lesions of the mucous membranes, an additional diet (with a predominance of liquid food in the diet) and local anesthesia are prescribed. If the decrease in neutrophils occurred as a result of taking medications, the way out of the situation is to stop using them and switch to alternative treatment.
Medicines
that lead to neutropenia:
- Anticonvulsants (Diazepam, Phenytoin, Sodium Valproate).
- Antidepressants (Clozapine, Haloperidol).
- Antibiotics (Doxycycline, Lincomycin, Cephalosporin, Penicillin, Vancomycin).
- Antiviral (Acyclovir, Zidovudine).
- Anthelmintics (Levomizole, Mebenzadol).
- Antituberculosis (Streptomycin, Rifampicin, Isoniazid, Ethambutol).
- Antifungals (Griseofulvin, Mycozolon, Amorolfine).
Including analgesics (Ibuprofen, Indomethacin, Amidopyrine, Acetylsalicylic acid, Phenylbutazone).
Treatment of febrile neutropenia
Febrile neutropenia requires immediate medical intervention (within 1 hour!).
For neutropenic fever the following is prescribed:
- Antimicrobial therapy;
- Emergency diagnostics (culture of blood, urine, secretions from the mouth, catheterization sites, vagina/urethra, ultrasound of internal organs, radiography).
The basis of modern antibacterial treatment for FN (febrile neutropenia) is beta-lactam antibiotics.
At the Spizhenko Clinic you can undergo a comprehensive study of neutropenia, determine the exact cause and receive the most correct treatment (if necessary).
Neutropenia is not a “sentence”, but also not a reason to leave this situation without competent medical attention.
Recommendations
- ^ a b c d f g gram h i j
Ohls , Robin (2012).
Hematology, immunology and infectious neonatology: issues and controversies
. Philadelphia, PA: Elsevier/Saunders. ISBN 978-1-4377-2662-6: Accessed by University of Pittsburgh. - ^ a b
Newburger PE, Dale DC (July 2013).
"Evaluation and management of patients with isolated neutropenia." Seminars on hematology
.
50
(3): 198–206. Doi:10.1053/j.seminhateol.2013.06.010. PMC 3748385. PMID 23953336. - ^ a b c
“Clinical picture of neutropenia: medical history, physical examination.”
emedicine.medscape.com
. Retrieved December 8, 2015. - "Neutropenia". National Center for Biotechnology, National Library of Medicine. Retrieved December 8, 2015.
- "Neutrophils". National Center for Biotechnology, National Library of Medicine. Retrieved December 8, 2015.
- ^ a b c
Fung M., Kim J., Marty F. M., Schwarzinger M., Koo S. (2015).
"Meta-analysis and cost comparison of empiric and preventive antifungal strategies in patients with hematologic malignancies with high-risk febrile neutropenia." PLOS One
.
10
(11): e0140930. Bibcode:2015PLoSO..1040930F. Doi:10.1371/journal.pone.0140930. PMC 4640557. PMID 26554923. - ^ a b
Horwitz MS, Corey SJ, Grimes HL, Tidwell T (February 2013).
"ELANE mutations in cyclic and severe congenital neutropenia: genetics and pathophysiology." Hematology/Oncology Clinics of North America
.
27
(1): 19–41, vii. doi:10.1016/j.hoc.2012.10.004. PMC 3559001. PMID 23351986. - Boxer L.A. (December 8, 2012). "How to approach neutropenia." Hematology.
American Society of Hematology. Educational program .
2012
(1): 174–82. doi:10.1182/asheducation.v2012.1.174.3798251. PMID 23233578. - Nakai, Yukie; Ishihara, Chikako; Ogata, Sagiri; Shimono, Tsutomu (July 2003). "Oral manifestations of cyclic neutropenia in a Japanese child: a case report with 5-year follow-up." Children's dentistry
.
25
(4): 383–388. ISSN 0164-1263. PMID 13678105. - Chazinski, Mary Fran (4 May 2012). Caring for a seriously ill child
. Elsevier Health Sciences. item 835. ISBN 978-0323086035. - Donadier J, Beaupin B, Fenneteau O, Bellannay-Chantelot S (November 2017). "Congenital neutropenia in the era of genomics: classification, diagnosis and natural history." British Journal of Hematology
.
