The trend towards an increase in the number of gynecological diseases in women of childbearing age is growing steadily from year to year, and even sadder is the fact that such diseases cause certain difficulties with pregnancy - apparently healthy young women cannot become pregnant for years, and in order to solve the problem they have to go to special centers.
Cost of services in our clinic
| Appointment with a gynecologist with the highest category | 1000 rub. |
| Consultative appointment with a doctor based on test results and ultrasound results | 500 rub. |
| Extended colposcopy | 1500 rub. |
| Amino test for bacterial vaginosis | 300 rub. |
| Medical abortion (all inclusive) | 4500 |
| Make an appointment by phone: 8-800-707-15-60 (toll-free) |
| *The clinic is licensed to remove tumors |
In part, the development of diseases of the female reproductive system is provoked by negative external factors - poor environment, unhealthy food, an abundance of stressful situations in life.
But there are also physiological factors that interfere with the normal process of egg fertilization. One of these factors includes endometrial hypoplasia. What kind of pathology is this and whether it is possible to cure it are the two main questions that will be addressed in this article.
Causes of thin endometrium in women
There is no generally accepted concept of “thin endometrium”. In medicine, it is customary to consider different parameters of endometrial thickness as an unfavorable criterion for pregnancy. Authors in the literature claim that tissue thickness less than 8 mm minimizes the chance of successful implantation of the embryo into the uterine cavity, and the effectiveness of IVF with this parameter is only 15%.
The main reasons for reducing the thickness of the endometrium:
- Chronic inflammation in endometrial tissue
- Inflammatory diseases of the pelvic organs
- Autoimmune endometritis as a consequence of inflammation
- Changes in hormonal balance
- Injuries suffered
- Follicular ovarian cyst
- Polycystic ovary syndrome
- Developmental defects of the uterus
- Previous instrumental abortions
- Adhesions (synechia) in the uterine cavity
- History of medical errors and surgical interventions on the uterus
The causes and treatment of “thin” endometrium are still being actively studied to formulate the most effective protocols for eliminating infertility and the success of IVF.
The Life Medic clinic uses only proven, modern methods of treating “thin” endometrium.
What factors can cause the development of endometrial hypoplasia?
First of all, these are disturbances in the normal functioning of the hormonal system of the female body. The second most important factor for the development of hypoplasia is infections that can be transmitted sexually. Also, the causes of the manifestation of pathology can be: inflammatory processes in the reproductive and genitourinary system, abortions with curettage, hereditary predisposition, circulatory disorders in the pelvic area, ovarian surgery.
Symptoms of endometrial hypoplasia include: late arrival of the first menstruation (after 16 years and older), various irregularities in the menstrual cycle and the very nature of menstruation - overly painful, prolonged or heavy menstruation can be signs of the development of pathology.
Symptoms of hypoplasia include anoragasmia, scanty hair growth in the area of primary sexual characteristics, underdevelopment of the pelvis and genital organs.
Endometrial hypoplasia is often treated with hormonal therapy, laser and current therapy. In some cases, homeopathic remedies and traditional medicine are prescribed.
ONLINE REGISTRATION at the DIANA clinic
You can sign up by calling the toll-free phone number 8-800-707-15-60 or filling out the contact form. In this case, we will contact you ourselves.
If you find an error, please select a piece of text and press Ctrl+Enter
Diagnosis of thin endometrium
Thinning of the endometrium may not be symptomatic. Clinical manifestations largely depend on the causes of the problem. With hormonal imbalances, there will be a delay in menstruation, with inflammation - moderate periodic pain, with anovulation - amenorrhea (absence of menstruation).
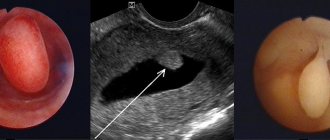
Ultrasound examination allows one to suspect endometrial hypoplasia. However, this alone is not enough. Using an ultrasound, the doctor will determine the discrepancy between the thickness of the endometrium and the phase of the menstrual cycle.
