pharmachologic effect
Antimicrobial medication. The active substance belongs to the group of fluoroquinolones . The principle of antimicrobial action is based on the ability of the active component to block DNA gyrase, to prevent the replication of the A-subunit of RNA, DNA, and to disrupt the synthesis of bacterial proteins.
Has a wide spectrum of anti- and anti-microbial effects. For gram-positive microorganisms, the antimicrobial effect appears only on cells that are in the process of mitosis.
In relation to gram-negative bacteria - into cells that are in the stage of division and in the stage of rest. The active substance is resistant to beta-lactamases.
Pefloxacin-akos 400 mg 10 pcs. film-coated tablets
Composition and release form Pefloxacin-akos 400 mg 10 pcs. film-coated tablets
Tablets - 1 tablet:
- Active substance: pefloxacin mesylate dihydrate (in terms of pefloxacin) - 400 mg;
- Excipients: corn starch, potato starch, lactose (milk sugar), low molecular weight povidone, microcrystalline cellulose, talc, calcium stearate;
- shell: hypromellose (hydroxypropyl methylcellulose), titanium dioxide (titanium dioxide), propylene lacol, macrogol (polyethylene oxide 4000, polyethylene olicol 4000), talc.
There are 10 pieces in a package.
Description of the dosage form
Film-coated tablets, round, biconvex, white or white with a yellowish tint.
Directions for use and doses
The drug is prescribed 1 tablet (400 mg) 2 times a day (every 12 hours), the average daily dose is 800 mg in 2 divided doses. The maximum daily dose is 1200 mg. The tablets are swallowed without chewing and washed down with plenty of water. When treating certain infections of the genitourinary system, the drug is prescribed 1 (400 mg) tablet per day.
For the treatment of gonorrhea in men and women, the drug is prescribed as a single dose of 800 mg.
In patients with impaired liver function, adjustment of the dosage regimen is required: for minor disorders, the drug is prescribed at a dose of 400 mg/day; for more severe disorders - every 36 hours; in case of severe liver pathology, the interval between administrations is extended to 2 days.
For patients with impaired renal function (with creatinine clearance below 20 ml/min), a single dose should be 50% of the average dose - with a frequency of administration 2 times a day, or the full single dose is administered 1 time a day.
Pharmacodynamics
Antimicrobial agent from the group of fluoroquinolones. It has a bactericidal effect and blocks DNA gyrase. In relation to gram-negative strains, it is effective on both dividing cells and cells in the resting stage; in the case of gram-positive strains - only on cells in the process of mitotic division. Has a wide spectrum of action.
Active against most aerobic gram-negative bacteria:
Escherichia coli, Klebsiella spp., indole-positive and indole-negative Proteus spp., incl. Proteus mirabilis, Enterobacter spp., Morganella morganii, Yersinia enterocolitica, Vibrio cholerae, Vibrio parahaemolyticus, Neisseria gonorrhoeae, Neisseria meningitidis, Haemophilus influenzae, Pseudomonas aeruginosa, Moraxella catarrhalis, Pasteurella multocida, Campylobacter spp., Serratia spp., Citro bacter spp., Salmonella spp., Shigella spp..
Aerobic gram-positive bacteria: Staphylococcus spp., (including those producing and not producing penicillinase and methicillin-resistant), Streptococcus spp., incl. Streptococcus pyogenes, Streptococcus agalactiac, Corynebacterium diphtheriae, Listeria monocytogenes; intracellular bacteria: Legionella spp. (including Legionella pneumophila), Brucella spp., Chlamydia spp., as well as against bacteria that produce beta-lactamases. Suppresses the life activity of Mycoplasma spp. and Helicobacter spp.
Moderately sensitive microorganisms:
Streptococcus pneumoniae, Acinetobacter spp., Clostridium perfringens, Pseudomonas spp., Chlamydia trachomatis. Resistant microorganisms: gram-negative anaerobes, Treponema spp., Mycobacterium tuberculosis. For diseases caused by moderately sensitive microorganisms, in vitro susceptibility testing is required. Inhibits microsomal oxidation in liver cells.
Pharmacokinetics
Absorption is high, 20 minutes after oral administration of a single dose (400 mg) 90% is absorbed. The time to reach the maximum concentration (4 mcg/ml) is 90-120 minutes, the therapeutic concentration is maintained for 12-15 hours. After repeated administration, the maximum concentration (Cmax) in the blood is 10 mcg/ml; concentration in the bronchial mucosa - 5 mcg/ml; the ratio between the concentration in the bronchial mucosa and blood is 100%. Bonding with plasma proteins is 25-30%.
Penetrates well into body tissues and fluids, incl. into bronchial secretions, lungs, prostate gland, cerebrospinal fluid and bone tissue. Volume of distribution - 1.5-1.8 l/kg. The concentration in the cerebrospinal fluid after 3 doses of 400 mg is 4.5 mcg/ml, when increasing the dose to 800 mg it is 9.8 mcg/ml; the concentration in cerebrospinal fluid is 89% of that in plasma. Concentration in other organs and tissues 12 hours after the last dose: thyroid gland - 11.4 mcg/g, salivary glands - 2.2 mcg/g, skin -7.6 mcg/g, nasopharyngeal mucosa - 6 mcg/g, tonsils - 9 mcg/g g, muscles -5.6 µg/g.
Metabolized in the liver by methylation to dimethylpefloxacin (has significant antibacterial activity), oxidized to N-oxide and conjugated with glucuronic acid to form pefloxacin glucuronide.
The half-life is 8-10 hours, with repeated administration - 12-13 hours. Excreted by the kidneys - 60%, with bile - 30% unchanged; partly in the form of metabolites. The concentration of unchanged drug in urine 1-2 hours after administration is 25 mcg/ml. after 12-24 hours - 15 mcg/ml. Unchanged pefloxacin and its metabolites are detected in the urine for 84 hours after the final administration. Poorly amenable to dialysis (extraction coefficient 23%).
Indications for use Pefloxacin-akos 400 mg 10 pcs. film-coated tablets
Treatment of infectious and inflammatory diseases caused by microorganisms sensitive to the drug:
- Kidney and urinary tract infections;
- infections of the pelvic organs (including adnexitis and prostatitis);
- infections of the gastrointestinal tract (including salmonellosis, typhoid fever);
- infections of the gallbladder and biliary tract (cholecystitis, cholangitis, empyema of the gallbladder);
- abdominal infections (intra-abdominal abscesses);
- infections of bones, joints;
- skin and soft tissues (including those caused by staphylococcus resistant to penicillin);
- lower respiratory tract infections;
- infections of JlOP organs (including chronic sinusitis, severe external otitis);
- gonorrhea;
- chlamydia, chancroid.
Contraindications
Hypersensitivity, glucose-6-phosphate dehydrogenase deficiency (hemolytic anemia).
Carefully.
Atherosclerosis of cerebral vessels, cerebrovascular accident, organic lesions of the central nervous system, epileptic syndrome of unknown etiology, renal and/or liver failure.
special instructions
For mixed infections, for infections of the pelvic organs, it is combined with drugs active against anaerobes (metronidazole, clindamycin).
During treatment, patients should receive large amounts of fluid while maintaining adequate diuresis (to prevent crystalluria). Due to the possible appearance of photosensitivity, you should not be exposed to UV radiation during the treatment period.
