Pharmacological properties of the drug Alflutop
Pharmacodynamics. The drug regulates metabolism in cartilage tissue. It has a chondroprotective effect by normalizing the biosynthesis of hyaluronic acid and type II collagen and inhibiting the activity of hyaluronidase and other enzymes that take part in the destruction of the intercellular matrix. These effects determine the activation of the processes of restoration of the structure of cartilage and prevent the destruction of the macromolecular structures of cartilage tissue. By restoring homeostasis in the joint, the drug inhibits the biosynthesis of inflammatory mediators, including cytokines. As a result, the drug has a pronounced anti-inflammatory and analgesic effect, which manifests itself on the 8th–10th day of treatment. Proteoglycans, which are part of the drug, have a trophic effect and a replacement effect, significantly increasing MRI indicators of hydrophilicity, cartilage height and bone tissue homogeneity. Pharmacokinetics. The effect of an extract of marine organisms is the combined effect of its active components, so the study of pharmacokinetic characteristics is impossible.
Registration number: P N012210/01 dated March 26, 2017. Dosage form: Solution for injection
The active component of the drug is a bioactive concentrate from small sea fish (North Sea sprat (Sprattus sprattus sprattus), Black Sea whiting (Odontogadus merlangus euxinus), Black Sea bellyfish (Alosa tanaica nordmanni), Black Sea anchovy (Engraulis encrassicholus ponticus)), obtained by extraction with subsequent deproteinization and delipidation.
Composition of the drug per 1 ml: Active ingredient: bioactive concentrate of small marine fish 0.1 ml; Excipients: phenol no more than 0.005 g, water for injection up to 1 ml.
Description Colorless or slightly brownish-yellow, or slightly yellow, transparent liquid.
Pharmacotherapeutic group: tissue repair stimulant of natural origin ATX code: M09AX
Pharmacological action Alflutol is a chondroprotector, the active component of which is a bioactive concentrate from small sea fish. The concentrate contains mucopolysaccharides (chondroitin sulfate), amino acids, peptides, sodium, potassium, calcium, magnesium, iron, copper and zinc ions. Alflutop prevents the destruction of macromolecular structures of normal tissues, stimulates restoration processes in interstitial tissue and articular cartilage tissue, which explains its analgesic effect. Anti-inflammatory effect and tissue regeneration are based on inhibition of hyaluronidase activity and normalization of hyaluronic acid biosynthesis. Both of these effects are synergistic and cause the activation of restoration processes in tissues (in particular, restoration of the structure of cartilage).
Indications for use Alflutop is used in adults with primary and secondary osteoarthrosis of various localizations (coxarthrosis, gonarthrosis, arthrosis of small joints), osteochondrosis and spondylosis.
Contraindications Hypersensitivity to the components of the drug. Pregnancy and lactation period. Childhood.
Caution It is not recommended to prescribe the drug in adolescence due to the lack of scientific clinical data in this category of patients.
Method of administration and dosage For polyosteoarthrosis and osteochondrosis, the drug is administered deeply intramuscularly: - 1 ml per day, the course of treatment is 20 injections (1 injection per day for 20 days) or - 2 ml every other day, the course of treatment is 10 injections ( 1 injection per day for 20 days). If large joints are predominantly affected, the drug is administered intra-articularly, 1-2 ml into each joint with an interval of 3-4 days. A total of 5-6 injections into each joint per course. A combination of intra-articular and intramuscular methods of administration is possible. It is advisable to repeat the course of treatment 6 months after consulting a doctor.
Side effects Itchy dermatitis, redness of the skin and a burning sensation at the injection site, and short-term myalgia are rarely observed. In some cases, with intra-articular injections, a transient increase in pain may occur. Very rarely, anaphylactic reactions may develop.
Interactions with other drugs Not identified to date.
Overdose In case of overdose, allergic reactions (sometimes severe) may occur in predisposed patients.
Special instructions In case of individual intolerance to seafood (sea fish), the risk of developing allergic reactions increases.
Release form Solution for injection. 1 ml or 2 ml in dark glass ampoules with a white break ring. A label is attached to each ampoule or prefilled syringe. 5 ampoules in cell polymer packaging coated with aluminum foil. 2 blister packs (1 ml or 2 ml ampoules) or 1 blister pack (2 ml ampoules) along with instructions for use in a cardboard pack.
Storage conditions: Protected from light at a temperature of 15°C to 25°C. Keep out of the reach of children.
Shelf life: 3 years. Do not use a drug that has expired.
Dispensed from pharmacies By doctor's prescription.