The gold standard for diagnosing thin endometrium is hysteroscopy followed by biopsy. The doctor conducts a visual examination of the uterus using a special device and selectively collects sections of tissue. After hysteroscopy, the material is examined by a pathologist, whose main task is to detect or exclude signs of chronic inflammation. Using modern methods, the number of endometrial receptors that recognize hormones and the number of protrusions (pinopodia) that recognize the embryo are assessed.
Relevance
In the available academic literature, the term “reproductive failures” is increasingly used, which collectively refers to primary and secondary infertility, implantation failures in assisted reproductive technology (ART) programs, miscarriage, and non-developing pregnancy [1].
According to the World Health Organization, the incidence of infertility in the modern world is on the verge of being demographically significant (up to 15%) and has no tendency to decrease [2]. According to official statistics (Rosstat, 2015), its level in Russia is 224.2 per 100 thousand women aged 18–49 years [3]. The loss of potential births associated with female infertility in 2014 amounted to 618.6 thousand. It is well known that the problem of infertile marriage goes far beyond the boundaries of the medical plane, since it entails demographic, social and psychological consequences [3]. On the other hand, the data on the high prevalence of spontaneous abortion in Russia remains disappointing - from 15 to 23% among all cases of registered pregnancies, about 50% of miscarriages occur due to recurrent miscarriage [3]. Interestingly, the risk of losing a desired pregnancy increases with the number of reproductive failures and is 38% after two previous miscarriages [4].
And finally, according to recent data, a common complication after ART is spontaneous abortion (12–29%), with 80% of them in the first trimester [5].
One of the global problems of modern reproductology, far from a final solution, according to researchers, is the disruption of implantation processes caused by a defect in the morphological substrate and/or dysfunction of the endometrial interface [2, 3, 5, 6]. This seems especially important from the point of view of predicting the outcome of pregnancy during in vitro fertilization (IVF), a still elusive goal of reproductive science, which has determined the active search for prognostic markers [5].
A systematic review and meta-analysis showed that with a pronounced lag in endometrial thickness (ET) from the lower normative limit in the middle and at the end of the follicular phase of the stimulated cycle in ART protocols, there is a sharp decrease in the probability of successful implantation, which is one of the reasons for the phenomenon of repeated reproductive failures (RIF – recurrent implantation failure) [7, 8]. It is in this context that maternal age, ovarian reserve, and endometrial receptivity (ER) markers have previously been assessed. Of course, RE is an integral part of implantation, so identification of an accurate marker of implantation would be very useful in ART cycles.
Despite a lot of research in the field of uterine implantology and embryology, today there is no ideal marker of EC. The lack of diagnostic accuracy, prognostic value and invasive nature of histological and biochemical markers of EC limit their clinical applicability [5].
The endometrium is a unique tissue of the body, which in women of reproductive age undergoes regular dynamic changes every month: processes of transformation, repair and regeneration [4]. All these transformations are aimed primarily at preparing for embryo implantation. Changes in the endometrium that occur during the menstrual cycle reflect the cumulative effects of ovarian cycle steroids. The functional layer of the endometrium begins to grow under the influence of estrogen (Es) until it reaches a maximum at the beginning of the peak release of luteinizing hormone (LH), and the pre- and postovulatory increase in progesterone (Pr) is responsible for the secretory transformation of the endometrium, which is accompanied by structural changes in the endometrial pattern [ 9].
It should be noted that, due to its non-invasiveness and universal availability, transvaginal sonography is the most commonly used and quite informative method for assessing ER in ART protocols. During a sonographic study in dynamics, an increase in TE in the follicular phase and its transformation from a hypoechoic strip into a three-layer compact form of a hyperechoic pattern in the postovulatory period can be tracked. It is believed that it is the presence of a three-layer structure of the endometrium on the day of introduction of the ovulation trigger that is associated with an increase in the likelihood of pregnancy [10].
In transvaginal sonography, TE is measured as the maximum distance between the echogenic interfaces of the myometrium and endometrium in a plane drawn through the central longitudinal axis of the uterine body. Today it has been proven that TE<7 mm in the absence of endometrial pattern structure at the time of embryo transfer is considered suboptimal in ART protocols [10].
