Endocrine therapy for breast cancer: carcinogenic effects of tamoxifen
Tamoxifen has remained the main anti-estrogenic drug for the treatment of patients with hormone-dependent breast cancer (BC) for many decades. The use of this selective estrogen receptor modulator in breast cancer is due to competitive binding to the estrogen receptor. Meanwhile, despite its proven anti-estrogenic activity, tamoxifen is characterized by a number of side effects, in particular it causes pathological proliferation of the endometrium, including polyps, hyperplasia and cancer of the uterine body. The article analyzes the carcinogenic properties of tamoxifen. Further research is needed into the possibility of a personalized approach to endocrine therapy for breast cancer with tamoxifen in order to reduce the risk of developing pathological proliferative processes of the endometrium. Identification of groups at high risk of developing endometrial pathology may help reduce the risk of serious adverse events during tamoxifen therapy.
Introduction
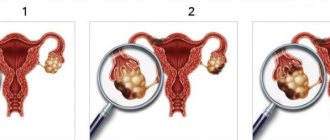
Currently, tamoxifen is the standard treatment for hormone-dependent breast cancer (BC) in women with preserved ovarian function. After five years of taking the drug, the risk of relapse is reduced by 41%, the risk of death from breast cancer by 34%. At the same time, tamoxifen is effective for metastatic breast cancer in pre-, peri- and postmenopause [1, 2]. Tamoxifen has long been considered a safe drug with few serious side effects. However, it later became clear that long-term use of the drug for adjuvant therapy of breast cancer is dangerous due to the development of secondary malignant neoplasms. The nature of the hormonal activity of tamoxifen is complex and depends on many factors, including the characteristics of the target organ, endogenous estrogen levels and pharmacogenetics. The antitumor effect of the drug is based on the formation of a complex with the estrogen receptor. This in turn leads to inhibition of tumor cell proliferation and, as a consequence, a decrease in the likelihood of recurrence and metastasis of a hormone-dependent breast tumor. It has been established that, despite reducing the risk of recurrence of hormone-positive breast cancer, as well as the risk of developing contralateral breast cancer, tamoxifen increases the risk of developing tumors in other locations by more than four times. This is especially true for postmenopausal patients, for whom taking tamoxifen for five years or more is associated with the development of uterine cancer and sarcoma, which are characterized by an unfavorable prognosis [3].
Mechanisms of tamoxifen-associated carcinogenesis
Tamoxifen-associated carcinogenesis is based on its estrogenic, epigenetic and genotoxic effects. The estrogenic mechanism is characterized by stimulation of the proliferation of endometrial cells expressing atypical variants of estrogen receptor alpha, as well as the membrane receptor GPR30 (GPER, GPER1), which activates G proteins [4, 5]. Tamoxifen is able to have a stimulating effect on cell growth in endometrial pathology due to partial agonism of estrogen receptor alpha or through GPER1, which is overexpressed after long-term treatment with tamoxifen [5]. This process promotes the activation of MMP-2/9, which leads to transactivation of EGFR. The GPER receptor can also sequentially activate MAPK and PI3K/Akt, inducing the expression of several genes associated with cell survival, proliferation, differentiation, migration, and invasion. Most likely, the estrogen-like effect of tamoxifen is realized through this mechanism [6].
Data on the correlation between the duration of hormone therapy with tamoxifen and the expression of GPER have already appeared in the scientific literature. In addition, tamoxifen promotes cytoskeletal remodeling and migration of endometrial cancer cells [7].
The epigenetic mechanism is based on the ability of tamoxifen to induce hypermethylation of the MGMT (O6-methylguanine-DNA methyltransferase) promoter. As a result, K-RAS and P53 mutations are formed, which cause an unfavorable prognosis for tumors that develop while taking tamoxifen [4]. High-frequency P53 mutations, which act as promoters of sporadic malignancy driver genes (PTEN and K-RAS), are associated with DNA changes in the form of microsatellite instability [8].