The legal entity in whose name the K.O. registration certificate was issued. Biotechnos S.A. st. Gorunului No. 3-5, Otopeni, Ilfov County, 075100, Romania
Manufacturer Manufacturer and primary packaging S.K. ZENTIVA S.A. Boulevard Theodore Palladi no. 50, district 3, Bucharest, 032266, Romania Secondary packaging and release quality control C.O. Biotechnos S.A. st. Gorunului No. 3-5, Otopeni town, Ilfov county, 075100, Romania
The organization that accepts consumer complaints is BIOTECHNOS LLC, 115432, Moscow, Andropova Ave., 18, bldg. 6, room XI, room 7 (office 6-07) Tel.
Special instructions for the use of Alflutop
The drug should be used with caution under strict medical supervision, especially in the case of autoimmune diseases (rheumatoid polyarthritis, ankylosing spondylitis, SLE, scleroderma). The drug may cause anaphylactic reactions. Application in pediatrics. It is not recommended to prescribe the drug in childhood and adolescence due to the lack of clinical data confirming the safety and effectiveness of its use in this age group. The drug does not affect the ability to drive a car or operate complex machinery.
Analgesic properties of the drug Alflutop in the treatment of chronic back pain
A.B.Danilov, T.R.Zharkova, L.T.Akhmetdzhanova Department of Nervous Diseases of the First Moscow State Medical University. I.M. Sechenova Pain in the lower back is the fifth most common reason for visiting a doctor. In most cases, it occurs against the background of degenerative-dystrophic diseases of the spine, which are found in 90-95% of the adult population. The main problem in treatment is chronic pain, which does not always correlate with the pathology of spinal structures detected by neuroimaging [1-3]. Alas, today there is no one universal effective method or drug that has clearly proven its effectiveness in the treatment of chronic back pain. This determines the search for new treatment options. One of these areas is the use of chondroprotectors. Interest in this group of drugs as potential analgesics is due to their anti-inflammatory properties and safety of use. The effectiveness of these drugs in the treatment of joint pathology has been well studied [4], while in the treatment of chronic back pain they are used much less frequently [5]. Special studies have shown that components of chondroprotective drugs such as glucosamine and chondroitin, in large doses, have certain anti-inflammatory effects and reduce pain [4, 6]. Traditionally, drugs of this series existing on the market are in tablet forms and are used for a long time in the complex treatment of joint diseases. In these cases, the therapeutic effect is usually expected within several months. Injectable drugs containing chondroprotective components look more attractive from the point of view of pain therapy.
In recent years, several studies have been published on the effectiveness of the injection drug Alflutop for osteoarthritis and back pain [7-13]. Alflutop is an original injection drug, which is an extract from marine fish that contains glycosaminoglycans, including hyaluronic acid, chondroitin sulfate, dermatan sulfate, keratan sulfate. The drug has a chondroprotective and anti-inflammatory effect, regulates metabolism in cartilage tissue. Its chondroprotective effect is associated with inhibition of the activity of hyaluronidase and other enzymes that take part in the destruction of the intercellular matrix and normalization of the biosynthesis of hyaluronic acid and type II collagen. Alflutop inhibits the biosynthesis of inflammatory mediators and reduces capillary permeability. The proteoglycans included in its composition have a trophic effect and have a replacement effect, significantly increasing the indices of magnetic resonance imaging (MRI), hydrophilicity, cartilage height and bone tissue homogeneity.