179
(4):557–574. doi:10.1111/bjh.14887. PMID 28875503. - Muturi-Kioi V, Lewis D, Launay O, Leroux-Roels G, Anemona A, Loulergue P, et al (4 August 2021). "Neutropenia as an adverse event after vaccination: results of a randomized clinical trial in healthy adults and a systematic review." PLOS One
.
11
(8):e0157385. Bibcode:2016PLoSO..1157385M. Doi:10.1371/journal.pone.0157385. PMC 4974007. PMID 27490698. - ^ a b c d e
Williams, Mark (2007).
Integrated hospital medicine - an evidence-based approach
. Philadelphia: Saunders Elsevier. ISBN 978-1-4160-0223-9; Access provided by University of Pittsburgh - Shvartsberg L.S. (January 1, 2006). "Neutropenia: etiology and pathogenesis." Clinical Cornerstone
. 8 Appendix 5: S5-11. Doi:10.1016/s1098-3597 (06) 80053-0. PMID 17379162. - via ScienceDirect (Subscription may be required or content may be available through libraries.) - Dale, DC, Bolyard A.A. (January 2021). "Update on the diagnosis and treatment of chronic idiopathic neutropenia." Current Opinion in Hematology
.
24
(1): 46–53. doi:10.1097/MOH.0000000000000305. PMC 5380401. PMID 27841775. - Makaryan V., Rosenthal E.A., Bolyard A.A., Kelly M.L., Boal J.E., Bamshad M.J. and others (July 2014). "Congenital neutropenia associated with TCIRG1". Human mutation
.
35
(7):824–7. Doi:10.1002/humu.22563. PMC 4055522. PMID 24753205. - Levene, Malcolm I.; Lewis, S. M.; Bain, Barbara J.; Imelda Bates (2001). Practical Hematology Dacie & Lewis
. London: W. B. Saunders. p. 586. ISBN 978-0-443-06377-0. - “Neutropenic patients and neutropenic regimens | Patients". Patient
. Retrieved December 8, 2015. - ^ a b
Shea M. M., Everhart J. E., Byrd-Holt J. D., Teasdale J. F., Rogers G. P. (April 2007).
"Prevalence of Neutropenia in the US Population: Age, Sex, Smoking Status, and Ethnic Differences." Annals of Internal Medicine
.
146
(7):486–92. Doi:10.7326/0003-4819-146-7-200704030-00004. PMID 17404350. - "Absolute Neutrophil Count Calculator." reference.medscape.com
. Retrieved December 8, 2015. - Schouten HC (September 2006). "Managing Neutropenia" (PDF). Annals of Oncology
. 17 App. 10 (Add. 10): x85-9. doi:10.1093/annonc/mdl243. PMID 17018758. - James R.M., Kinsey S.E. (October 2006). "Research and treatment of chronic neutropenia in children." Archives of Childhood Diseases
.
91
(10):852–8. doi:10.1136/adc.2006.094706. PMC 2066017. PMID 16990357. - Agranulocytosis - Advances in Research and Treatment: 2012 Edition: ScholarlyBrief
. Scientific publications. December 26, 2012. p. 95. ISBN 9781481602754. - Jubelirer SJ (6 April 2011). "The benefits of the neutropenic diet: fact or fiction?" Oncologist
.
16
(5): 704–7. Doi:10.1634/theoncologist.2011-0001. PMC 3228185. PMID 21471277. - Johansen HK, Gøtzsche PC (September 2014). "Fat-soluble formulations of amphotericin B versus amphotericin B in neutropenic cancer patients." Cochrane Database of Systematic Reviews
(9): CD000969. Doi:10.1002/14651858.cd000969.pub2. PMC 6457843. PMID 25188673. - Johansen HK, Gøtzsche PC (September 2014). "Amphotericin B versus fluconazole for the control of fungal infections in patients with neutropenic cancer." Cochrane Database of Systematic Reviews
(9): CD000239. Doi:10.1002/14651858.cd000239.pub2. PMC 6457742. PMID 25188769. - "Neutropenia." eMedicine
. MedScape. Retrieved December 8, 2015. - Andersen K.L., Tesfa D., Sirsma V.D., Sandholdt H., Hasselbalch H., Bjerrum O.V. and others (June 2016). "Prevalence and clinical significance of neutropenia detected on routine complete blood count: a longitudinal study." Journal of Internal Medicine
.
279
(6):566–75. Doi:10.1111/joim.12467. PMID 26791682.