For patients with severe liver and kidney diseases, dose adjustment is required in proportion to the degree of damage.
If severe and prolonged diarrhea occurs during or after treatment with pefloxacin, it is necessary to exclude the development of pseudomembranous colitis (immediate discontinuation of the drug and administration of appropriate treatment is required).
During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Overdose
Symptoms: nausea, vomiting, confusion, mental agitation; in severe cases - loss of consciousness, convulsions.
Treatment: gastric lavage, activated carbon. It is necessary to ensure medical monitoring of the patient’s condition and sufficient fluid intake into the patient’s body; If necessary, carry out symptomatic therapy. Hemodialysis is not an effective method for removing quinolone derivatives from the body.
Side effects Pefloxacin-akos 400 mg 10 pcs. film-coated tablets
From the nervous system: depression, headache, dizziness, increased fatigue, insomnia, increased convulsive readiness, anxiety, agitation, tremor, rarely - convulsions.
From the digestive system: nausea, vomiting, diarrhea, abdominal pain, loss of appetite, flatulence, pseudomembranous colitis, transient increase in liver transaminases, cholestatic jaundice, hepatitis, liver necrosis.
From the urinary system: crystalluria, rarely glomerulonephritis, dysuria.
Allergic reactions: skin rash, itching, urticaria, skin hyperemia, photosensitivity, rarely - angioedema, bronchospasm, arthralgia. From the hematopoietic system, leukopenia, neutropenia, thrombocytopenia, agranulocytosis, eosinophilia.
Other: tachycardia, myalgia, tendinitis, candidiasis.
Drug interactions
Pefloxacin reduces the metabolism of theophylline in the liver, which leads to an increase in the concentration of theophylline in the plasma and central nervous system (to avoid the development of intoxication, the dose of theophylline must be reduced). Significantly reduces the prothrombin index (in patients taking indirect anticoagulants, constant monitoring of the blood picture is necessary). Cimetidine and other microsomal oxidation inhibitors increase the half-life, reduce total clearance, but do not affect the volume of distribution and renal clearance.
Coating drugs slow down absorption.
The simultaneous use of beta-lactam antibiotics helps prevent the development of resistance during the treatment of staphylococcal infections. Aminoglycosides, piperacillin, azlocillin, ceftazidime enhance the antibacterial effect (including for infections caused by Pseudomonas aeruginosa).
Drugs that block tubular secretion slow down the elimination of pefloxacin.
Pharmacodynamics and pharmacokinetics
The antimicrobial agent is quickly absorbed from the lumen of the digestive tract after taking the tablet orally. 20 minutes after a single use of 400 mg of Pefloxacin, about 90% of the dose taken is absorbed, and the Cmax is 4 mcg/ml and is achieved in 1.5-2 hours.
After intravenous infusion, Cmax is achieved faster - after 1 hour. After repeated use, the concentration of the active antimicrobial substance in the bronchial mucosa is 5 μg/ml.
Pefloxacin is capable of binding to plasma proteins by 25-30%. The active ingredient penetrates well into all tissues in the body and fluids:
- sputum;
- heart;
- bronchial secretion;
- cerebrospinal fluid;
- bile;
- bone;
- lungs;
- peritoneal fluid;
- prostate.
In the above tissues and fluids, the concentration of the antimicrobial substance is higher than in the blood.
After 3 doses of 400 mg, the concentration of Pefloxacin in the cerebrospinal fluid is 4.5 mcg/ml; when the dose is doubled, this figure reaches 9.8 μg/ml, and the ratio between the concentrations of the antibiotic in plasma and liquid is 89%.
Metabolism occurs in the hepatic system, where the active substance, as a result of methylation, is converted to dimethylpefloxacin, whose antibacterial activity is much more pronounced than that of its predecessor.
The metabolite is able to oxidize to N-oxide, and then conjugate with glucuronic acid to form pefloxacin glucuronide.
When taken orally, the T1\2 indicator is 8-10 hours, and when used repeatedly - 12-13 hours. After a single intravenous infusion, T1\2 is 7.2-13 hours, and with repeated administration - 14-15 hours. It is excreted partially in the form of metabolites, as well as unchanged in feces and urine (60%) through the renal system.
After a single use of the drug, its metabolites can be detected in the urine for 84 hours. During hemodialysis, the extraction coefficient of the active substance is 23%.
Pharmacological properties of the drug Pefloxacin
Pefloxacin is a synthetic broad-spectrum antimicrobial agent of the fluoroquinolone group. It acts bactericidal, inhibiting the replication of bacterial DNA, affecting RNA and protein synthesis in microbial cells. Shows activity against microorganisms resistant to other antimicrobial agents. Sensitive to pefloxacin: Escherichia coli, Klebsiella spp., Enterobacter spp., Serratia spp., Proteus mirabilis , indole-positive Proteus, Citrobacter spp., Salmonella spp., Shigella spp., Haemophilus spp., Staphylococcus spp., Neisseria gonorrhoeae , moderately sensitive : Streptococcus spp., Pseudomonas spp., Acinetobacter spp., Clostridium perfringens, Mycoplasma spp., Chlamydia spp . Gram-negative anaerobes, Spirochaeta spp., Mycobacterium tuberculosis . Unchanged pefloxacin and its metabolites can be detected in urine 84 hours after the last dose. Pefloxacin is quickly and almost completely absorbed from the digestive tract (90–100%). The maximum concentration in blood plasma after oral administration is achieved within 1–1.5 hours; when taken at a dose of 400 mg, it is 4.4 mcg/ml. The half-life is approximately 8 hours. It penetrates well into tissues and organs, including the myocardium, central nervous system, bronchial mucosa, bone tissue, bone marrow, etc. The half-life averages 12 hours (8–15 hours), about 20 –30% of the active substance binds to blood plasma proteins. Pefloxacin is largely metabolized in the liver. It is excreted in urine and bile, mainly unchanged, partly in the form of active metabolites - dimethyl-pefloxacin (norfloxacin), pefloxacin-N-oxide and pefloxacin glucuronide. 60–70% of pefloxacin is excreted in the urine in the form of metabolites and 7–9% unchanged; About 25% is excreted in feces (unchanged and in the form of metabolites).
Indications for use
Pefloxacin is prescribed for infectious lesions caused by a microorganism sensitive to the antibiotic:
- soft tissue diseases;
- typhoid fever;
- salmonellosis;
- empyema of the gallbladder;
- prostatitis;
- adnexitis;
- cholangitis;
- nosocomial infections;
- chlamydia;
- gonorrhea;
- cholecystitis;
- intra-abdominal abscesses;
- skin diseases;
- eye infections;
- endocarditis;
- sepsis;
- peritonitis;
- diseases of joints and bones;
- surgical infections;
- epididymitis;
- lesions of the paranasal sinuses;
- osteomyelitis;
- middle ear lesions;
- chancroid ; _
- diseases of the ENT organs;
- respiratory tract infections;
- lesions of the larynx, pharynx.
The drug can be used to prevent infectious diseases after surgical interventions.