Evidence that P53-positive endometrial tumors are more common in the group of patients receiving tamoxifen (31.4 vs. 18.2%; p = 0.05) and less likely to express estrogen receptors (60.8 vs. 26.2%; p = 0.05); p ≤ 0.001), confirm the epigenetic mechanism of tamoxifen-induced carcinogenesis [9]. However, it is possible that endometrial cells with pre-existing mutations have an advantage in growth and proliferation [10]. This explains the fact that postmenopausal women and women with typical risk factors, including obesity, are at higher risk of developing tamoxifen-associated endometrial cancer than premenopausal women without risk factors.
The genotoxic mechanism of the carcinogenic effect of tamoxifen is based on the formation of derivative DNA adducts [4]. Tamoxifen is a prodrug that, when ingested, is broken down into the active metabolites N-desmethyl-tamoxifen, 4-hydroxy-N-desmethyl-tamoxifen (endoxifen) and 4-hydroxy-tamoxifen via the cytochrome P450 (CYP) system [11]. In a number of studies, polymorphic forms of P450 were found in the endometrium, capable of generating reactive metabolites of tamoxifen, which bind to DNA in the endometrium, causing a genotoxic effect and, accordingly, provoking the development of endometrial cancer [12].
Research results are contradictory. Analysis of DNA adducts in endometrial tissues of women with breast cancer taking tamoxifen as endocrine therapy did not show convincing results. The formation of DNA adducts in endometrial tissues was detected at extremely low levels and only in a few patients [10]. However, S. Shibutani et al., as well as EA Martin et al. succeeded in detecting substances identified as trans- and cis-epimers of R-(N2-deoxyguanosinyl) tamoxifen in the endometrium of women receiving tamoxifen therapy [13, 14]. Such reactive metabolites can be detected in endometrial, myometrial and breast tissues in small quantities even after a single dose of tamoxifen [15].
Thus, the described mechanisms indicate that tamoxifen is a carcinogen with the potential to increase the risk of developing metachronous tumors.
Clinical trial data
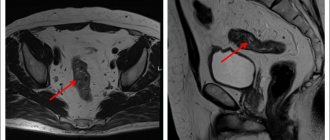
The incidence of endometrial cancer during five years of tamoxifen use does not exceed 0.3% [16]. However, some data suggest that patients with tamoxifen-induced endometrial cancer may have lower survival rates [9].
The first studies of the carcinogenic effect of tamoxifen on the endometrium appeared in the late 1980s, but these were isolated observations or analyzes of relatively small groups of patients.
B. Fisher et al. in 1998, published data from the NSABP-P-1 study (National Surgical Adjuvant Breast and Bowel Project P-1 Study) regarding chemoprevention of breast cancer. In the study, tamoxifen reduced the risk of breast cancer by almost 50% in 13,388 randomized women (moderately high risk) while increasing the risk of endometrial cancer (hazard ratio (HR) 2.53; 95% confidence interval (CI) 1.35–4.97). The increased risk of developing endometrial cancer occurred primarily in women aged 50 years and older. In all observations, endometrial cancer in the tamoxifen group was detected at stage I; no cases of death from endometrial cancer were recorded. In this study, there was no increase in the incidence of liver, colon, ovarian, or other cancers [17].
In the randomized ATLAS (Adjuvant Tamoxifen: Longer Against Shorter) trial, which included 12,894 breast cancer patients, an increase in the risk of endometrial cancer was also noted during a ten-year period of treatment with tamoxifen (RR 1.74). The cumulative risk of developing endometrial cancer over 5–14 years was 3.1% and the risk of death was 0.4% for women prescribed long-acting tamoxifen therapy. In the control group, similar figures were 1.6 and 2%, respectively (an absolute increase in mortality of 0.2%). But in general, the clinical advantage of prolonged use of tamoxifen in terms of the effectiveness of breast cancer treatment turned out to be significantly higher than the risk of side effects. The maximum benefit from prolonged use of tamoxifen in the form of a reduction in the risk of death from breast cancer by 2.8% was obtained by premenopausal patients. They had a disproportionately lower risk of death from endometrial cancer (0.4% in women who took tamoxifen for ten years, 0.2% in those who took tamoxifen for five years) [18].