With regard to sonographic assessment of structure and ER [11], the appearance of a (three-layered) triple endometrial line on the day of the ovulation trigger is defined as class A, or receptive (receptive), while a homogeneous phenotypic appearance, or multi-layered endometrium, is classified as class C - non-receptive (immune).
The secretion of progesterone (Pr) initiates changes in the endometrium, which is reflected in the homogeneity of the connecting uterine zone and the clarity of the central echogenic line, in this anatomical pattern defined as class B [11].
It should be noted that the main parameters used to assess the thickness and structure of the endometrial pattern using traditional two-dimensional ultrasound have a significantly lower diagnostic informativeness and significance compared to three- and four-dimensional ones.
With the advent of appropriate sonographic equipment, additional opportunities have emerged to increase the prognostic value of this research method in ART protocols. These include measurement of endometrial volume and Doppler ultrasonography of uterine and subendometrial blood flow [9, 10].
In light of the above, the results of M.P.’s study turned out to be very interesting. Plyasunova (2018), where the role of hemodynamic disorders in the implementation of the “thin” endometrial syndrome was shown. The author argues that reliable Doppler criteria for the above endometrial dysfunction should be considered an increase in the index of resistance (IR)≥0.80 and the absence of its decrease in the 2nd phase of the menstrual cycle, an increase in the systolic-diastolic ratio (S/D)>4.5 and a decrease in the final diastolic velocity (Vmin) <2.5 cm/s in endometrial and subendometrial vessels [12]. However, TE and endometrial structure still remain the most studied and in demand parameters for assessing its failure.
The first key question naturally arises: what do we understand today by the term “thin endometrium” in the absence of such a diagnosis in the International Classification of Diseases, 10th revision. So, the endometrium is considered “thin” when its thickness during sonographic scanning does not exceed 7 mm [13–18], although, according to other researchers, M-echo 6 [19–21] and 8 mm are also classified as hypoplastic endometriopathies [ 22]. It is this TE that is used to predict the possibility of pregnancy in ART cycles. Regardless of the causative factor, thin endometrium is often associated with low success rates after IVF [22–23].
At the same time, numerous studies have proven the possibility of pregnancy even with TE at the time of embryo transfer of 4 and 5 mm [23–25]. This suggests that RE is not always related to its thickness.
According to K.V. Krasnopolskaya (2016), TE less than 8 mm by the time of completion of the natural (in natural cycles), artificial (when using folliculogenesis inducers) or simulated (with hormone replacement therapy Es) follicular phases should be considered “thin”. But at the same time, the very phenomenon of insufficient development (growth) of the endometrium in the proliferative phase of the cycle without signs of hypoestrogenism should be considered as a manifestation of resistance to estrogen stimulation [26].
Thin endometrium can be the result of various factors, the most common of which are inflammatory and iatrogenic. Poor vascularity and low estradiol levels may also lead to poor endometrial growth. The endometrium can also be inherently “thin” in some women, which many researchers call idiopathic hypoplasia [27].
If we consider the main causes of “thin” endometrial syndrome, we can conditionally divide them into three main groups.
Inflammatory causes: Acute or chronic infection can lead to damage to the basal layer of the endometrium. For example, in India, the most common cause of thin endometrium is genital tuberculosis. Since regeneration occurs during pathological fibrosis, this leads to destruction of the endometrium and obliteration of the uterine cavity. Regeneration of the endometrium even after complete treatment is very difficult, because fibrosis destroys the basal layer of the endometrial pattern, along with it destroying hopes of restoring its receptivity.
Iatrogenic causes: surgical abortion, repeated curettage of the mucous membrane of the uterine cavity damage the basal layer of the endometrium. Hysteroscopic myomectomy, polypectomy or laparoscopic myomectomy with entry (with penetration) into the uterine cavity can lead to intrauterine adhesions.
Idiopathic causes: “thin” endometrium may not necessarily be secondary to the process of disease formation. This may be a result of individual uterine architecture [28] or intrinsic properties of the endometrium that influence its growth [29].
But still, if we are more specific about the gynecological reasons that negatively affect EC, then we should pay attention to the diseases presented in the table [30].
It should be noted that there is still no clear pathogenetic picture of the implementation of the named endometriopathy.