Very intriguing is the analgesic effect of the drug, which, according to many authors, manifests itself quite quickly. An open multicenter study assessing the effectiveness and safety of Alflutop in patients with vertebrogenic cervicobrachialgia demonstrated its ability to reduce the severity of pain and increase mobility in the cervical spine and shoulder joint [9]. In general, a positive result was observed in 82% of patients, and the analgesic effect appeared within the first 2 weeks after the start of treatment. In a special double-blind, placebo-controlled study on the use of the drug Alflutop for chronic lumbar sciatica, its high effectiveness was also demonstrated [10]. In these and other studies on the use of the drug Alflutop, it was clearly demonstrated that the onset of the analgesic effect begins in the 2nd week of therapy [7, 8, 12, 13]. It is unlikely that the reduction in pain during these periods is associated with the restoration of cartilage tissue or other structural changes in the tissues of the spine or joint. Thus, the question remains insufficiently clear about the mechanisms of the analgesic effect of the drug Alflutop, which appears quite quickly and persists throughout the entire course of treatment and after its completion. Effectiveness research
The purpose of this study is to clarify the mechanisms of the analgesic effect of the drug Alflutop in the treatment of patients with chronic pain in the lower back. Material
The study involved 30 patients (7 men and 23 women, mean age 40.6±10.8 years). Inclusion criteria: 1) patient age from 25 to 70 years; 2) chronic pain syndrome in the lower back lasting at least 3 months, not due to specific causes (oncology, infection, etc.) against the background of confirmed degenerative changes in the spine according to radiography, computed tomography (CT) and MRI; 3) pain intensity of at least 4 points on the visual analogue scale (VAS). In 13.3% of patients, protrusion of the intervertebral disc without root compression was diagnosed, in 23.3% - facet atropathy, in 16.6% - pathology of the iliosacral joint, in 10% - radiculopathy due to disc herniation. 12 patients had pain at the level of the neck and shoulder girdle, and 18 patients had pain at the lumbar level. The control group included 15 practically healthy people (8 men and 7 women), whose average age was 44.37±10.3 years. Treatment
All patients received monotherapy with Alflutop 1 ml intramuscularly daily for 20 days. Before therapy, patients received non-steroidal anti-inflammatory drugs - NSAIDs (83.3%), muscle relaxants (60%), physiotherapeutic treatment (53.3%). 3 days before the start of the study, in accordance with the protocol, previous pharmacotherapy for pain was stopped. Physiotherapy, massage and manual therapy were not used during treatment. Research methods
The intensity of pain was assessed using VAS at each week of treatment and 1 month after its completion. The presence of a neuropathic component of the pain syndrome was assessed using the DN4 questionnaire before and after treatment [14]. The Beck Inventory was used to determine the level of depression. To assess the level of reactive and personal anxiety, the Spielberger test, modified by Hanin, was used. The Quality of Life questionnaire (SF 36) used in the study assessed the degree of adaptation disorders of patients [15]. To assess the degree of activity impairment, the Roland-Morris Activity Impairment Scale was used [16].
To assess the functional state of nociceptive and antinociceptive systems before and after treatment, the nociceptive flexor reflex (NFR) technique was used [17, 18]. NFR belongs to the group of protective reflexes and is interesting because it allows you to objectively and quantitatively assess a person’s pain threshold. It has been proven that in a healthy person there is a close connection between the threshold of subjective pain sensation and the threshold for the occurrence of this reflex. This reflex also allows us to assess the state of nociceptive and antinociceptive systems in humans, to study the role and influence of various neurotransmitters and drugs involved in pain control. Description of the NFR technique
The subject should sit in a comfortable chair, legs as relaxed as possible, knees bent at an angle of 130o, and the foot at the ankle joint should be at an angle of 90o. To reduce emotional stress, it is necessary to inform the subject about the conditions of the experiment. Stimulating electrodes are placed behind the ankle or slightly lower along the peroneal nerve, at a distance of 2 cm from each other, the cathode is proximal, the anode is distal. Recording electrodes are placed on the abdomen of m. biceps femoris capitis brevis (cathode) and on the tendon of this muscle (anode). The ground electrode is placed midway between the stimulating and recording electrodes. The stimulus used is a trend (packs) of stimuli with a total duration of 20 ms, with a frequency of 300 Hz and a duration of each stimulus of 1 ms. To avoid habituation, it is recommended that the stimulus packets be presented in an irregular order. The study begins with low-intensity stimuli, gradually increasing it, and observing the appearance of muscle responses. When a response appears, its threshold (reflex threshold - Pr) is recorded, i.e. the magnitude of the electric current at which it appeared. The threshold of subjective pain (pain threshold - Pb) is also recorded, i.e. the magnitude of the electrical stimulus at which the patient first indicates the appearance of localized acute pain in the area where the stimulating electrodes are located. In healthy individuals, the pain and reflex thresholds usually coincide or the first is slightly lower than the second. To accurately determine the relationship between pain and reflex threshold, the coefficient is calculated - pain threshold/reflex threshold (Pb/R), which in healthy people is approximately 0.9-1.0.
After treatment, a general assessment of the results of therapy was carried out by the patient and the doctor using a 10-point system: 0 - no effect, 10 - very high effectiveness. Statistical processing was carried out using the standard software package Statistica 6. Research results
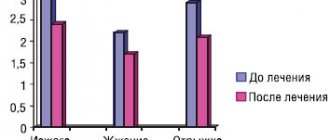
The average duration of the disease in the group ranged from 3 months to 2 years (8.6±5.5 months), the pain intensity was 5.7±1.3. The patients' complaints included the following types of pain: shooting (10%), pressing (33.3%), aching (50%), nagging pain (6.7%). The dynamics of pain intensity according to VAS during treatment with Alflutop by week are shown in Fig. 1. A significant decrease in pain according to VAS was noted already in the 2nd week of treatment. During further treatment, a decrease in pain intensity to an average of 3.2 points was noted. 1 month after completion of treatment, pain intensity did not differ significantly from VAS parameters immediately after completion of the course of injections.
