Pharmacodynamics
Noopept® has nootropic and neuroprotective properties. Improves learning ability and memory, acting on all phases of processing: initial information processing, consolidation, retrieval. Prevents the development of amnesia caused by electric shock, blockade of central cholinergic structures, glutamatergic receptor systems, and deprivation of the paradoxical phase of sleep.
The neuroprotective (protective) effect of the drug Noopept® is manifested in increasing the resistance of brain tissue to damaging influences (trauma, hypoxia, electroconvulsive, toxic) and reducing the degree of damage to brain neurons. The drug reduces the volume of the lesion in a thrombotic stroke model and prevents the death of neurons in cultured tissue of the cerebral cortex and cerebellum exposed to neurotoxic concentrations of glutamate and free radical oxygen.
Noopept® has an antioxidant effect, blocks voltage-gated calcium channels in neurons, weakening the neurotoxic effect of excess calcium, improves the rheological properties of blood, having antiaggregation, fibrinolytic, and anticoagulant properties.
The nootropic effect of the drug is associated with the formation of cycloprolylglycine, which is similar in structure to the endogenous cyclic dipeptide, which has antiamnestic activity, as well as the presence of a choline-positive effect.
Noopept® increases the amplitude of the transcallosal response, facilitating associative connections between the cerebral hemispheres at the level of cortical structures. Helps restore memory and other cognitive functions impaired as a result of damaging influences - brain injury, local and global ischemia, prenatal damage (alcohol, hypoxia).
The therapeutic effect of the drug in patients with organic disorders of the central nervous system manifests itself starting from 5–7 days of treatment. Initially, the anxiolytic and mild stimulating effects present in the spectrum of activity of the drug Noopept® are realized, manifested in the reduction or disappearance of anxiety, increased irritability, affective lability, and sleep disturbances. After 14–20 days of therapy, a positive effect of the drug on cognitive functions, parameters of attention and memory is revealed.
Noopept® has a vegetative-normalizing effect, helps reduce headaches, orthostatic disorders, and tachycardia.
When discontinuing the drug, no withdrawal syndrome is observed. Does not have a damaging effect on internal organs; does not lead to changes in the cellular composition of the blood and biochemical parameters of blood and urine; does not have immunotoxic, teratogenic effects, does not exhibit mutagenic properties.
Noopept in the treatment of dyscirculatory encephalopathy with moderate cognitive impairment
According to epidemiological studies, mild and moderate cognitive impairment, not reaching the level of dementia, in older age groups occurs in 12–17% of people over 65 years of age [10, 15, 18]. In a significant proportion of cases, these disorders are of vascular origin and become progressive, eventually transforming into vascular or mixed (vascular and neurodegenerative) dementia.
Impaired cognitive functions, along with other neuropsychic and motor disorders, largely determine the severity of cerebrovascular disorders in dyscirculatory encephalopathy (DE). A feature of cognitive deficit in DE is the predominance of neurodynamic and regulatory cognitive impairments. This is manifested by slowness of mental activity, weakening of attention, decreased speech activity, disruption of planning, organization and control of activities. Memory impairment, as a rule, is moderate and is of a secondary nature (this is evidenced by a deficit in free reproduction with relatively intact recognition and the effectiveness of mediating techniques) [16, 18].
Neuropsychological testing in mild cognitive disorders reveals predominantly neurodynamic disorders in the form of slowness, decreased performance, exhaustion, and fluctuations in attention [7, 9, 10, 12, 19]. As DE progresses, the severity of cognitive impairment increases. Along with neurodynamic disorders, regulatory disorders are identified (dysregulatory or subcortical-frontal cognitive syndrome) [7, 9, 12, 21, 22]. Moderate cognitive impairment, although it does not lead to a significant limitation in the professional, everyday and social independence of patients, can make it difficult to perform complex (usually instrumental) types of daily activities and contribute to a decrease in the quality of life of patients [10, 12, 15, 16, 18, 26 , 27]. Further progression of vascular damage to the brain leads to severe cognitive impairment, reaching the level of dementia, disrupting professional, everyday and social adaptation [6, 10, 12, 15–18, 26].