An increased incidence of endometrial cancer was also the most serious side effect of long-term tamoxifen treatment in the aTTom (adjuvant Tamoxifen – To offer more?) study. A total of 6934 patients participated in the study. The analysis of the study results was carried out twice: in 2008 and 2013. The first assessment of the incidence of endometrial cancer showed a twofold increase in rates with ten years of tamoxifen compared with five years (76 versus 35 cases, respectively) [19]. According to the results of the second analysis, 102 cases of endometrial cancer were registered with ten years of treatment and 45 cases with five years of treatment (RR 2.2). Thus, over a ten-year period of drug use, the risk of death increased (p = 0.02). Death from endometrial cancer was recorded in 37 (1.1%) and 20 (0.6%) patients, respectively [20]. It is clear that the duration of tamoxifen therapy significantly affects the risk of developing endometrial cancer.
In the large STAR (Study of Tamoxifen and Raloxifene) study conducted by the NSABP Breast and Colorectal Cancer Study Group, 19,747 postmenopausal women (mean age 58.5 years) with an increased risk of developing breast cancer received oral tamoxifen (20 mg/day). day) or the antiestrogen raloxifene (60 mg/day) for five years. There were more cases of endometrial cancer in the tamoxifen group than in the raloxifene group (RR 0.62; 95% CI 0.35–1.08). The annual incidence of endometrial cancer was 1.99% with tamoxifen and 1.25% with raloxifene (RR 0.62; 95% CI 0.35–1.08). The seven-year cumulative incidence was 14.7% in the tamoxifen group and 8.1% in the raloxifene group (p = 0.07). However, endometrial hyperplasia, a risk factor for endometrial cancer, was much more common in the tamoxifen group, and the difference was statistically significant (RR 0.16; 95% CI 0.09–0.29). There were significantly fewer patients undergoing hysterectomy for reasons other than cancer in the raloxifene group (RR 0.39; 95% CI 0.30–0.50). Notably, the study did not describe the clinical outcomes of endometrial hyperplasia. Moreover, there was no increase in the risk of its transformation into endometrial cancer. However, differences between groups of patients who underwent hysterectomy for reasons unrelated to the tumor probably led to an underestimation of the true risk of developing tamoxifen-associated uterine pathology and a decrease in the significance of differences between groups [21, 22].
The randomized, placebo-controlled IBIS-I trial included 7154 both premenopausal and postmenopausal women aged 35–70 years with an increased risk of developing breast cancer. The risk of endometrial cancer was found to increase in the tamoxifen group compared with the placebo group during the first five years of follow-up (RR 3.76; 95% CI 1.20–15.56). However, after stopping treatment, the risk of developing endometrial cancer decreased to normal (for 5–10 years of follow-up RR 0.64; 95% CI 0.21–1.80; for follow-up ≥ 10 years RR 1.40; 95% CI 0 .38–5.61) [23]. Thus, the cumulative carcinogenic effect of tamoxifen is obvious.
Although there is some uncertainty about the gynecological toxicity data in the studies described, the risk of endometrial cancer can be considered a serious adverse effect of tamoxifen therapy. In a meta-analysis that included 32 studies of tamoxifen as endocrine therapy for breast cancer (n = 52,929), the relative risk of developing endometrial cancer in women treated with tamoxifen was 2.7 compared with patients in the control group (RR 2.70; 95% CI 1. 94–3.75). The development of endometrial cancer was recorded in patients in 23 studies. A total of 185 cases of endometrial cancer were reported over a mean follow-up period of 5.4 years. Postmenopausal patients were at greatest risk. A study of the risk of developing tumors in other locations, except breast cancer and endometrial cancer, did not show a statistically significant role for tamoxifen (RR 1.04; 95% CI 0.92–1.17). According to data from 16 studies, a small statistically significant increase in the risk of tumor development was found in the gastrointestinal tract (RR 1.31; 95% CI 1.01–1.69). The remaining studies, including the single largest trial (NASBP P-1), did not report a significant increase in the risk of developing gastrointestinal tumors [24].