B. Xu et al. (2015) in an attempt to find pathophysiological signs of “thin” endometrium, found that “thin” endometrium is characterized by poor growth of glandular epithelium, high resistance in the uterine arteries, decreased expression of the vascular endothelial growth factor VEGF (Vascular endothelial growth factor) and poor vascular formation [31 ]. High blood flow resistance in the radial arteries may be a trigger that adversely affects the growth of the glandular epithelium and, as a result, a decrease in the level of vascularization in the endometrium. Numerous studies suggest that RE is regulated not only by the local concentration of sex steroids, but also by many factors, including determining endometrial blood flow [31–33].
At the same time, any pathological process that disrupts the functional state of the endometrium is accompanied by a decrease in the production of fertile factors with a violation of the adaptive properties of the body and can lead to disruption of the implantation process.
According to V.A. Krutova (2016), under the “mask” of infertility of unknown origin, extranosological structural and functional changes in the endometrium may be hidden, reducing its receptivity, causing a decrease in its thickness and a discrepancy between the structure of the endometrium and the day of the menstrual cycle [34].
These data do not contradict the results of the study by A.V. Lvovoy (2018), during which it was shown that the pathogenesis of “thin” endometrium is based on dyschronous maturation of endometrial glands against the background of a pronounced depletion of its vascular network, compaction of the stromal component, asynchrony and acceleration of pinopodia maturation, tight intercellular contacts, smooth apical surface, atypical cells of the microenvironment and decreased expression of growth factors such as leukemia inhibitory factors LIF (Leukemia Inhibitory Factor), LIF-R, CD34, VGEF A (Vascular Endothelial Growth Factor) [35].
Of no less interest are the studies of M.I. Bazina (2016), where it was shown that the genesis of the implementation of morphofunctional defects of the endometrium with a violation of its receptivity lies in progressive local inflammation, the latter being triggered by an increase in lymphoplasmacytic infiltration of the endometrium, which contributes to a decrease in the level of expression of receptors for Es in the glands and early overexpression of receptors for PR, leading to the progression of stromal fibrosis of the basal endometrial pattern [1].
Thus, the presented data allow us to conclude that the available literature does not have clear ideas about the pathogenetic mechanisms of the formation of “thin” endometrium.
Speaking about possible diagnostic methods, it should be noted that office hysteroscopy, increasingly used in modern outpatient practice, with regard to the accuracy of verification of chronic endometritis, incl. and “thin” endometrium, demonstrates insufficiently high sensitivity (40%) and specificity (80%) [4]. This method in itself is not a “gold” standard, and it must be supplemented by morphological research.
For indirect assessment of ER, in addition to histological assessment of its structure, receptivity markers such as αvβ3 integrin, mucin-1, LIF, and HOXA10 gene expression have recently been used. An Endometrial Function Test allows you to evaluate the immunohistochemical state of the endometrium using special markers - the mitotic regulator cyclin E and p27 (cyclin E inhibitor). The use of EC markers has not yet found routine use, since the clinical benefit of this diagnostic method is very limited. Perhaps in the future, molecular assessment of EC will become more promising than histological assessment [4].
It should be recognized that there are no absolute morphological criteria for verifying RE disorders.
The world scientific literature describes markers such as integrins, glycodelin, LIF, HOXA genes, heparin-binding factor, factor-like epidermal growth factor (HB-EGF - heparin-binding epidermal growth factor-like growth factor), VEGF, colony-stimulating factor ( CSF – colony stimulating factor), IL-15 and some others. However, none of them is a universal marker of EC. Probably, fundamental science has only yet to come closer to understanding implantation and numerous studies in this area are required [4].
Domestic researchers have proposed to determine the expression levels of LIF, TGF-β1 (Transforming growth factor beta 1) and VEGF in the endometrium using an immunohistochemical method [36] using sets of antibodies and a computer analysis system for microscopic images. In the process of such analysis, the relative area of expression and optical density of LIF, TGF-β1 in the glandular and superficial epithelium and in the endometrial stroma as a marker of EC is assessed [26].