The leading role of frontal dysfunction in the structure of neuropsychological disorders is also manifested in the frequent combination of cognitive and emotional-personal disorders [8, 10, 12, 18, 23]. The latter, in the earlier stages of DE, are represented predominantly by affective disorders (irritability, emotional lability, anxiety, depression); at a later stage, they may be accompanied by pronounced personality and behavioral disorders in the form of apathetic-abulsic disorders, disinhibition, explosiveness, as well as psychotic disorders.
To treat cognitive impairment in DE, nootropic drugs are widely used - compounds that act on the central nervous system, enhancing cognitive capabilities (improving memory, attention, orientation, etc.). The main characteristic of nootropics is their activating specific effect on the higher integrative functions of the brain and the restoration of disorders of higher nervous activity. The main mechanisms of the therapeutic action of nootropic drugs are: increased synthesis of adenosine triphosphoric acid (ATP), increased absorption of glucose by brain cells; strengthening of synaptic transmission processes in the central nervous system; normalization of neurotransmitter disorders (the effect of a number of nootropic drugs is mediated through the neurotransmitter systems of the brain, among which the most important are monoaminergic, acetylcholinergic, glutamatergic, GABAergic); membrane-stabilizing effect [4, 19]. The mechanisms of action of nootropic drugs have been intensively studied at the neurophysiological level. The influence of nootropics on the late components (400–800 ms) of evoked potentials in people when solving problems has been shown, which is considered as direct confirmation of the effect of nootropics on cognitive processes [11].
Considering that the need for nootropics in clinical practice is great, obtaining and introducing into practice new highly effective nootropic drugs is an important and urgent task. Peptide drugs are interesting as potential nootropics, since neuropeptides play a major role in the regulation of cognitive functions and, in most cases, peptide drugs are more effective than drugs from other chemical groups [1, 2, 4, 5].
Noopept is an ethyl ester of N-phenyl-acetyl-L-prolylglycine, a dipeptide with nootropic and neuroprotective properties [1, 13].
The neuroprotective (protective) effect of Noopept is manifested in increasing the resistance of brain tissue to damaging influences (trauma, hypoxia, electroconvulsive and toxic damage). The drug reduces the volume of the lesion in an experimental thrombotic stroke model and prevents the death of neurons in cultured tissue of the cerebral cortex and cerebellum exposed to toxic concentrations of glutamate and free radical oxygen [1, 13]. Noopept has an antioxidant effect, an antagonistic effect on the effects of excess calcium entering cells, and improves the rheological properties of blood. The nootropic effect of the drug is associated with the formation of cycloprolylglycine, which is similar in structure to the endogenous cyclic dipeptide, which has antiamnestic activity and is also characterized by the presence of acetylcholinergic action.
The presence of a wide spectrum of nootropic activity in Noopept was the basis for conducting clinical studies on its use in adults with impairments of memory, attention and other intellectual and mnestic functions after traumatic brain injury and chronic cerebrovascular insufficiency. The therapeutic effect of the drug in patients with organic disorders of the central nervous system manifests itself starting from 5–7 days of treatment. Initially, the anxiolytic and mild stimulating effects present in the activity spectrum of Noopept are realized, manifested in the reduction or disappearance of anxiety, increased irritability, affective lability, and sleep disturbances. After 14–20 days of therapy, a positive effect of the drug on cognitive functions, parameters of attention and memory is revealed [1, 13]. It has been established that the drug is well tolerated in therapeutically effective daily doses [13].
An analysis of the dynamics of the electroencephalogram (EEG) spectrum showed that Noopept causes an increase in the power of the alpha rhythm and a weakening of the delta rhythm, confirming the presence of a nootropic effect in the drug [1, 11, 13]. Of particular interest is the assessment of the effect of Noopept on cognitive impairment associated with cerebrovascular insufficiency.
The purpose of this study was to evaluate the clinical effectiveness, safety and tolerability of the use of the drug Noopept for DE with moderate cognitive impairment.
