Hemostatic gelatin sponges Cutanplast
Absorbable absorbent hemostatic (hemostatic) sponges Cutanplast based on gelatin 99.3% and Na lauryl sulfate 0.7%.
More details Request price
Hemostatic agents "Emosist"
Hemostatic material Emosist produced by Mascia Brunelli (Italy) is made on the basis of oxidized regenerated cellulose. Used to stop capillary, venous and weak arterial bleeding.
More details Request price
offers a wide selection of hemostatic agents to stop bleeding; one of the effective methods is sponges. The hemostatic agent is presented in the form of a dry porous mass, has a faint vinegary odor and a whitish color. This material is resistant to high temperatures (up to 75 degrees), does not dissolve in organic solutions and water.
Features of wound healing after tooth extraction
Before moving directly to hemostatic sponges, what they consist of and how to use them, you need to understand how the wound heals after removal and what can go wrong here. More on this later.
Why is there bleeding after removal?
Bleeding is a natural reaction to surgery. After all, when a tooth is removed, the smallest capillaries that penetrate the gums and ligamentous apparatus - the periodontium, which attaches the roots to the bone socket - are torn. And with difficult removal, the bleeding may be even stronger, because... The dentist makes incisions in the gums and drills into the jaw bone - the tissue is more damaged.
Bleeding is a natural reaction to surgery
Read on the topic: How long can a tooth hurt after extraction - normal and pathological.
How long does bleeding last normally?
Normally, the blood stops within 15-20 minutes (less often, within 45 minutes) and a blood clot forms in the socket. It seals the hole like a cork, preventing germs from penetrating inside. If the patient has problems that affect blood clotting, then the bleeding time is prolonged, and a clot may not form at all. And this is already fraught with inflammation of the bone socket (the pathology is called “alveolitis”). Therefore, according to indications, the dentist applies a hemostatic drug to the area of the extracted tooth.
On a note! Bleeding may appear again for a short time (and there will be quite a bit of blood) after the anesthetic wears off - if it contained adrenaline. This substance narrows the blood vessels in the intervention area, and as they expand normally, slight bleeding occurs. It goes away quickly.
Why does the bleeding not stop for a long time?
As a rule, after tooth extraction, the dentist says that you should not eat or drink for 2 hours. But few people say that you should not rinse your mouth or suck drinks out of a straw. In the first days, only oral baths are allowed, and it is better to drink in the usual way. Also, do not touch the wound with a brush. It is better to chew food on the healthy “side” of the jaw. Otherwise, there is a risk of moving or “pulling” the clot out of the hole - which will provoke new bleeding or even inflammation, which will result in long-term treatment.
The following groups of patients are also at risk:
- who has not formed a blood clot,
- with blood diseases,
- who takes blood clotting medications,
- having high blood pressure,
- women during menstruation or menopause,
- exposing themselves to physical activity immediately after removal and during the recovery process,
- if there was already inflammation at the extraction site (for example, pericoronitis above a wisdom tooth),
- during complex operations: when several teeth were removed at the same time, or manipulations were performed with increased tissue trauma.
Bleeding may occur during physical exertion after removal.
When you need urgent help
If the bleeding is profuse and does not stop for a long time, if after normalization of the condition the blood begins to ooze heavily again, this is a reason to consult a dentist. In a situation where the patient, in the presence of continuous bleeding, feels weakness, apathy, if the temperature has risen sharply (above 39 degrees Celsius), it is better to call an ambulance or go to the hospital on your own.
Any surgical intervention is inevitably associated with bleeding. At the same time, the main source of blood loss in operations on the liver, spleen and kidney when large vessels are intact is capillary-parenchymal bleeding, to stop which they resort to a variety of hemostatics: local hypothermia, thermo- and ultrasonic coagulation, laser irradiation, the use of hemostatic films, adhesives, gels , sponges, etc. [1].
The use of hemostatic materials alone in practical surgery is limited due to their low hemostatic activity and the occurrence of adhesions in the abdominal cavity. Toxicity, traumatic and antigenic properties, as well as the locally irritating effect of other drugs (for example, based on oxycellulose) contribute to the occurrence of an inflammatory process, necrosis of organ parenchyma, which predisposes to the development of infection [2]. However, according to many authors, sponges based on collagen, the most well-known and long-used traditional hemostatic agent, in combination with various substances, turn out to be the most effective compared, for example, with gauze packing (a physical method of hemostasis) and make it possible to stop bleeding in 1. 5-2, sometimes 3 times faster [3, 4]. In this regard, the development and study of new hemostatic sponges based on collagen, containing various substances that affect hemostasis, are urgent tasks with the goal of increasing the rate of onset of hemostasis, as well as expanding the scope of effective use of application procedures.