Contraindications
- hemolytic anemia;
- period of gestation ;
- individual intolerance to fluoroquinolones;
- glucose-6-phosphate dehydrogenase deficiency;
- breast-feeding;
- epilepsy;
- Manufacturer's age limit is up to 18 years.
Relative contraindications:
- convulsive syndrome , the etiology of which is unknown;
- pathology of blood circulation in the brain;
- organic changes/lesions of the central nervous system;
- atherosclerosis of the vessels of the head.
Pefloxacin-Akos
Release form, composition and packaging
Tablets, coated in white or white with a yellowish tint, round, biconvex. 1 tab. pefloxacin mesylate 200 mg. Excipients: corn or potato starch, polyvinylpyrrolidone, lactose, calcium stearate, microcrystalline cellulose, talc. Shell composition: hydroxypropylcellulose, polyethylene oxide 4000, titanium dioxide, 1,2-propylene glycol, talc or Opadry.
Tablets, coated in white or white with a yellowish tint, round, biconvex. 1 tab. pefloxacin mesylate 400 mg. Excipients: corn or potato starch, polyvinylpyrrolidone, lactose, calcium stearate, microcrystalline cellulose, talc. Shell composition: hydroxypropylcellulose, polyethylene oxide 4000, titanium dioxide, 1,2-propylene glycol, talc or Opadry.
The solution for infusion is clear, yellowish or greenish-yellowish in color. 1 ml 1 amp. Pefloxacin (as mesylate) 80 mg 400 mg. Excipients: ascorbic acid, benzyl alcohol, sodium pyrosulfite, disodium salt of ethylenediaminetetraacetic acid, sodium carbonate, water for injection.
Clinical and pharmacological group: Antibacterial drug of the fluoroquinolone group.
pharmachologic effect
A broad-spectrum antimicrobial drug from the group of fluoroquinolones. It has a bactericidal effect by blocking DNA gyrase, disrupting the replication of the A-subunit of DNA and RNA and the synthesis of bacterial proteins. In relation to gram-negative bacteria, it acts on cells that are in the stage of rest and division, in relation to gram-positive bacteria - only on cells that are in the process of mitotic division. Resistant to β-lactamases.
Has a wide spectrum of action. The drug is active against aerobic gram-negative bacteria: Escherichia coli, Klebsiella spp., indole-positive and indole-negative Proteus spp. (including Proteus mirabilis), Enterobacter spp., Helicobacter pylori, Morganella morganii, Yersinia enterocolitica, Vibrio cholerae, Vibrio parahaemolyticus, Neisseria gonorrhoeae, Neisseria meningitidis, Haemophilus influenzae, Pseudomonas aeruginosa, Moraxella catarrhalis, Pasteurella multocida, Campylobacter spp. , Serratia spp., Citrobacter spp., Salmonella spp., Shigella spp., Haemophilus spp., Neisseria gonorrhoeae; aerobic gram-positive bacteria: Staphylococcus spp., (including those producing and not producing penicillinase and methicillin-resistant), Streptococcus spp. (including Streptococcus pyogenes, Streptococcus agalactiae), Corynebacterium diphtheriae, Listeria monocytogenes; intracellular bacteria: Legionella spp. (including Legionella pneumophila), Brucella spp., Chlamydia spp., Mycoplasma spp. The following are moderately sensitive to the drug: Pneumococcus spp. (Streptococcus pneumoniae), Acinetobacter spp., Clostridium perfringens, Pseudomonas spp., Chlamydia trachomatis. Gram-negative anaerobic bacteria - Bacteroides spp. - are resistant to the drug. (except B.fragilis), Treponema spp., Mycobacterium tuberculosis.
Pharmacokinetics
Suction
After oral administration, pefloxacin is rapidly absorbed from the gastrointestinal tract. After a single oral dose of 400 mg of pefloxacin, 90% of the dose is absorbed after 20 minutes, while Cmax is reached after 90-120 minutes and is 4 mcg/ml. After repeated administration, Cmax in blood plasma is 10 mcg/ml, concentration in the bronchial mucosa is 5 mcg/ml; the ratio between the concentration in the mucous membrane and in the blood is 100%. After intravenous administration, Cmax is achieved within 60 minutes.
Distribution
Plasma protein binding is 25-30%. Vd - 1.5-1.8 l/kg. Pefloxacin penetrates well into tissues and body fluids (bronchial secretions, lungs, prostate gland, cerebrospinal fluid, peritoneal fluid, bone tissue, heart, sputum, urine, bile). The concentration in the listed fluids and tissues is higher than the concentration in blood plasma. Concentration in the cerebrospinal fluid after three doses of 400 mg is 4.5 mcg/ml, when increasing the dose to 800 mg it is 9.8 mcg/ml; the ratio between plasma and liquid concentrations is 89%. Concentration in other organs and tissues 12 hours after the last dose: thyroid gland - 11.4 mcg/g, salivary glands - 2.2 mcg/g, skin - 7.6 mcg/g, tonsils - 9 mcg/g, muscles - 5.6 mcg/g.
Metabolism
Metabolized in the liver by methylation to dimethylpefloxacin (has significant antibacterial activity), oxidized to N-oxide and conjugated with glucuronic acid to form pefloxacin glucuronide.
Removal
T1/2 after oral administration is approximately 8-10 hours, with repeated administration - 12-13 hours; T1/2 after intravenous administration of a single dose is 7.2-13 hours, with repeated administration - 14-15 hours. It is excreted in the urine (60%) and in feces unchanged, partially in the form of metabolites. The content of unchanged pefloxacin in the urine 1-2 hours after administration is 25 mcg/ml, after 12-24 hours – 15 mcg/ml. Unchanged pefloxacin and its metabolites are detected in the urine within 84 hours after the last dose of the drug. The extraction coefficient of pefloxacin during hemodialysis is 23%.
Indications
Treatment of infectious and inflammatory diseases caused by microorganisms sensitive to the drug:
- kidney and urinary tract infections;
- gastrointestinal infections (including salmonellosis, typhoid fever);
- infections of the gallbladder and biliary tract (including cholecystitis, cholangitis, empyema of the gallbladder);
- abdominal infections (including intra-abdominal abscesses, peritonitis);
- infections of the pelvic organs (including adnexitis, prostatitis);
- infections of bones and joints (including osteomyelitis);
- skin and soft tissue infections;
- lower respiratory tract infections;
- infections of the ENT organs (including the middle ear, paranasal sinuses, pharynx and larynx);
- eye infections;
- sepsis, septicemia;
- bacterial endocarditis;
- meningoencephalitis;
- skin and soft tissue infections;
- gonorrhea;
- chlamydia;
- prostatitis;
- epididymitis;
- chancroid;
- surgical and nosocomial infections;
- prevention of infectious complications after surgical interventions.
Dosage regimen
They are set individually, depending on the location and severity of the infection, as well as the sensitivity of microorganisms. For uncomplicated infections, adults are prescribed 400 mg orally 2 times a day, the average daily dose is 800 mg.
The tablets should be taken orally, on an empty stomach, without chewing, with plenty of water. For severe infections, the drug is prescribed intravenously (in 250 ml of 5% glucose solution for 1 hour): the first dose is 800 mg, then 400 mg every 12 hours. The average duration of treatment is no more than 1-2 weeks.