Interestingly, “thin” endometrium is more often observed in women of the older age group, probably due to decreased subendometrial blood flow. The prevalence of this type of endometriopathy has been reported in 5% of women aged <40 years and up to 25% over 40 years in natural cycles [9].
In 2009, as part of a randomized clinical trial [14], the characteristics of ART cycles and their outcomes were studied in 175 patients with TE<7 mm on the day of oocyte retrieval. The study stratified patients into three groups based on TE≥4 and <5 mm; ≥5 and <6 mm; ≥6 and <7 mm. Pregnancy rate (PNR) and implantation rate did not show a statistically significant difference between the compared groups, although a trend towards an increase in the above indicators was still observed as TE increased. The cumulative clinical pregnancy rate (CPR) and miscarriage rate of the study group were compared with that of 5573 patients who underwent IVF during the same period and whose TE was ≥7 mm. It turned out that the CNNB was 26 and 51% (p <0.0001), the miscarriage rate was 31 and 17% (p = 0.02) in patients with TE <7 and >7 mm, respectively. Interestingly, the researchers compared CNBC and live birth rate. In patients with “thin” endometrium, they were further assessed depending on age, number of oocytes retrieved and embryos transferred.
It turned out that significantly better results were obtained when the patients were under 35 years of age, or the number of oocytes retrieved was >5, or the number of embryos transferred was 3 or more. The authors concluded that if TE is <7 mm on the day of oocyte retrieval and the number of embryos is ≥3, then patients should be offered an embryo cryopreservation program [14]. Although this study supports the notion that TE <7 mm reduces the likelihood of pregnancy, results based on stratification by age, number of oocytes, and embryos transferred indicate that TE is not the sole determinant of outcome.
A completely opposite point of view is described in a systematic review and meta-analysis by A. Kasius et al. (2014), covering 1170 patients included in ART programs. The authors believe that TE in ART protocols cannot be used as a marker for canceling the embryo transfer protocol, incl. and cryopreserved [33].
According to these authors, the frequency of “thin” endometrium (≤7 mm) was low and amounted to 2.4% (260 out of 10,724). However, there was also a trend towards a decrease in CNBC and live birth rate in these women (odds ratio [OR] = 0.38; 95% confidence interval [CI] - 0.09-1.5), because the probability of clinical pregnancy with TE≤7 mm was significantly lower than with >7 mm (23.3 vs. 48.1%, or OR=0.42; 95% CI – 0.27–0.67). The positive predictive value for clinical pregnancy was 77%, negative - 48%. From this review we can conclude: TE can predict our likelihood, but cannot be a factor in predicting pregnancy [33].
Meanwhile, it should be emphasized that some authors describe the negative impact of controlled ovarian stimulation on ER and, to improve implantation, recommend cryopreservation of embryos with subsequent transfer to cycles of hormone replacement therapy [14].
In light of the above, it was interesting to study the significance of TE for CCNB and the outcomes of cryopreserved embryo transfer cycles. For example, in studies by T. El-Toukhy et al. (2008) found that TE=9–14 mm, measured on the day of initiation of progesterone support, is associated with higher implantation and CNNB compared with TE=7–8 mm [13].
No less interesting are the results of E. Dix and JH Check (2010), who in a retrospective analysis studied the pregnancy rate in patients with TE<6 mm [37]. Of the 35 patients, only 3 became pregnant and two gave birth. The pregnancy rate in the study cohort was 8.5%.
At the same time, the results of a recent study by the same group of researchers [38], who compared CNNB between fresh and cryoprotocols with TE<6 mm, turned out to be pessimistic; CNNB at TE=4–5 mm in the fresh protocol compared with the cryoprotocol was 10.6 versus 27 .2% (p=0.079).
Egg donation (oocyte donation) cycles are ideal for measuring the independent effect of TE as a parameter of endometrial receptivity because there is lower variability in embryo quality. In connection with the above, the data of L. Dain et al. (2013), who studied the effect of TE on reproductive outcome in oocyte donation cycles using 6.0 and 8.2 mm as the end point for inclusion in the group with thin endometrial syndrome [27]. There were no statistically significant differences in CNNB (29.6 vs. 30.0%) and live birth rate (16.7 vs. 23.6%) in women with TE<6 mm compared with TE>6 mm, respectively. However, the researchers emphasized that in the group with TE no more than 8.2 mm, a higher proportion of live births was observed than in those with a “thinner” endometrium. Perhaps the “thin” endometrium is not able to support the development of pregnancy after implantation, which leads to a greater number of miscarriages due to intrauterine fetal death.