Achieving this goal involved consideration of known methods for assessing the effectiveness of the use of local hemostatic agents.
However, during the study of the problem, the presence of generalizing indicators was not revealed that would make it possible to give an integral assessment of the application of the corresponding method. For the most part, quantitative indicators are used here, expressed in the form of bleeding time, changes in sponge mass and similar characteristics. During the course of this work, no other indicators were found. In this regard, the task was set to develop a methodology that would allow assessing the effectiveness of using collagen sponges using quantitative, qualitative and integral indicators.
The purpose of the study was to study the effectiveness of new samples of hemostatic implants based on collagen and hemostatic agents deposited in them; development of a method for assessing the effectiveness of collagen hemostatic sponges in stopping parenchymal bleeding in surgical practice; study of new drugs based on collagen sponges in a comparative aspect.
As materials for experimental studies, samples of hemostatic collagen sponges with a length, width and height of material of 1 cm were used, weighed on a torsion balance, containing: hemoblock 2%; hemoblock 5% [5]; hemoblock and tranexamic acid; platelets and tranexamic acid [6, 7]; cryoprecipitate 10%; cryoprecipitate and tranexamic acid; chitosan and caprofen [8, 9]; tranexamic acid 9%. Hemostatic collagen sponges Belkozin and Zelenaya Dubrava were used as comparative samples.
In vivo experiments
were carried out on 110 white Wistar rats of both sexes weighing 180-200 g. For the study, animals without external signs of the disease were used, which underwent a quarantine regime in the vivarium of the Federal State Budgetary Educational Institution of Higher Education "Kursk State Medical University". All animals were kept under identical conditions on a standard diet.
Experiments on animals were carried out under aseptic conditions in the operating unit of the Department of Operative Surgery and Topographic Anatomy named after. HELL. Myasnikov Federal State Budgetary Educational Institution of Higher Education "Kursk State Medical University".
Operations and manipulations with animals were carried out using general anesthesia (xylazine hydrochloride, zoletil), and removal from experiments was carried out by overdose of anesthesia, in accordance with the Convention for the Protection of Vertebrate Animals Used for Experimental or Other Scientific Purposes (adopted by the Council of Europe in 1986, Strasbourg, France), and Council Directive 86/609/EEC of 24.11.86 on the harmonization of the laws, regulations and administrative orders of the Member States regarding the protection of animals used for experimental and other scientific purposes.
For a comparative assessment of the time to stop bleeding and the amount of blood loss in an acute experiment, rats underwent a median laparotomy under general anesthesia (Fig. 1,
Rice. 1. Simulation of a liver wound. 2
).
Rice. 2. Simulation of a spleen wound.
The liver and spleen were removed alternately into the surgical wound. After careful hemostasis of the edges of the laparotomy wound and its delimitation with polyethylene, a dry sterile napkin with a known mass was placed under the intended area of organ injury.
The liver wound was made on one of its lobes by cutting off the edge in such a way that the length of the wound was 1 cm (tangential to the circumference of the liver lobe), and the depth was 0.5 cm (in the place furthest from the edge) (Fig. 3).
Rice. 3. Application of hemostatic material to the area of the liver wound. The spleen wound at one of its poles was modeled similarly (length - 0.5 cm, depth - 0.3 cm) (see Fig. 1,
2
).
The resulting capillary-parenchymal bleeding was immediately stopped by applying hemostatic material to the wound area. After the experiment, the sponge was weighed (see Fig. 3, 4
).
Rice.
4. Application of hemostatic material to the area of the spleen wound. As a control, we used the above technology without the use of hemostatic materials, using a gauze pad, Belkozin collagen sponge, and Zelenaya Dubrava collagen sponge. Simultaneously with the application of the wound and the application of hemostatic material, time was started using a stopwatch to record the period of bleeding cessation. To determine the time of final bleeding cessation, the moment of cessation of bleeding from the drug or control wound was noted.
The amount of blood loss was measured using the method of E.M. Levite. To do this, the difference in the mass of the sterile material before the operation and after it was soaked in blood during the operation was determined and calculated by the expression:
m2–m1=m,
where m2 is the mass of the material after complete hemostasis (the blood flowing from the wound was absorbed into the material); m1 is the mass of the material before the experiment; m is the amount of blood loss.