For patients with impaired liver function, dosage regimen adjustment is required. For minor disorders, the drug is prescribed 400 mg 2 times a day, for more severe disorders - every 36 hours, for severe liver diseases, the interval between doses is extended to 2 days. The course of treatment is no more than 30 days.
For patients with impaired renal function (with CC less than 20 ml/min), a single dose is 50% of the average dose with a frequency of administration 2 times a day, or the full single dose is administered 1 time a day.
For elderly patients, the dose of the drug is reduced by 1/3.
Side effect
- From the side of the central nervous system: depression, headache, dizziness, insomnia, increased fatigue, increased convulsive activity, anxiety, agitation, tremor; rarely - convulsions.
- From the digestive system: nausea, vomiting, diarrhea, abdominal pain, anorexia, flatulence, pseudomembranous colitis, transient increase in the activity of liver transaminases, cholestatic jaundice, hepatitis, liver necrosis.
- From the urinary system: crystalluria; rarely - glomerulonephritis, dysuria.
- From the hematopoietic system: leukopenia, neutropenia, thrombocytopenia (at doses of 1.6 g/day), agranulocytosis, eosinophilia. Allergic reactions: skin rash, itching, urticaria, skin hyperemia, photosensitivity; rarely - Quincke's edema, bronchospasm, arthralgia. Other: tachycardia, myalgia, tendonitis, tendon rupture, candidiasis. Local reactions: phlebitis.
Contraindications
- epilepsy;
- hemolytic anemia;
- glucose-6-phosphate dehydrogenase deficiency;
- pregnancy;
- lactation (breastfeeding);
- children and adolescents up to 18 years of age;
- hypersensitivity to fluoroquinolones.
The drug is prescribed with caution for atherosclerosis of cerebral vessels, cerebrovascular accidents, organic lesions of the central nervous system, and convulsive syndrome of unknown etiology.
Pregnancy and lactation
The drug is contraindicated for use during pregnancy and lactation (breastfeeding).
Use for liver dysfunction
For patients with impaired liver function, dosage regimen adjustment is required. For minor disorders, the drug is prescribed 400 mg 2 times a day, for more severe disorders - every 36 hours, for severe liver diseases, the interval between doses is extended to 2 days. The course of treatment is no more than 30 days.
Side effects
Nervous system:
- convulsions (rarely recorded);
- migraine type headaches;
- fast fatiguability;
- anxiety;
- limb tremors;
- emotional arousal;
- dizziness;
- depression.
Digestive tract:
- cholestatic jaundice;
- abdominal pain;
- diarrhea syndrome;
- increased levels of ALT, AST;
- pseudomembranous colitis;
- flatulence;
- vomit;
- hepatic necrosis;
- nausea;
- hepatitis.
Urinary tract:
- glomerulonephritis (rarely reported);
- crystalluria;
- dysuria.
Other reactions:
- phlebitis (injection forms);
- Quincke's edema;
- eosinophilia;
- neutropenia;
- skin itching;
- candidiasis;
- arthralgia;
- leukopenia;
- myalgia;
- tachycardia;
- tendon rupture;
- tendinitis;
- photosensitivity;
- hyperemia of the skin;
- skin rashes;
- bronchospasm.
Instructions for use of Pefloxacin (Method and dosage)
The treatment regimen and the selection of a single dose of Pefloxacin are carried out on an individual basis, taking into account concomitant pathology, the severity of the infectious disease, the localization of the inflammatory process, and the sensitivity of the flora.
Instructions for the use of Pefloxacin for uncomplicated infectious diseases : 400 mg twice a day (the average daily dosage is 800 mg of pefloxacin). The tablets must not be chewed. It is recommended to drink plenty of liquid. It is preferable to take on an empty stomach.
For severe infectious diseases, the medication is administered intravenously by drip, having previously dissolved the medication in a 5% glucose solution (250 ml). Initially, a single dose of 800 mg is administered, then 400 mg every 12 hours. The duration of antimicrobial therapy should not exceed 1-2 weeks.
In case of pathology of the hepatic system, the treatment regimen is adjusted. For uncomplicated diseases, 400 mg is prescribed twice a day; for severe infectious lesions - 400 mg every 36 hours. The interval between antibiotic uses for severe lesions of the hepatic system is up to 2 days. The duration of antimicrobial therapy is no more than 30 days.
In case of pathology of the renal system, only 50% of the intended dose can be administered once. Elderly patients are prescribed 2/3 of the expected dose.
Use of the drug Pefloxacin
The average dose for adults and children over 15 years of age is 800 mg/day. Orally prescribed with meals, 400 mg 2 times a day (morning and evening). For urinary tract infections, 400 mg/day is prescribed. To prevent recurrence of urinary tract infections, 400 mg can be prescribed once a week. Parenterally administered as a slow (over 1 hour) intravenous infusion 2 times a day (morning and evening), 400 mg diluted in 250 mg of 5% glucose solution. If it is necessary to quickly achieve an effective concentration of pefloxacin, treatment begins with an initial dose of 800 mg. Patients with impaired liver function are prescribed at the rate of 8 mg per 1 kg of body weight as an infusion over 1 hour, for jaundice - once a day, for ascites - every 36 hours, for a combination of jaundice and ascites - every 48 hours.
Interaction
In the treatment of staphylococcal infectious diseases, the combination of Pefloxacin with beta-lactam antibacterial agents helps prevent the development of resistance.
Antibacterial synergism is observed with simultaneous therapy with Azlocillin, Piperacillin, Aminoglycosides, Ceftazidime .
This effect is also recorded in relation to diseases caused by Pseudomonas aeruginosa .
A decrease in the prothrombin index is observed with simultaneous treatment with indirect anticoagulant medications, which requires constant monitoring of blood clotting .
Pharmacokinetic interactions
Antacids containing Mg and/or Al hydroxide interfere with the absorption of the antibacterial component of the drug. The elimination of the active substance is slowed down when treated with medications that can block tubular secretion.
The drug slows down the metabolism of Theophylline in the hepatic system, which may lead to an increase in its concentration in the central nervous system and plasma, which may require adjustment of the treatment regimen.
Cimetidine does not affect renal clearance and volume of distribution, but it can reduce the half-life and total clearance of the antibiotic.
Pharmaceutical interactions
The medication is incompatible with Heparin . Mixing with solutions containing Cl ions is prohibited due to the risk of precipitation.
Efficacy of pefloxacin in the treatment of urological infections
About the article
1744
0
Regular issues of "RMZh" No. 25 dated December 15, 2005 p. 1679
Category: General articles
Authors: Mazo E.B. , Popov S.V. 1 1 St. Petersburg State Budgetary Healthcare Institution Clinical Hospital of St. Luke, St. Petersburg; FSBEI HE SPbSU, St. Petersburg
For quotation:
Mazo E.B., Popov S.V. Efficacy of pefloxacin in the treatment of urological infections. RMJ. 2005;25:1679.