The quality of embryos plays an important role in implantation and is one of the determining factors for pregnancy outcome. JA Gingold et al. (2015) tried to evaluate the relationship between TE and ER on pregnancy outcome after transfer of euploid embryos [22]. Transferring only euploid embryos after preimplantation genetic screening, the authors found that TE (≤8 vs >8 mm) on the day of ovulation triggering did not have a statistically significant correlation with CNBC or clinical outcomes in all age groups (23.4–44.4 years , average – 36.1±4.0 years) neither in fresh nor in cryoprotocols.
Methods used to treat thin endometrium may include hysteroscopic adhesiolysis, cyclic hormonal therapy with two agents - estrogen (E) and progesterone (Pr), vasoactive drugs, and technologies including physical therapy, intrauterine infusion of granulocyte colony-stimulating growth factor and regenerative medicine [ 36]. Of the above methods, treatment with exogenous ES is the most commonly used and convenient, allowing to increase TE and improve reproductive outcomes [1, 12, 39].
It is known that morphological changes in the constituent compartments of the endometrium normally occur during the menstrual cycle under the influence of Es and Pr. Estradiol (E2), the most active Es, binds to estradiol α-receptors and causes an increase in the thickness of endometrial tissue [32].
It should be remembered that it is E2 that provides an increase in tissue sensitivity to PR by inducing the expression of receptors for PR [32]. Endometrial epithelial cells are estrogen sensitive, but they do not proliferate as a result of the direct action of E2. Es act on stromal cells, promoting the synthesis of growth factors of epithelial cells, which leads to an increase in DNA synthesis and the proliferation of neighboring epithelial cells. In contrast to the proliferative effect of E2 Pr, it promotes clear differentiation of the endometrium [32, 40].
The most significant result of this kind of influence of Pr is the inactivation of E2 through stromal receptors for Pr and ensuring the preparation of the endometrium for embryo implantation. The production and secretion of glycogen-rich substances by epithelial cells is also induced by Pr [40].
There are several routes of administration of E2 prescribed for the treatment of thin endometrial syndrome (oral, transdermal and vaginal). Oral administration of E2 is well tolerated by patients, however, it should be noted that after oral administration, E2 is actively metabolized in the intestinal mucosa and in the liver. At the same time, it is easily converted into estrone (E1) and estrone sulfate (E1S) with the blood level of E1 being 3–6 times higher than E2. E1 is a weak Es with low affinity for α- and β-Es receptors. Thus, the so-called primary passage through the liver can significantly reduce the expected activity of E2, while at the same time increasing the thrombotic risk [41].
It is very important to emphasize that, according to some authors, there is a concept of a “minimum period” during which the receptive endometrium can grow [40, 41]. It is 5–7 days, so the duration of the E2 prescription for 10–14 days is not absolute. If TE does not reach the desired parameters during this period, the use of E2 drugs can be continued [42].
The results of numerous domestic studies have convincingly shown that transdermal forms of Es can cause adequate endometrial growth, despite the lower concentration of E2 in the blood compared to oral delivery routes. The researchers concluded that transdermal use of E2 has a beneficial effect on EC [41, 43].
Experts from the Interdisciplinary Association of Reproductive Medicine Specialists (MARS) agree with the opinion stated above.
The protocol states that when TE is detected less than 8 mm on the 21st–24th day of the cycle, cyclic hormonal therapy with two agents has become widespread - estrogen and progesterone. The E2 drug is recommended to be prescribed transdermally, not only in the first, but also in the second half of the cycle. In the second phase of the cycle, a progesterone drug is added to the treatment according to a scheme that coincides with NLF therapy. The dose of estradiol is selected individually depending on the thickness of the uterine mucosa, on average 1–4 mg/day [44]. At the same time, it is important for the clinician to remember that drugs should be prescribed in doses that do not contradict the official instructions for use. In this regard, it should be noted that the transdermal forms of estradiol registered in Russia (Estrogel and Divigel) have different restrictions on the maximum permissible daily dosage. For Estrogel, the maximum daily dose is 3 mg [45], while for Divigel, the maximum permissible daily dose, according to the instructions, is only 1.5 mg/day [51]. Thus, the use of the drug Estrogel in a bottle with a dispenser pump allows the use of higher doses, while remaining within the legal framework. [45].