The obtained data were statistically processed with the calculation of average values, standard errors of means and significance of differences. Differences for which the p
was less than or equal to 0.05. The significance of the differences was assessed using the Mann-Whitney test, as well as by calculating the confidence interval [10, 11].
In addition, a visualization coefficient was introduced, which made it possible to numerically express the ability of a particular sponge to absorb blood during the corresponding physical process. This coefficient (hereinafter K) was calculated as the difference between the rate of blood flow (ic) from the wound and the rate of its absorption by the sponge (pg). In this case, the rate of blood flow was determined as the volume of blood expended per unit of time, and the rate of blood absorption by the sponge was determined as the ratio of the difference in the weight of the sponge before and after the experiment to the time of bleeding.
TO
=
C
ik–
C
pgmg/s;
In order to track the dynamics of changes in the rate of blood flow per unit of time, we introduced an indicator of the deceleration of blood flow (hereinafter - H), which was calculated as the ratio of the K indicator to the bleeding time.
When studying in an acute experiment on rats in the comparative aspect of the time to stop bleeding on a model of experimental injuries of the liver and spleen using the studied samples, the results presented in table were obtained. 1,
Table 1. Average indicators of time to stop bleeding in a standard liver injury model, average K- and H-indicators (M±m), statistical analysis Note. Here and in the table. 2:* - p - significance of differences in mean values in relation to control groups (1, 2, 3, 4) was calculated using the Mann-Whitney test. Data were considered reliable at p≤0.05; ** - significant differences relative to control groups with a probability of 95% when comparing confidence intervals. CI—confidence interval, TC—tranexamic acid. 2
.
Table 2. Average indicators of time to stop bleeding in a standard splenic injury model, average K- and H-indicators (M±m), statistical analysis
Analysis of the results showed that the drugs have hemostatic properties, significantly reducing bleeding time relative to the control. After a comparative assessment, it was concluded that a highly effective drug is a hemostatic sponge with tranexamic acid 9% ( p
≤0.05), since this drug received high ratings for quality properties (Table 3)
Table 3. Assessment of the qualitative properties of the drug Note.
A - adhesion to the wound surface (adhesion); AbP—absorption capacity in experiments on the liver, mg; AbS - on the spleen, mg; U - ease of use; * - light drug; ** - crumbles, crumbles when cutting or using. Rating scale: 1 - absence of attribute; 2 - unsatisfactory; 3 — satisfactory; 4 - good; 5 - excellent. and has the lowest K-indices both in experiments on the liver (1.072±0.130832) and in experiments on the spleen (1.08±0.044835) with a bleeding time of 50.7±1.9 s and 68.4 ±2.9 s respectively. The least effective drug was a hemostatic sponge with chitosan and caprofer (K = 1.981 ± 0.054921, K = 2.424 ± 0.135248 for the liver and spleen, respectively), which also had a low rating for use, since it crumbled, crumbled when cut, stuck to gloves, was extremely light in weight, and poorly applied to the wound surface. At the same time, the H-index in experiments on the liver in both cases was approximately the same (≈0.02), while the K coefficient and the average bleeding time differed by almost 2 times.
The current situation indicates that, under conditions of the same rate of slowing down blood flow, a sponge with chitosan and caprofer absorbs 2 times more blood volume, since it requires 2 times more time to create hemostasis than a sponge with tranexamic acid 9% . This fact indicates the insufficient effectiveness of the active substance included in this sponge. The results obtained in experiments on the spleen confirm this fact (bleeding time 131.5±15, K=2.424±0.135248).
The next most effective drug is a hemostatic sponge with cryoprecipitate 10%, which, among other things, has one of the highest H-values (H = 0.025) in experiments on a standard spleen injury model (reliable data). This is followed by a hemostatic sponge with cryoprecipitate and tranexamic acid ( p
≤0.05; unreliable differences in splenic injury when compared with Belkozin). With minimal differences, next comes a hemostatic sponge with a hemoblock of 5%, which, like the sponge with cryoprecipitate 10%, has H = 0.025 in experiments on the spleen. It is inferior to a hemostatic sponge with a hemoblock and tranexamic acid (unreliable differences in liver injury in relation to Belkozin, Zelenaya Dubrava). Despite the fact that it has the highest H-value in experiments on the liver (H = 0.035), it also has a fairly high K coefficient, which indicates that it absorbs a large volume of blood while slowing down the rate of bleeding from the wound quite quickly (Fig. 5) .