Patients and methods
We examined 360 patients aged 50–80 years (115 men and 245 women), average age 69.8 ± 8.3 years, with stage I and II DE of atherosclerotic and hypertensive origin with moderate cognitive impairment. The diagnosis of DE was made in accordance with generally accepted criteria [8].
The study was conducted with the participation of specialists from 12 medical institutions in Russia: the Department of Nervous Diseases of the Moscow Medical Academy. I. M. Sechenova (chief researcher of Academician of the Russian Academy of Medical Sciences, Prof. N. N. Yakhno); Department of Neurology and Neurosurgery, Russian State Medical University; Rehabilitation Clinic No. 7, Moscow (chief researcher Prof. A. N. Boyko); Medical Center of the Presidential Administration, Moscow, Central Clinical Hospital (chief researcher Prof. V. I. Shmyrev); Department of Neurology, St. Petersburg State Medical University. acad. I. P. Pavlova (chief researcher of Academician of the Russian Academy of Medical Sciences, Prof. A. A. Skoromets); Regional Neurological Center of the State Healthcare Institution ROCH, Rostov-on-Don (chief researcher Prof. Yu. V. Trinitatsky); Department of Neurology and Neurosurgery of Samara State Medical University (chief researcher Prof. I. E. Poverennova); Clinical Institute of the Brain, Yekaterinburg (chief researcher Prof. A. A. Belkin); Department of Neurology, Novosibirsk State Medical University (chief researcher Prof. B. M. Doronin); Department of Neurology and Rehabilitation of Kazan State Medical University (chief researcher Prof. E. I. Bogdanov); Department of Neurology and Neurosurgery, Irkutsk State Institute for Advanced Medical Studies, Irkutsk (chief researcher Prof. V.V. Shprakh); Department of Neurology, Novokuznetsk State Institute for Advanced Medical Studies (chief researcher, Ph.D. M. G. Zhestikova).
All patients underwent a clinical neurological examination. Assessment of changes in the severity of clinical manifestations of DE during treatment with Noopept was carried out using the Clinical Global Impression of Change Scale (CHI-I), assessment of cognitive functions was carried out using the Mini-Mental State Examination Scale (MMSE) [20], and the clock drawing test. (TRCH) with an assessment on a 10-point system, literal and categorical associations [24].
All patients received Noopept at a dose of 10 mg twice daily (20 mg/day) for 2 months.
All adverse events that occurred to the patient after taking the first dose of Noopept and until the day of the final visit were recorded. Statistical processing of the results was carried out using the statistical package SPSS 13.0 for Windows. To assess the reliability of intergroup differences in dependent samples, the parametric Student's t-test was used. The significance level for differences in the studied parameters was set at p < 0.05.
results
As a result of the study, moderate cognitive impairment was identified in patients. During examination, patients presented asthenic complaints: fatigue, irritability, absent-mindedness, memory loss, headaches with mental and physical stress. When conducting a neuropsychological study, patients found it difficult to concentrate on performing tests, they quickly became exhausted when performing a more complex task (“counting” according to the MMSE, literal and categorical associations). These disorders reflected a violation of the regulatory level and were manifested by impulsiveness in decision making and the occurrence of characteristic errors, impaired voluntary attention, decreased speech activity, and slow performance of neurodynamic tests. Memory suffered moderately, its impairments were modally nonspecific according to the MMSE data.
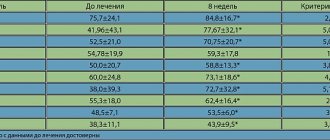
The study showed a positive effect of Noopept therapy on the cognitive functions of the examined patients: attention, memory, neurodynamic functions, which confirms a significant improvement in the total MMSE score during Noopept treatment in patients with DE and moderate cognitive impairment (Fig. 1).
A significant improvement in the TFC indicator reflects an improvement in constructive praxis (Fig. 2).
An improvement in neurodynamic functions and speech production was revealed, which is confirmed by a significant improvement in the performance of the categorical association test and a positive trend obtained when comparing the results of the literal association test (Fig. 3).