Urinary tract infections (UTIs) are among the most common infectious diseases. UTI-related problems account for more than 7 million physician visits per year in the United States, of which 2 million are related to acute cystitis [6,11]. UTIs account for about 15% of all outpatient antibiotics prescribed in the United States, with annual treatment costs of approximately $1 billion. UTIs are also responsible for more than 100,000 hospitalizations per year (most often due to acute pyelonephritis). UTIs account for more than 40% of all nosocomial infections and in most cases they are catheter-associated [11]. The prevalence of UTI varies with age and gender. It is estimated that approximately 50% of women can report having at least one episode of UTI in their lifetime. Meanwhile, among men aged 20 to 50 years, the most common disease is chronic prostatitis, accounting for 8% of outpatient visits to a urologist. With age, the frequency of this disease increases and reaches 30–73%. Moreover, chronic bacterial prostatitis (CKD) accounts for 5–15% of cases of the disease [2]. By age 50, the incidence of UTIs in men and women becomes approximately the same.
Classification of UTIs According to the anatomical classification, upper urinary tract infections include acute and chronic pyelonephritis. Acute cystitis is the most common manifestation of lower urinary tract infection. Based on the condition of the urinary tract and the presence of concomitant diseases, infections are divided into uncomplicated and complicated [6]. Uncomplicated UTIs occur in the absence of obstructive uropathy and structural changes in the kidneys and urinary tract, as well as in patients without serious concomitant diseases. Complicated UTIs occur in patients with obstructive uropathy, against the background of instrumental (invasive) methods of examination and treatment, and severe concomitant diseases (diabetes mellitus, neutropenia). Any UTI in men is considered complicated. Etiology of UTIs The most common causative agents of uncomplicated UTIs are gram-negative bacteria of the Enterobacteriaceae family. The most common strains are Escherichia coli, which are found in 80–90% of infections. Other representatives of the family Enterobacteriaceae (Proteus spp., Klebsiella spp., Enterobacter spp., etc.), as well as coagulase-negative staphylococci (Staphylococcus saprophyticus) are detected in the remaining 10% [11]. Complicated UTIs are caused by a wide variety of gram-negative and gram-positive microorganisms. The spectrum of pathogens of complicated UTIs is determined by the geographic region and also depends on the profile of the department, even within the same medical institution. According to 4 large-scale studies of bacterial pathogens of nosocomial UTIs (SENTRY, 1998; ESGNI-003, 2000; PEP, 2003 and a study of the structure of hospital UTIs in Straubing, 2001), in 70–80% the etiological agents of these infections were such gram-negative microorganisms as Escherichia coli, Pseudomonas spp., Proteus spp., Klebsiella spp., Enterobacter spp. Gram-positive bacteria caused complicated UTIs in 15–30% of cases and were represented by enterococci and staphylococci [13]. The most common etiological agents of CKD are members of the Enterobacteriaceae family of gram-negative bacteria. The most common strains are Escherichia coli, which are found in 65–80% of infections. Pseudomonas aeruginosa, Serratia spp., Klebsiella and Enterobacter aerogenes spp., and Acinetobacter spp. are detected in the remaining 10–15%. Enterococci account for 5 to 10% of confirmed prostate infections. The role of gram-positive bacteria such as Staphylococcus spp is controversial. and Streptococcus spp. with CKD. A number of researchers believe that these microorganisms are not a common cause of prostate infection, others suggest the etiological role of coagulase-negative staphylococci [2]. Antimicrobial therapy for UTIs The main goals of therapy for both uncomplicated and complicated UTIs are: rapid and effective response to treatment, prevention of relapses, and prevention or at least delaying the development of microbial resistance to antimicrobial drugs. Factors influencing the choice of an antimicrobial drug for the treatment of UTIs include: the individual characteristics of the patient, the characteristics of the infection, the sensitivity of the identified microorganism to the antibiotic, its pharmacokinetic properties and the availability of information on the effectiveness and safety of the drug proven in clinical studies, the level of local and regional resistance of pathogens . Fluoroquinolones, which are currently the drugs of choice for the treatment of urological infections, meet the above requirements [6]. Since their appearance on the world market, fluoroquinolones have quickly become a relevant group of antimicrobial drugs for the treatment of UTIs and CKD. A feature of the antibacterial action of fluoroquinolones is the presence of two targets of action in the bacterial cell, which are enzymes (type II topoisomerases) responsible for changes in the spatial configuration of bacterial DNA: DNA gyrase and topoisomerase IV. DNA gyrase supercoils bacterial DNA, and topoisomerase IV separates daughter chromosomes during replication. The key point in the action of fluoroquinolones is the formation of a three-component complex (bacterial DNA–enzyme–fluoroquinolone). This complex prevents bacterial DNA replication. Due to the fact that topoisomerases have cleaving activity, the DNA molecule is destroyed (Fig. 1). After oral administration of fluoroquinolones, concentrations of drugs are created in the urine, kidney tissues and male genital organs that significantly exceed the MIC values for most bacterial pathogens of UTI (Table 1) [3]. This ensures a high degree of eradication of uropathogens. Moreover, the clinical effectiveness of fluoroquinolone therapy in most cases correlates with eradication of the pathogen. The importance of pefloxacin in the treatment of urological infections For more than 15 years, the second generation fluoroquinolone pefloxacin has been successfully used in clinical practice for the treatment of UTIs. A representative of pefloxacin has recently appeared on the Russian market - Pelox 400 produced by Wockhard Ltd. (India) compared to other drugs of this INN Pelox 400, which has a more affordable price with a decent level of quality. The quality of the drug meets international standards and is confirmed by a GMP certificate. Pefloxacin (Pelox 400) is a broad-spectrum antimicrobial drug that is active against most gram-negative and some gram-positive microorganisms. Pefloxacin is a 1-ethyl-6-fluoro-7-(4-methyl-1-piperazinyl)-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid in the form of a dihydrate mesylate salt for the preparation of dosage forms in the form of tablets and injection solutions (Fig. 2). Pefloxacin (Pelox 400) has a bactericidal effect, inhibiting bacterial DNA replication through interaction with the DNA gyrase subunit, which controls the topological organization of the DNA molecule. Pefloxacin is well absorbed from the gastrointestinal tract, reaching maximum concentrations in the blood after 1–2 hours. This drug is also detected in the blood serum for a long time (up to 48 hours). The high volume of distribution of pefloxacin (110–140 l) indicates its good penetration into various organs and tissues. The advantages of the drug Pelox 400 also include its low dependence on the pH of the environment, which is of great importance in the treatment of urological infections, since the urine pH is normal and in various diseases can fluctuate between 4.5–8.5. The pharmacokinetic parameters of pefloxacin are shown in Table 2 [7]. The antibacterial effect of most antimicrobial drugs consists of both the action of the drug itself and its active metabolites, whose pharmacological, antibacterial and chemical properties sometimes differ significantly from the properties of the metabolized compound. Typically, significant amounts of metabolites are excreted in the urine and are the main component of the drug's antimicrobial activity in the treatment of UTIs [1]. Pefloxacin, like other fluoroquinolones, is extensively metabolized to form active metabolites that are excreted in the urine. The main metabolites of pefloxacin in blood serum are pefloxacin N-oxide and norfloxacin. In the urine, along with unchanged pefloxacin, metabolites of this drug are also found (Table 3) [3]. It was found that the pharmacokinetic