Rice. 5. Average values of H- and K-indices in experiments on a model of standard liver injury. In experiments on the spleen, this material performs worse: it has one of the lowest H values against the background of a high K value (Fig. 6).
Rice. 6. Average values of H- and K-indices in experiments on a model of standard spleen injury. The hemostatic sponge with platelets and tranexamic acid performed somewhat worse (an unreliable difference in the data is determined only for Belkozin). In seventh place is a hemostatic sponge with a hemoblock of 2% ( p
>0.05 relative to Belkozin; non-significant differences are determined in case of spleen injury with Belkozin, Zelenaya Dubrava).
in vivo studies
in an acute experiment on rats, when studying in a comparative aspect the time to stop bleeding, the amount of blood loss on a model of experimental standard injuries of the liver and spleen, quantitative, qualitative and integral indicators allow us to conclude that all experimental samples have reliable hemostatic properties, expressed to varying degrees. The activity of the latter is associated with the hemostatic agents included in the sponges. Contrary to the expected results, monocomponent sponges turned out to be more effective: hemostatic sponges with tranexamic acid 9% and cryoprecipitate 10% performed better, followed by their combination. The same pattern was revealed with sponges containing hemoblock: a single drug (hemoblock 5%) turned out to be more effective than the combination of hemoblock and tranexamic acid. Apparently, these phenomena are associated with the mechanism of drug preparation, their interaction (destruction), and the nature of the substrate impregnation (layer-by-layer or continuous). The low hemostatic properties of the hemostatic sponge with chitosan and caprofer can be associated with the absorption properties of the polysaccharide included in the composition, which needs time for the fibers to swell.
These samples can be used during operations on parenchymal organs, which will significantly reduce postoperative complications associated with the presence of delayed bleeding. However, to make a final decision, it is necessary to carry out a wider range of studies in order to study such properties as biodegradation, bioinertness, the severity of the adhesive process at the implantation site, the presence of local and systemic effects after using materials. The methodology for assessing the quality of hemostatic sponges developed during the study can be used in similar studies, and the qualitative K-index can serve as a criterion for classifying sponges and act as a marker of the degree of sorption.
Based on the results of the study, we can conclude that all presented samples reliably have hemostatic properties, most of which are superior to analogues available on the market. A new technique has been developed that makes it possible to evaluate the properties of hemostatic sponges in an acute experiment using integral indicators.
The authors declare
no conflict of interest.
Information about authors
Bezhin A.I. (Bezhin AI)
— Doctor of Medical Sciences, Head of the Department of Operative Surgery and Topographic Anatomy named after. HELL. Myasnikov KSMU; Kursk, st. K. Marksa, 3, 305041; e-mail
Soldatova Daria Sergeevna (Soldatova DS)
- doctor - pediatric surgeon of the Regional Children's Hospital No. 2, candidate of the Department of Operative Surgery and Topographic Anatomy named after. HELL. Myasnikov KSMU; Kursk, st. Khutorskaya, 43 "a" 305029; e-mail;
https://orcid.org/0000-0002-9278-2737
Litvinenko I.V. (Litvinenko IV)
— 3rd year student of KSMU; Kursk, st. K. Marksa, 3, 305041; e-mail
Ryzhov A.S. (Ryzhov AS)
— 3rd year student of KSMU;
Kursk, st. K. Marksa, 3, 305041; e-mail
Istranova E.V. (Istranova EV)
— Ph.D., leading researcher at the Institute of Regenerative Medicine, Sechenov University; Moscow, st. Trubetskaya, 8, building 2, 119991;
Istranov L.P. (Istranov LP)
— Doctor of Pharmaceutical Sciences, Professor, Chief Researcher at the Institute of Regenerative Medicine, Sechenov University; Moscow, st. Trubetskaya, 8, building 2, 19991; e-mail: [email protected]
What is a hemostatic sponge
Now let's move on to why a hemostatic sponge is placed in the hole left after tooth extraction? Let's first understand the terminology. “Hemo” means “blood” and “stasis” means stopping. Those. a hemostatic sponge is needed to stop bleeding. In simple terms, this is a kind of replacement for a blood clot. The hemostatic sponge in the tooth socket not only seals the open cavity, but also has a hemostatic effect. Firstly, it puts pressure on small vessels, stopping bleeding. Secondly, it absorbs excess blood, increasing several times. The bleeding stops within 2-3 minutes.
On a note! By the way, local hemostatic agents are used not only in dentistry, but also in other areas of medicine - for example, hemostatic bandages successfully replace tourniquets that are applied for bleeding.