Thus, when comparing the results of a neuropsychological examination in patients with DE while taking Noopept for 60 days at the indicated dose, a significant improvement in the total MMSE score, constructive praxis (TCP), speech production (categorical associations) was revealed (p < 0 .05). A positive trend was noted when studying literal associations (0.05 < p < 0.06) (
.).
The study revealed good tolerability of Noopept. In 14 patients, adverse events were noted while taking Noopept, manifested in four cases in the form of increased difficulty falling asleep, in four cases in the form of increased headache, in two cases in the form of increased non-systemic dizziness, in one case in the form of a feeling of “heaviness” in the head, in two cases in the form of single increases in blood pressure in patients with hypertension, stopped by taking antihypertensive drugs, and in one case in the form of an allergic reaction such as urticaria. In 13 cases, the severity of adverse events was mild and did not require discontinuation of the drug. In one case, when an allergic reaction occurred, Noopept was stopped and antihistamines were prescribed, after which the allergic reaction regressed. A connection with Noopept was probable in all cases.
The overall assessment of the clinical effectiveness of Noopept according to the Clinical Global Impression of Change Scale (CGI-I) corresponds to a moderate improvement.
Thus, during treatment with Noopept, improvements in speech fluency, constructive praxis, neurodynamic functions, and memory were noted. Neuropsychological disorders in the examined patients with DE were mainly due to lack of attention and existing modality-nonspecific memory impairments of a dynamic nature. The noted improvement in neuropsychological functions during treatment with Noopept is associated with its nootropic properties. The drug has a high safety profile, is well tolerated, does not cause significant unwanted side effects, and can be recommended for the correction of cognitive impairment in patients with DE.
Literature
- Avedisova A. S., Yastrebov D. V. Comparative effectiveness of Noopept and piracetam in the treatment of asthenic disorders and disorders of organic genesis // Russian. honey. Journal 2007. No. 5. pp. 434–437.
- Arsenyeva K. E. Nootropic drugs in the treatment of cerebrovascular diseases // Russian. honey. magazine 2007. No. 4. pp. 225–228.
- Voronina T. A. Hypoxia and memory. Features of the effects and use of nootropic drugs // Bulletin of the Russian Academy of Medical Sciences. 2000. No. 9. pp. 27–34.
- Voronina T. A., Seredenin S. B. Nootropic drugs, achievements and new problems // Expert. and wedge. Pharmacol. 1998. T. 61, no. 4. pp. 3–9.
- Gudasheva T. A., Ostrovskaya R. U., Trofimov S. S. et al. Peptide analogues of piracetam as ligands of putative nootropic receptors // Chem. Pharmacol. magazine 1985. No. 1. P. 1322–1329.
- Damulin I.V. Vascular dementia // Neurol. magazine 1999. No. 4. P. 4–11.
- Damulin I.V., Zakharov V.V., Yakhno N.N. Cognitive disorders: differential diagnosis and treatment methods. Method. rec. M., 2000. 44 p.
- Damulin I.V., Parfenov V.A., Skoromets A.A., Yakhno N.N. Circulatory disorders in the brain and spinal cord / In the book: Diseases of the nervous system. A guide for doctors. T. 1. Ed. N. N. Yakhno. 4th ed., revised. and additional M.: Medicine, 2005. pp. 232–303.
- Zakharov V.V., Damulin I.V. Diagnosis and treatment of memory disorders and other higher brain functions in the elderly. Method. rec. Ed. N. N. Yakhno. M., MMA, 1997. 44 p.
- Zakharov V.V., Yakhno N.N. Syndrome of moderate cognitive impairment in old age: diagnosis and treatment // Russian. honey. magazine 2004. No. 10. pp. 573–576.
- Krapivin S.V. Neurophysiological mechanisms of action of nootropic drugs // Journal. neuropathol. and psychiatry. 1993. T. 93, no. 4. pp. 104–107.