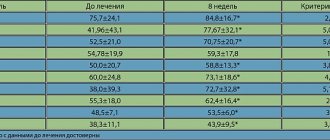
A highly significant decrease in the degree of activity impairment was revealed during treatment according to the Roland-Morris activity impairment scale (before treatment 4.9±2.8, after treatment 3.3±1.4; p<0.009). There were no significant decreases in the severity of anxiety and depression during treatment (Table 1).
According to the SF36 questionnaire, during treatment there was a significant improvement in the quality of life of patients (before treatment 82.2±8.9, after treatment 86.7±4.9; p<0.02). When analyzing the data from the DN4 questionnaire, the average score among the examined patients was 1.93±1.87. The presence of a neuropathic component was detected in 20% of patients. There was no significant decrease in the number of points, according to the DN4 questionnaire, during treatment, but there was a tendency towards its decrease (before treatment 1.93±1.87, after treatment 1.6±1.5; p=0.4) .
According to the NFR study, pain thresholds and nociceptive reflex in the examined group were significantly lower than in healthy people. The Pb/Pr ratio did not differ significantly from healthy people. After treatment, there was a significant increase in pain and reflex thresholds to the level of normative values. The results of NFR before and after treatment are shown in Fig. 2 and in table. 2. According to the doctor’s assessment, the effectiveness of treatment on average for the group was 7.8±1.6 points, the assessment of the effectiveness of treatment by the patient himself reached 7.9±1.7 points.
The drug was well tolerated by patients. No side effects were identified during the study. Discussion
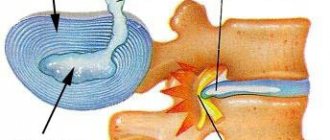
One of the important peripheral pathogenesis factors that predetermine the tendency to chronic back pain is the destruction of cartilage tissue, involving both intervertebral discs and intervertebral joints. It causes persistent biomechanical disorders that contribute to the constant resumption of pain, and provokes further progression of the pathological process in the structures of the spine, closing a vicious circle in spinal osteochondrosis. In this regard, the use of chondroprotectors in the complex treatment of spinal osteochondrosis seems logical. However, at present there are few studies that would confirm the effectiveness of chondroprotective drugs for spinal osteochondrosis [5]. Recent Russian studies of the effectiveness of one of the complex chondroprotectors, the drug Alflutop, have shown that in patients with chronic algic vertebrogenic syndromes, the drug helps to permanently reduce pain, increase spinal mobility and expand the functional capabilities of patients [9, 10]. The therapeutic effect of Alflutop was evident within the first 2 weeks of treatment and increased throughout the course of therapy.
The results of our study using the NPR technique showed that in patients with chronic pain, pain thresholds and nociceptive reflex were significantly reduced with a normal Pb/Pr ratio. This indicates an increase in peripheral nociceptive afferentation and a lack of antinociceptive control in these patients. After the end of treatment, a significant increase in both pain thresholds and reflex was obtained to the level of normative values. These results indicate normalization of the functional state of pain control systems, primarily due to the reduction of nociceptive afferentation. This analgesic effect, obtained in a fairly short period of treatment (3 weeks), allows us to discuss the role of nonspecific inflammation (peripheral sensitization) as one of the known significant mechanisms for maintaining chronic pain [1-3]. Alflutop probably has an analgesic effect in a fairly short time due to components with anti-inflammatory properties. Many studies emphasize that chondroprotective drugs containing glucosamine sulfate, chondroitin, etc., have anti-inflammatory properties and can reduce pain regardless of the structure-modifying effect [4, 5, 8, 11, 12]. However, the use of oral forms of chondroprotective drugs does not always have an analgesic effect [6]. Perhaps, due to the injection form and the content of a complex of components with chondroprotective and anti-inflammatory properties, Alflutop reduces the intensity of pain much faster than oral chondroprotectors. Good tolerability of the drug and its safety are also important, which is its significant advantage over NSAIDs.