profiles of pefloxacin after intravenous administration at a dose of 8 mg/kg in patients with insufficiency of renal function did not differ significantly from the profiles in healthy people. The half-life of the drug in patients with creatinine clearance more than 10 mg/min. increased, but total plasma clearance remained within normal values. In another study, 20 patients with severe renal failure were given pefloxacin by infusion at a dose of 1 mg/kg or administered orally at a dose of 800 mg twice daily for 5 days. After 5 days, the maximum and minimum concentrations of the drug in the blood were comparable to those obtained in healthy people [12]. In connection with the above, pefloxacin in the treatment of urological infections is especially important as the drug most indicated for patients with chronic renal failure. Important advantages of pefloxacin (Pelox 400) are high oral bioavailability, significant volume of distribution, post-antibiotic effect, exceeding against Pseudomonas spp. These effects of ciprofloxacin and ofloxacin, a high degree of accumulation in sufficient concentration in the secretion of the prostate gland and ejaculate, determine the modern successful use of this antimicrobial drug in the treatment of urological infections. Clinical studies have confirmed the effectiveness of pefloxacin in the treatment of patients with uncomplicated UTIs [9,10]. Randomized, double-blind, multicenter studies assessed the efficacy and safety of oral pefloxacin 400 mg and cotrimoxazole 160/800 mg daily for 6 to 10 days. The studies were carried out on 180 patients. Clinical effectiveness of use was registered in 86 and 80% of cases, respectively, and bacteriological in 85 and 80% of cases. Both drugs were well tolerated. In another study conducted by the staff of the nephrology clinic in Ljubljana (Slovenia), pefloxacin administered orally to 40 outpatients with UTI was also more effective than co-trimoxazole (95% to 75% eradication of the pathogen) [7]. In a large-scale study, 356 patients with complicated acute, chronic and recurrent UTIs were prescribed pefloxacin 800 mg per os daily. Cure was registered in 72% of patients, therapy was ineffective in 7%, and relapses developed in 21%. In 44 patients, relapses were caused by activation of the original pathogen, in 32% by superinfection. When treated with pefloxacin, eradication of 295 strains of bacteria was observed, including 146 strains of Escherichia coli. The use of pefloxacin 400 mg twice daily led to clinical recovery in 72% of cases, and the microbiological effectiveness of therapy was 81%. Of particular interest are data from a study of the effectiveness of pefloxacin in nephrological patients with immunosuppression [8]. In this study, 35 patients received oral pefloxacin. The drug was effective in 100% of patients after kidney transplantation, in 77% with renal failure and in 57% of patients on hemodialysis. The parenteral form of pefloxacin was prescribed to 14 patients, and its effectiveness was 80% in patients with renal failure and 67% in patients taking immunosuppressants. Studying the effectiveness of pefloxacin in the treatment of chronic nonspecific prostatitis is a particularly pressing problem. According to Segal A.S. et al., 1991, the effectiveness of the antibacterial action of pefloxacin in the complex treatment of 27 patients with chronic nonspecific prostatitis and urethroprostatitis was 85% [5]. We used pefloxacin in 2002 in the complex treatment of CKD in 28 patients aged 24 to 44 years [4]. Pefloxacin was prescribed to all patients 400 mg 2 times a day with meals. The total duration of treatment was 4 weeks. Along with antimicrobial therapy, patients were prescribed anti-inflammatory therapy (suppositories with diclofenac 50 mg 2 times a day for 2 weeks) and transrectal laser therapy. Clinical and bacteriological monitoring of the effectiveness of the therapy was carried out after 4 weeks. taking the drug. During a control examination of 28 patients receiving the drug, according to a bacteriological study, eradication of pathogens was achieved in 25 (89.3%) patients. During treatment of patients with CKD with pefloxacin, we noted a significant decrease and disappearance of symptoms, as well as normalization of the number of leukocytes in the secretion of the prostate gland, a decrease in prostate volume according to ultrasound, and an increase in the maximum volumetric flow rate of urine according to uroflowmetry. The drug was well tolerated by patients and the side effects that occurred in 2 of them did not necessitate discontinuation of this antibiotic. One of these patients, starting from the second week of therapy with pefloxacin, developed nausea after taking the drug, and the second patient, at week 3, after a three-hour exposure to the sun, received a burn, although he had previously been warned about possible photosensitivity and warned against prolonged ultraviolet irradiation. The above symptoms disappeared on their own after completing the course of treatment. In general, pefloxacin is well tolerated by patients. Adverse reactions develop in an average of 9% of patients. The most common side effect of pefloxacin therapy is gastrointestinal disorders [6]. Elderly patients and patients with impaired liver function require dose adjustment of the drug. Conclusion Today, pefloxacin (Pelox 400), a second-generation fluoroquinolone classified as a broad-spectrum antibacterial drug, continues to be an effective antimicrobial drug for the treatment of urological infections. The use of pefloxacin in the treatment of UTI is relevant (due to the characteristics of its metabolism and excretion) in patients with concomitant chronic renal failure. The pharmacokinetic equivalence of oral and intravenous pefloxacin allows for interchangeability and transfer from one route of administration to another. This drug also continues to be successfully used in the treatment of chronic bacterial prostatitis caused by gram-negative bacteria. A number of undoubted advantages of the drug, such as good penetration through the hematoprostatic barrier and sufficient accumulation in the prostate secretion and ejaculate, determine the possibility of further successful use of pefloxacin in this disease. Thus, pefloxacin continues to successfully occupy a special place in the arsenal of an intensively developing group of antimicrobial drugs - fluoroquinolones. Literature 1. Lopatkin N.A., Sinyukhin V.N., Kondratyeva E.M., Derevyanko I.I. Prediction of the effectiveness of abactal therapy based on pharmacokinetic data. // Urology and nephrology. – 1991. – Appendix. – pp. 44–46. 2. Mazo E.B., Popov S.V., Karabak V.I. Antimicrobial therapy of chronic bacterial prostatitis. // Russian Medical Journal. – 2004. – T.12, No. 12. – P. 737–740. 3. Padeiskaya E.N., Yakovlev V.P. Fluoroquinolones. – M.: Bioinform, 1995. – 220 p. 4. Popov S.V., Mazo E.B. Abactal (pefloxacin) in the treatment of patients with chronic bacterial prostatitis. // Russian Medical Journal. – 2002. – T.10, No. 26. – P. 1234–1236. 5. Segal A.S., Permyakov A.N., Dunaevsky Ya.L., Balashova L.D., Dolgopyatov D.G. Abactal in the complex treatment of chronic nonspecific prostatitis and urethroprostatitis. // Urology and nephrology. – 1991. – Appendix. – pp. 65–66. 6. Ushkalova E.A. Fluoroquinolones in the treatment of urinary tract infections: current state of the issue. // Pharmateka. – 2005. – No. 16. – P. 15–22. 7. Drinovec J. Klinicno testiranje pefloksacina. – Ljubljana: Lek, Indok, 1987. 8. Drinovec J. Klinicne izkusnje s pefloksacinom pri infekcijah secil in pri imunsko kompromitiranih bolnikih. – Ljubljana, 1988. 9. Guibert J., Mazeman E., Colau JC et al. Acute cystitis in women over 50 years of age. Efficacy of pefloxacin with single dose and norfloxacin for 10 days. // Presse Med. – 1993. – Vol. 22 (7)– P.288–292. 10. Guibert J. Efficacy of single dose of ciprofloxacin and pefloxacin in the treatment of female acute cystitis. // Presse Med. – 1995. – Vol. 24 (6)– P.304–308. 11. Naber KG, Morrissey I, Ambler JE Urinary Tract Infections and Fluoroquinolones. –Science Press Ltd, 2000. 12. Jungers P., Ganeval D., Hannedouche T., Prieur B., Montay G. Steady–state levels of pefloxacin and its metabolites in patients with severe renal impairment. //Eur. J. Clin. Pharm. – 1987. – Vol. 33– P.463–467. 13. Wagenlehner FME, Weidner W., Naber KG Emerging drugs for bacterial urinary tract infections. // Expert Opin.Emerging Drugs – 2005. – Vol. 10 (2) – P.275–298.