- Levin O. S., Golubeva L. V. Heterogeneity of moderate cognitive disorder: diagnostic and therapeutic aspects // Consilium Medicum. 2006. No. 12. pp. 106–110.
- Neznamov G. G., Teleshova E. S. Results of a comparative study of noopept and piracetam in the treatment of patients with mild cognitive impairment in organic diseases of the brain of vascular and traumatic origin // Journal. neurol. and a psychiatrist. 2008. T. 108, No. 3. P. 33–42.
- Semina I. G., Semina I. I., Azancheev N. M. et al. On the issue of membrane mechanisms of action of nootropic drugs // Biological membranes. 2001. T. 18, No. 5. pp. 363–369.
- Yakhno N. N. Cognitive disorders in a neurological clinic // Neurologist. magazine 2006. T. 11, supplement No. 1. P. 4–12.
- Yakhno N.N., Damulin I.V. Dyscirculatory (vascular) encephalopathy // Ross. honey. magazine 1999. No. 5. P. 3–7.
- Yakhno N. N., Zakharov V. V. Memory impairments in neurological practice // Neurologist. magazine 1997. No. 4. pp. 30–35.
- Yakhno N. N., Zakharov V. V., Lokshina A. B. et al. Tanakan (EGb 761) in the treatment of moderate cognitive impairment (multicenter study) // J. neurol. and psychiatry. 2006. No. 12. pp. 48–53.
- Bowler JV, Hachinski V. The concept of vascular cognitive impairment / In: Vascular cognitive impairment. T. Erkinjuntti, S. Gauthier (eds.). NY: Martin Dunitz, 2002. pp. 9–26.
- Folstein MF, Folstein SE, McHugh PR Mini-mental state exam: a practical method for grading the cognitive status of patients for the clinician // J. Psychiatr. Res. 1975. Vol. 12. P. 189–198.
- Galluzzi S., Sheu C.-F., Zanetti O. et al. Distinctive clinical features of mild cognitive impairment with subcortical cerebrovascular disease // Dement. Geriatr. Cogn. Discord. 2005. Vol. 19. R. 196–203.
- Geroldi C., Ferrucci L., Bandinelli S. et al. Mild cognitive deterioration with subcortical features: Prevalence, clinical characteristics, and association with cardiovascular risk factors in community–dwelling older persons (The InCHIANTI Study) // J. Amer. Ger. Soc. 2003. Vol.51. P. 1064–1071.
- Monfort V., Pouthas V., Ragot R. Role of frontal cortex in memory for duration: an event-related potential study in humans // Neurosci. Lett. 2000. Vol. 286. R. 91–94.
- Lezak M. Neuropsychological assessment. NY: Oxford University Press, 1983. pp. 768–170.
- Petersen RS, Smith GE, Waring SC et al Mild cognitive impairment: clinical characterization and outcome // Arch. Neurol. 1999. Vol. 56. P. 303–308.
- Rockwood K., Wentzel C., Hachinski V. et al. Prevalence and outcomes of vascular cognitive impairment // Neurology 2000. Vol. 54. R. 447–451.
- Roman G.C., Erkinjuntti T., Wallin A. et al. Subcortical ischemic vascular dementia // Lancet Neurology. 2002. Vol.1. R. 426–436.
N. N. Yakhno , Doctor of Medical Sciences, Professor, Academician of the Russian Academy of Medical Sciences I. V. Damulin , Doctor of Medical Sciences, Professor L. M. Antonenko , Candidate of Medical Sciences
MMA im. I. M. Sechenova, Moscow
Table.
Pharmacokinetics
N-phenylacetyl-L-prolylglycine ethyl ester, absorbed in the gastrointestinal tract, enters the systemic circulation unchanged, penetrates the BBB, and is determined in the brain in higher concentrations than in the blood. Tmax averages 15 minutes. T1/2 from blood plasma - 0.38 hours. Partially stored unchanged, partially metabolized with the formation of phenylacetic acid, phenylacetylproline and cycloprolylglycine. It has high relative bioavailability (99.7%), does not accumulate in the body, and does not cause drug dependence.