One of the latest large meta-analyses by S. Mocellin et al. was devoted to assessing the effectiveness of chemoprophylaxis in 50,927 women with an increased risk of developing breast cancer. Scientists analyzed the results of using selective estrogen receptor modulators (tamoxifen and raloxifene) and aromatase inhibitors (exemestane and anastrozole). Three studies involving 22,832 women reported a reduced risk of breast cancer in the tamoxifen group compared with placebo (RR 0.68; 95% CI 0.62–0.76). In terms of side effects, in two studies involving 20,361 women, tamoxifen was associated with an increased incidence of adverse events compared with placebo (RR 1.28; 95% CI 1.12–1.47). In particular, patients in the tamoxifen group had a significantly higher incidence of endometrial cancer (RR 2.26; 95% CI 1.52–3.38) [25].
In addition to endometrial cancer, tamoxifen has been reported to be associated with an increased risk of uterine sarcoma [26]. Several studies have shown that tamoxifen significantly increases the risk of developing non-endometrioid tumors. Mortality associated with these morphological variants is 2.3–5.4 times higher than with endometrioid cancer of the uterine body [8, 27].
Most tamoxifen-related sarcomas reported in the literature were MMMT (malignant mixed mesodermal tumor) in structure [28].
Adenofibromas, adenosarcomas and carcinosarcomas (malignant mixed Müllerian tumors) have also been described with the use of tamoxifen [29, 30]. There is likely an association between uterine carcinosarcoma and tamoxifen therapy, especially long-term therapy. However, it is not clear whether a similar correlation exists with adenofibroma and adenosarcoma, as only a few cases have been reported. It has been suggested that periglandular stromal condensation, often found in endometrial polyps during treatment with tamoxifen for breast cancer, in some cases can transform into adenosarcoma [31]. Despite this, genomic analysis has shown that there are no global significant differences between tamoxifen-induced, endometrioid or non-endometrioid tumors and tumors arising in patients not taking tamoxifen [32, 33].
conclusions
So, it is obvious that an increase in the frequency of pathological changes in the reproductive organs in women and the development of secondary tumors in various locations are manifestations of the carcinogenic effect of tamoxifen. The presented data may be useful for gynecological monitoring and counseling during treatment with tamoxifen in patients with breast cancer for the purpose of early detection of secondary tumors of the female reproductive system and, possibly, prevention of gynecological cancer in this group of patients. However, the issue of identifying groups of patients with the maximum risk of developing endometrial pathology has still not been resolved, which excludes the possibility of personalizing antiestrogen therapy.
The use of tamoxifen for breast cancer, especially the prescription of prolonged endocrine therapy or endocrine prophylaxis with this antiestrogen, requires a careful discussion of all potential benefits and risks. Because of gynecologic toxicity, it is necessary to find a way to predict carcinogenic effects and determine which women are most likely to receive the greatest benefit with minimal side effects from tamoxifen therapy. It seems necessary to search for additional data to identify groups of patients who are candidates for this type of therapy. An important issue remains the choice of the optimal dose of tamoxifen, taking into account the metabolism of each individual patient.
Thus, the problem of achieving a balance between harm and benefit from a particular therapy is actively discussed by the global oncology community and requires further study.
Conclusion
Tamoxifen is characterized by the presence of side effects, but its clinical significance does not decrease due to this. However, any potential negative impact of therapy on overall patient survival, provided that cure for hormone-positive breast cancer is possible, requires active identification of life-threatening adverse events, such as secondary malignancies. This is extremely important when monitoring patients during or after endocrine therapy and making decisions about the use of tamoxifen not only for therapeutic but also for preventive purposes.
Despite the long history of studying the carcinogenic properties of tamoxifen, especially in relation to the development of pathology of the female reproductive system, a way to prevent them has not been found. Obviously, patients taking tamoxifen should be informed about the risks of endometrial hyperplasia, endometrial cancer and uterine sarcoma, as well as any abnormal vaginal bleeding, spotting from the vagina. Postmenopausal women taking tamoxifen should be closely monitored by oncologists and gynecologists.
It is necessary to develop a strategy for assessing the risks of developing endometrial pathology in breast cancer patients receiving tamoxifen therapy. Such tactics involve studying the initial state of the hormonal status of patients, their individual, as well as structural and molecular genetic characteristics, and characteristics of drug metabolism.