Content is licensed under a Creative Commons Attribution 4.0 International License.
Share the article on social networks
Recommend the article to your colleagues
special instructions
For diagnosed mixed infectious lesions of the pelvic and abdominal organs, Pefloxacin is recommended to be prescribed together with other antibiotics active against anaerobic infection ( Clindamycin, Metronidazole ).
Prevention of crystalluria during antibacterial therapy is to drink plenty of fluids. It is recommended to limit exposure to ultraviolet radiation due to possible photosensitization. Severe pathology of the renal system requires an individual approach and selection of a treatment regimen.
If prolonged, severe diarrhea develops, it is necessary to conduct examinations to exclude pseudomembranous colitis . If the diagnosis is reliably confirmed, Pefloxacin is discontinued and syndromic treatment is carried out.
Abactal (pefloxacin) in the treatment of patients with chronic bacterial prostatitis
P
The problem of diagnosis and treatment of chronic prostatitis continues to be relevant for urologists observing men aged 20 to 50 years.
It is during this period of life that approximately half of males notice various manifestations of the pathology in question [1,5]. It is noteworthy that only 5–20% of patients with chronic prostatitis can confidently identify the causative agent of the infection [6]. The most important role in the etiology of chronic bacterial prostatitis belongs to gram-negative bacteria of the Enterobacteriaceae family,
among which the undisputed leader belongs to
Escherichia coli
(about 80% of cases) [4].
Microorganisms such as Klebsiella pneumoniae, Proteus mirabilis
, etc. are much less common. Enterococci also play a certain role in the development of the disease.
The etiological involvement of Staphylococcus aureus
remains conclusively unproven [4,8].
Penetration of infection into the prostate gland most often occurs in an ascending manner
, from the posterior urethra. Infection of the prostate gland through the lymphogenous route is possible in the presence of inflammatory diseases in neighboring organs (epididymis, rectum, etc.). The abundant system of anastomoses between the venous plexuses of the pelvic organs predetermines the possibility of hematogenous infection in the presence of an inflammatory process in one of these organs. The canalicular route of infection of the prostate gland is usually realized through the transfer of microorganisms from inflammatory foci in the epididymis and seminal vesicles [3]. It should be noted that for the development of bacterial inflammation in the prostate gland, the presence of an infectious agent in itself is clearly not enough. According to a number of researchers [7,10], factors of dynamic bladder dysfunction and intraprostatic reflux of infected urine can play a certain role in the occurrence, maintenance and recurrence of a chronic inflammatory process [7]. Venous congestion, caused by the structure of the pelvic vein system, is also traditionally considered a factor predisposing to the development of inflammation in the gland [1,3].
Chronic bacterial prostatitis (CKD)
manifested by pain in the pelvic region, urination disorders, various erection and ejaculation disorders, and in some cases – changes in the fertilizing ability of sperm. The leading place in the diagnosis of CKD belongs to microbiological research, namely the Stamey localization test (four-glass test) to determine the sensitivity of microflora to antibiotics [8].
After a comprehensive examination and identification of the pathogen, it becomes necessary to prescribe adequate antibacterial therapy to the patient.
The criteria influencing the choice of an antimicrobial drug for the treatment of chronic bacterial prostatitis are: the sensitivity of the identified microorganism to the antibiotic, the ability of the latter to penetrate the hematoprostatic barrier in sufficient concentration and accumulate in the gland tissue, as well as overcome the extracellular polysaccharide shell formed by microcolonies of bacteria [ 9].
Ideal antibiotic for the treatment of chronic bacterial prostatitis
should be fat-soluble, slightly alkaline, with a dissociation coefficient that helps create the maximum concentration of the drug in the prostate gland [1,3]. A necessary condition for effective treatment of CKD is long-term (up to 4–12 weeks) administration of an antibiotic. [1,4]. Moreover, during the first 2–4 weeks, treatment is carried out under clinical and microbiological control. In cases where effectiveness is achieved, therapy is extended to 8–12 weeks, and in the absence of a positive result, there is a need to discontinue the drug and reconsider further treatment tactics.
Fluoroquinolones
are the only group of antibacterial drugs that, on the one hand, are active against gram-negative bacteria that cause bacterial prostatitis, and on the other hand, are capable of affecting microorganisms enclosed in an extracellular polysaccharide shell [4].
Abactal (pefloxacin),
, has been successfully used for a number of years . Abaktal exhibits high activity against etiologically significant gram-negative flora. Pefloxacin has a bactericidal effect - it inhibits bacterial DNA replication and also affects RNA and the synthesis of bacterial proteins. Its undoubted pharmacokinetic advantage is its long (8–10 hours, with repeated administration – up to 12–13 hours) half-life, which allows the drug to be administered twice a day. Abaktal also has other advantages that are important for the treatment of infections of the reproductive system in men: almost complete (up to 100%) bioavailability when administered orally, the ability to quickly penetrate organs, tissues and body fluids (volume of distribution - 1.5–1.8 l /kg). This creates high concentrations in the secretions of the prostate gland and seminal fluid [2].
Material and methods
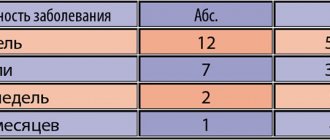
In the urological clinic of the Russian State Medical University, Abaktal was used in the complex treatment of chronic bacterial prostatitis in 28 patients aged 24 to 44 years (24–29 years – 5 patients; 29–34 years – 8; 34–39 years – 10; 39–44 years – 5). The diagnosis of the disease was based on physical examination, digital rectal examination, results of laboratory tests (clinical blood and urine tests, microscopy of prostate secretions) and microbiological examination (a simplified version of the Stamey test: prostate secretions and a portion of urine after prostate massage were analyzed) with sensitivity determination isolated microflora to antibiotics. All patients underwent transabdominal and transrectal ultrasound examination of the prostate gland and uroflowmetry, as well as, to exclude other diseases of the genitourinary organs, ultrasound examination of the kidneys and bladder with determination of the amount of residual urine.
In all patients, scrapings from the urethra and prostate secretions were examined to identify atypical intracellular microorganisms using the polymerase chain reaction method. These were not found either in scrapings from the urethra or in the secretion of the prostate gland. As a result of a bacteriological study (a simplified version of the Stamey test), the growth of microorganisms was detected in the secretion of the prostate gland and the third portion of urine (Table 1). It should be noted that according to the antibiogram data, all identified microorganisms were sensitive in vitro
to pefloxacin and a number of other antimicrobial drugs.