One of the results of the study was a significant decrease in the degree of impairment of the patient’s functional abilities during treatment, assessed on the Roland-Morris scale. From our point of view, this result is extremely important, since the fastest return of the patient to daily physical activity is the main factor in the prevention of relapses and prevents the chronification of pain [1-3]. It is known that in case of chronic back pain, prolonged bed rest (“lying down”) and refusal of any physical activity are associated with a poor prognosis in terms of recovery and reduction in pain intensity. Often, patients, most often because of pain and fear of its intensification, choose such tactics (passive coping strategy), which further shapes their “pain behavior,” leading to poor recovery and maladjustment [3]. In our work, we showed that a gradual decrease in pain intensity, starting from the 2nd week of treatment with Alflutop, allowed patients in the study group to quickly restore their motor functional abilities. In general, the treatment led to a significant improvement in the quality of life indicator according to the SF36 questionnaire. Conclusion A course of treatment with Alflutop for 3 weeks (1 intramuscular injection daily) led to a significant decrease in the intensity of pain in the study group of patients with chronic back pain. A decrease in pain was noted from the 2nd week of injections, reaching a maximum at the end of the course. The analgesic effect persisted for 1 month after the end of treatment. As a result of therapy, a significant improvement in the motor functions of patients was noted, which is an important factor in the prevention of relapses and chronic pain. Data from NFR studies indicate the role of peripheral nociceptive afferentation (peripheral sensitization) in the mechanisms of chronic pain maintenance. It can be assumed that the analgesic effect of the drug is associated with a reduction in peripheral nociceptive mechanisms due to the anti-inflammatory properties of this drug. We also cannot exclude the possible analgesic properties of other components of the Alflutop drug. Thus, the results of our study allow us to consider Alflutop as an effective and safe drug for the treatment of chronic back pain, both as monotherapy and in complex treatment. Literature 1. Voznesenskaya T.G. Pain in the back and limbs. Pain syndromes in neurological practice. Ed. A.M.Veina. M.: Medpress, 1999; With. 217-83. 2. Danilov A.B. Pain syndromes. In the book: Neurology. National leadership. 2009; With. 423-41. 3. Podchufarova E.V. Chronic back pain: pathogenesis, diagnosis, treatment. RMJ. 2003; 11 (25): 32-7. 4. Badokin V.V. The importance of inflammation in the development and course of osteoarthritis. Cons. Med. 2009; 11 (9): 91-5. 5. Gorislavets V.A. Structure-modifying therapy of neurological manifestations of spinal osteochondrosis. Cons. Med. 2010; 12 (9): 62-7. 6. Walsh AJ, O'neill CW, Lotz JC. Glucosamine HCl alters production of inflammatory mediators by rat intervertebral disc cells in vitro. Spine J 2007; 7 (5): 601-8. 7. Groppa L.G., Mynzatu I. Karasawa M. et al. Efficacy of alflutop in patients with deforming arthrosis. Wedge. rheumatol. 1995; 3:20-2. 8. Korshunov N.I., Marasaev V.V., Baranova E.Ya. and others. The role of inflammation and assessment of the chondroprotective effect of Alflutop in patients with osteoarthritis according to magnetic resonance imaging of the knee joint. RMJ. 2003; 11 (23): 1320. 9. Levin O.S. and others. Efficacy of Alflutop in vertebrogenic cervicobrachialgia (Open multicenter study). Pharmateka. 2008; 6: 48-54. 10. Levin O.S. The effectiveness of Alflutop in chronic vertebrogenic lumbar ischialgia according to a double-blind, placebo-controlled study. Scientific-practical rheumatol. 2004; 4:80-4. 11. Lukina G.V., Sigidin Ya.A. Experience with the use of the drug alflutop in the treatment of osteoarthritis. Wedge. rheumatol. 1996; 4:40-3. 12. Svetlova M.S. Ignatiev V.K. The use of alflutop in the treatment of patients with osteoarthritis. Wedge. honey. 2004; 82 (6): 52-5. 13. Khodyrev V.N., Golikov L.G. Clinical effectiveness of alflutop for spinal osteochondrosis (12-month study). Scientific-practical neurol. 2003; 3: 104. 14. Bouhassira D et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005; 114 (1-2): 29-36. 15. Raigorodsky D.Ya. Practical psychodiagnostics. Samara: Bakhrakh-M, 2004. 16. Roland M, Morris R. A study of the natural history of back pain: part I: development of a reliable and sensitive measure of disability in low-back pain. Spine 1983; 8: 141-4. 17. Danilov A.B., Danilov A.B., Vein A.M. Nociceptive flexor reflex: a method for studying brain mechanisms of pain control. Journal neuropathol. and a psychiatrist. them. S.S. Korsakov. 1996; 1: 101-7. 18. Sandrini G, Serrao M, Rossi P et al. The lower limb flexion reflex in humans. Prog Neurobiol 2005; 77: 353-95. 19. Wilkens P et al. Effect of Glucosamine on Pain-Related Disability in Patients With Chronic Low Back Pain and Degenerative Lumbar Osteoarthritis JAMA 2010; 304(1):45-52. SOURCE CONSILIUM-MEDICUM NEUROLOGY No. 2/2010