There are surgical technologies for improving the reparative function of the endometrium, such as the endometrial “scratching” procedure using office hysteroscopy, which involves applying longitudinal scratches (each 20 mm long and 2 mm deep) with biopsy forceps on both lateral and posterior surfaces of the uterus. It has been established that trauma to the endometrium during biopsy enhances the regenerative potential and has a positive effect on the incidence of clinical pregnancy in IVF cycles [4]. Presumably, there are several mechanisms that provide improvement in endometrial receptivity when it is damaged during a biopsy in the proliferative phase. Local trauma likely induces decidualization of the endometrium, thereby increasing the likelihood of implantation. The presence of a wound surface during endometrial biopsy serves as a factor that intensifies repair processes, initiating additional secretion of cytokines and growth factors (IL-11, LIF, HB-EGF) involved in the implantation process [4, 46].
It should be noted that today the search for physical methods of preconceptional preparation of the endometrium in case of disruption of its receptivity continues. For example, irrigation of the uterine cavity with cavitated solutions for hypoplastic endometrium [35, 47], complex synchronous electrotherapy, magnetic laser-light therapy for chronic endometritis with an autoimmune component, leading to an increase in TE and positive dynamics of the morphological picture [48], have been proposed.
In the unique work of E.S. Silantieva (2008) proved the clinical and morphological effectiveness of the therapeutic effect of physiotherapy for “thin” endometrial syndrome. The author made the following conclusion: the use of physical methods of treatment in the complex treatment of reproductive function disorders contributes to its restoration in 55.3% of patients; the pregnancy rate in the first cycle of IVF use in infertile women associated with hypoplastic endometrial dysfunction with previous failures reached 41.1 %, while the frequency of pregnancy carrying to term with habitual fetal loss syndrome in early pregnancy was recorded in 82.4% of patients [48].
Similar positive changes after the use of complex physiotherapeutic treatment were obtained in a study by M.P. Plyasunova (2018). The author showed a significant increase in TE>7.0 mm during the “implantation window”, the disappearance of endometrial heterogeneity, hyperechoic inclusions in the endometrium according to ultrasound, as well as a decrease in IR<0.80 and S/D<4.5 and an increase in Vmin> 2.5 cm/s with Doppler measurements of blood flow in the uterine arteries [12].
Promising for “thin” endometrial syndrome is the use of vitamin E, L-arginine, PRP (Platelet Rich Plasma) therapy, sildenafil citrate for the correction of trophic disorders: the growth of glandular epithelium and improvement of vasculogenesis, expression of vascular growth factors are histologically proven, but there are not enough randomized ones yet clinical trials [39, 49]. It is advisable for “thin” endometrial syndrome, but therapy with aspirin and omega-3 has not been proven for verified insufficiency of uterine perfusion [4, 50].
Conclusion
Thus, options for improving the preparation of women for embryo implantation after failures in ART cycles are actively discussed in the scientific literature, however, from the standpoint of analyzing the “thin” endometrial syndrome, this topic is far from a final solution. There is still no unified methodology for determining EC in the world - all diagnostic markers have an insufficient evidence base in terms of interpretation of results and are difficult to reproduce in routine clinical practice. Improving diagnostic capabilities for EC will make it possible in the future to increase the effectiveness of overcoming reproductive failures associated with thin endometrial syndrome.
Effective methods for treating thin endometrium
There is no single approach to the treatment of patients with thin endometrium. The variety of treatment methods indicates insufficient global knowledge of the problem.