One could assume the presence of hospital flora in 5 patients who had previously undergone ureterolithoextraction in our clinic for a stone in the lower third of the ureter. In 1 of these patients, the growth of mixed flora (Escherichia coli and Morganella) was detected, and in 4 others, only Escherichia coli was detected. The level of microbial bodies in 1 ml of the test fluid was more than 100,000 in all patients. The disease was manifested by pain of various types in the pelvic region in 26 patients, frequent and painful urination in 15 patients.
Microscopic examination of prostate secretion in all patients revealed an increase in the number of leukocytes (more than 12 per field of view) and a decrease in the number of lecithin grains. In the examined secretions of 5 patients, presumably infected with hospital flora, leukocytes covered all fields of view. In the analyzes of the remaining 23 patients, the following number of leukocytes was determined in the visual fields: 15–20 – in 8 patients; 30–40 – in 7; 50–60 – in 8. Transabdominal and transrectal ultrasound examination revealed an increase in the volume of the prostate gland (average volume 36.8 cm3), as well as focal and diffuse hyperechogenicity of the prostate tissue. The average maximum urine flow rate according to uroflowmetry was 12.5 ml/sec. Antibacterial therapy was carried out with the drug Abaktal, which was prescribed per os
All patients: 400 mg 2 times a day with meals. The total duration of treatment was 4 weeks. Along with antibiotic therapy with Abaktal, patients were prescribed anti-inflammatory therapy (suppositories with NSAIDs 2 times a day for 2 weeks) and transrectal laser therapy. Rectal exposure to laser radiation (Mustang-2000 device) was carried out using a KLO 3 emitting head in the red optical range with a wavelength of 0.63 μm and a power of 20 mW. The exposure time was 10 minutes. The course of laser therapy consisted of 10 daily procedures.
Clinical and microbiological monitoring of the effectiveness of the therapy was carried out 4 weeks after the start of treatment.
Research results and discussion
A control microbiological study (a simplified version of the Stamey test) in 28 patients taking Abactal showed that complete eradication of pathogens was achieved in 25 (89.3%) patients. In 1 patient (3.5%) with an etiologically significant combination of E. coli
and
Proteus mirabilis,
as a result of treatment with Abaktal, the pathological process was reduced to a mono-infectious form (only Proteus infection remained). In 2 patients (7.1%) - with Escherichia coli isolated in the secretion of the prostate gland before treatment - a control microbiological study revealed epidermal staphylococci. This phenomenon can be explained by the fact that after the destruction of the etiologically responsible pathogens, colonization of the prostate gland occurred with the above bacteria, which are usually weakly virulent for individuals without impaired function of the immune system and factors of nonspecific resistance of the body. In this case, it was decided to abandon the use of antibiotics. During a control microbiological study (prostate secretion and a portion of urine after massage of the gland), carried out after 8 weeks, no bacteria were found.
Treatment with Abaktal in patients with CKD made it possible to state the disappearance or significant reduction of symptoms, normalization of the number of leukocytes in the secretion of the prostate gland, a decrease in prostate volume (ultrasound data) and an increase in the maximum volumetric flow rate of urine according to uroflowmetry (Table 2).
In general, Abactal was well tolerated by patients. The side effects that occurred in 2 patients did not necessitate discontinuation of the antibiotic. One of these patients experienced nausea in the second week after starting the drug. Another patient, in the 3rd week of a course of antibiotic therapy, after three hours of exposure to the sun, complained of manifestations of photodermatitis (although he was informed about the possibility of photosensitization, which can develop during treatment with any of the fluoroquinolones and was warned against prolonged ultraviolet irradiation). The above symptoms disappeared on their own after completing the course of treatment.
Thus, the results of the study indicate that Abaktal continues to be a highly effective antimicrobial drug for the treatment of chronic bacterial prostatitis and demonstrates significant activity against gram-negative pathogens responsible for the development of bacterial inflammation in the prostate gland. Administration of Abaktal to 28 patients suffering from CKD led to eradication of pathogens in 25 (89.3%) patients, elimination or significant reduction of symptoms, elimination of inflammatory changes in the secretion of the prostate gland, reduction of its volume (according to transabdominal and transrectal ultrasound), uroflowmetrically confirmed increasing the maximum volumetric flow rate of urine and, ultimately, improving the quality of life of the observed patients. Side effects that occurred in 2 (7.1%) patients did not require discontinuation of the antibiotic and went away on their own after completion of the course of treatment.
Conclusion
The antibiotic Abaktal (pefloxacin) from the fluoroquinolone group retains its high clinical effectiveness in the treatment of patients with chronic bacterial prostatitis. The microbiological effectiveness of the drug is 89.3%. The advantages of Abaktal specific to this pathology: good penetration through the hematoprostatic barrier, the ability to influence microorganisms in biofilms in combination with other advantages inherent in pefloxacin (antimicrobial spectrum adequate to the etiology of prostatitis, almost complete bioavailability when taken orally
;
long half-life, allowing the drug to be prescribed 2 times a day; low incidence of side effects) justify the possibility of further successful use of Abaktal in the treatment of chronic bacterial prostatitis. Literature:
1. Mazo E.B., Stepensky A.B., Gamidov S.I., Grigoriev M.E., Krivoborodov G.G., Belkovskaya M.N. Pharmacotherapy of chronic prostatitis. RMJ 2001; Vol.9, No. 23.
2. Padeiskaya E.N., Yakovlev V.P. Fluoroquinolones. Moscow. Bioinform. 1995. P. 93–95
3. Petrov S.B., Babkin P.A. Bacterial prostatitis. Clinical Antimicrobial Chemotherapy 1999; 3: 95–100.
4. Practical guide to anti-infective chemotherapy. Edited by L.S. Strachunsky, Yu.B. Belousov, S.N. Kozlov. Moscow.2002.P.73–78; 247.
5. Stewart C. Prostatitis. Emerg. Med. Clin.North. Am 1988; 6:391–402.
6. Weidner W., Schiefer H. G., Krauss H. et. al. Chronic prostatitis: A through search for etiologically involved microorganisms in 1461 patients. Infection 1991; 19(3):119–125.
7. D.Caropreso, TDMoon a–Blockers: An Effective Treatment for Prostatitis? Current Urology Reports 2000,1:148–154
8. G. Luzzi The prostatitis syndromes. Int STD and AIDS 1996; 7:471–478
9. Falagas ME et al. Practice guidelines: prostatitis, epididymitis and urethritis// Infectious Diseases in Clinical Practice.1995;4 (5) 325–32
10. Ghobiсh A. Voiding dysfunction associated with chronic bacterial prostatitis. Eur. Urol. 2002; 42:159–162
Analogs
Level 4 ATX code matches:
Siflox
Hyleflox
Leflobakt
Lefoccin
Gatifloxacin
Ofloxacin
Faktiv
Tigeron
Lebel
Zanotsin
Lomefloxacin
Eleflox
Lomflox
Tsiprobay
Sparflo
Tariwid
Zoflox
Abaktal
Moxifloxacin
Levofloxacin
- Pelox-400;
- Peflacin;
- Abaktal;
- Peflobid;
- Perflox;
- Perti;
- Unikpef;
- Pefloxacin-Acos;
- Peflacine.