The Life Medic clinic uses groups of drugs that have proven their effectiveness:
- Antibacterial
- Immunostimulants
- Estrogen preparations
- Circulatory stimulants
The approach to patients is individual and aimed at eliminating the cause of endometrial thinning. Girls with anovulation (an egg is not formed) have a tissue thickness of 5-6 mm, but when deciding whether to resume physiological ovulation, the tissue thickens and returns the ability to fix the embryo.
Symptoms of endometrial hyperplasia
The main symptom of endometrial hyperplasia is non-cyclical bleeding . This discharge appears after slight delays in menstruation or during the intermenstrual period. The discharge, unlike normal menstruation, is spotting, in rare cases it can be profuse with clots (this is typical for patients with adolescent hyperplasia). Prolonged bleeding of this nature can lead to anemia (anemia).
The second symptom is the absence of ovulation (due to excess estrogen) and, as a consequence, the absence of pregnancy. In case of any unusual discharge and absence of pregnancy for a long period with constant unprotected sexual activity, you must contact a highly qualified gynecologist, since endometrial hyperplasia does not go away on its own.
How to grow the endometrium for conception?
The search for a clear answer to the question: how to grow a thin endometrium is still ongoing, but some effective techniques are already available in modern reproductive health clinics.
During the normal menstrual cycle, the thickness of the endometrium constantly changes. This is taken into account during treatment.
Normal indicators of endometrial thickness in different phases of the menstrual cycle
| Cycle phase | Changes in endometrial tissue | Cycle day | Normal thickness values (mm) |
| Follicular phase | Bleeding | 1-2 | — |
| 3-4 | 1-3 | ||
| Proliferation | 5-7 | 4-6 | |
| 8-10 | 5-10 | ||
| Luteal phase | Secretion | 11-14 | 8-14 |
| 15-18 | 10-15 | ||
| 19-23 | 10-15 | ||
| 24-28 | 10-15 |
The approach to growing the endometrium should be comprehensive. Therapy is carried out in 2 stages:
- Identifying causes and eliminating them. Most often you have to deal with chronic tissue inflammation and use drugs: antibiotics, immunomodulators, anti-inflammatory drugs. The funds are used both systemically and locally. In case of hormonal changes, the cause of the problem is determined. Adhesions, cysts, and hormone-producing tumors are eliminated surgically.
- Restoring the functionality of the endometrium. A long phase that can take 2-3 months. Traditionally, this stage includes physical therapy. It helps to increase blood circulation, accelerate metabolic processes, rapid regeneration, as well as reflexology and hirudotherapy.
Cystic endometrial hyperplasia
Cystic endometrial hyperplasia is an increase in the number of endometrial tissue cells , which is accompanied by cystic neoplasms. As a result of cystic endometrial hyperplasia, the volume of the uterus greatly increases. Cystic endometrial hyperplasia manifests itself in the same way as other types of hyperplasia (dysfunctional uterine bleeding, lack of ovulation, infertility).
But do not forget that cystic endometrial hyperplasia can occur with virtually no symptoms, so if there is unusual discharge, immediately consult a highly qualified doctor for a quality examination.
Is it possible to get pregnant with thin endometrium?
Pregnancy and thin endometrium are compatible concepts, since it cannot be said that this problem completely excludes the possibility of embryo implantation into the uterine tissue. However, even the attachment of a fertilized egg to the thin endometrium does not guarantee a normal pregnancy with a successful birth, the same applies to IVF.
The main complications that may occur in pregnant women with thin endometrium:
- Spontaneous abortion in the early stages
- Late premature birth
- Delayed physical development of the fetus
- Placental insufficiency
- Preeclampsia (dangerous late gestosis)
This means that when a diagnosis of “thin endometrium” is made, it is better not to take risks, but to undergo a course of treatment before planning a pregnancy.
About endometrial hyperplasia
Endometrial hyperplasia is a growth of the intrauterine layer of the endometrial uterus of a benign nature.
This formation leads to an increase in volume and thickening of the endometrial walls. The cause of this neoplasm is the strong proliferation of stromal and glandular elements of the endometrium. Endometrial hyperplasia can become a favorable background for the development of endometrial cancer . Endometrial hyperplasia can appear in a woman at any age, but most often during adolescence or the premenopausal period, since it is during these periods that hormonal changes occur in the body.