Hemostatic collagen sponge with boric acid and furatsilin
The hemostatic collagen sponge contains a solution of collagen obtained from cattle, boric acid and furatsilin.
The hemostatic collagen sponge is a dry, porous mass of yellow color with a faint odor of acetic acid; it absorbs liquid well and swells slightly.
Pharmacological properties
The hemostatic collagen sponge has a hemostatic and antiseptic effect, stimulates tissue regeneration. Left in a wound or cavity, it is completely absorbed.
Indications for use
The hemostatic collagen sponge is used as a hemostatic agent for domestic injuries to stop capillary bleeding, during surgical interventions in an outpatient or inpatient setting. For filling defects of parenchymal organs and stopping parenchymal bleeding, for tamponade of the dural sinuses, for stopping bleeding from the bone marrow canal, in the practice of an ENT doctor, an ophthalmologist, in dentistry, when completing first aid kits (car).
Application
The hemostatic collagen sponge is removed from the bag, applied to the bleeding area, pressed against it for 1–2 minutes, or the bleeding cavity is tightly tamponed. After soaking in blood, the sponge fits tightly to the bleeding surface. If the bleeding does not stop, a second layer of sponge is applied.
The hemostatic effect of the sponge is enhanced if it is additionally moistened with a thrombin solution. After the bleeding has stopped, the sponge is not removed, as it subsequently completely resolves.
Contraindications
The use of a hemostatic collagen sponge is contraindicated in case of arterial bleeding, intolerance to the nitrofuran series (furacilin).
Release form
Collagen hemostatic sponge is produced in sterile plastic bags measuring 50x50 mm, 90x90 mm, 10 mm x 10 mm with a thickness of 7+2 mm.
Shelf life: 5 years.
The hemostatic collagen sponge has undergone clinical trials in the leading clinics of the country: Research Institute of Traumatology and Orthopedics named after N.N. Pirogov, City Clinical Hospital No. 7, Moscow Dental Institute, 2nd Medical Institute named after N.I. Pirogov, Clinic of Eye Diseases named after V.P. Odintsov, Surgical Clinic KMINGPK, Hospital named after Academician N.N. Burdenko.
INN: Boric acid+Nitrofural+(Collagen)
Appearance and release form
The hemostatic sponge looks like a cube or plate, which looks like a small sponge or a piece of yellow or white foam rubber. Although in fact it consists mainly of natural collagen with various medicinal additives. The plate is quite soft and easy to cut, which allows the dentist to cut the desired size according to the shape of the hole.
The plate is quite soft and easy to cut
Manufacturers produce both small (1x1 centimeter) and large sponges (10x10 cm). The plates are tightly sealed in transparent bags, and the cubes are sealed in dark, opaque bottles. Hemostatic powders and gels are also produced. Absolutely all collagen hemostatics dissolve on their own in the wound over time.
Types of hemostatic sponges
According to the manufacturing method, hemostatic sponges for teeth are of the following types:
- from donor blood plasma - amben,
- from bones/tendons/blood of cattle - collagen and gelatin.
You need to understand that a hemostatic agent not only stops bleeding (due to aminocaproic acid, for example) and stimulates the formation of a blood clot, but also has an antiseptic effect. It can also accelerate regeneration and relieve inflammation. It all depends on the active components that make up the hemostatic sponge. The drug can be impregnated with the following substances:
- antiseptics: chlorhexidine, furatsilin, silver suspension, boric acid, iodoform,
- local anesthetics: for example, lidocaine,
- preparations for bone regeneration: calcium phosphate,
- antibiotics: metronidazole, chloramphenicol, neomycin, gentamicin, etc.,
- natural substances: propolis, eugenol, etc.
Hemostatic sponges for teeth come in different types
Experience in using collagen dressings and Meturacol sponges in surgical practice
The prevalence of trophic ulcers of venous etiology is about 70% of all ulcers of the lower extremities. Trophic ulcers occur in 1–2% of the adult population. Diabetic micro-, macroangiopathy and distal neuropathy cause trophic ulcers in 3% of patients with diabetes mellitus (DM). The number of patients with foot ulcers reaches 15%. Every year, 0.6–0.8% of patients with diabetes undergo lower limb amputation at various levels. Moreover, in 85% of cases, amputations are preceded by ulcers [6, 10]. It is known that the course of the wound process is more complex and varied against the background of diabetes. Diabetic foot syndrome (DFS) is one of the most important problems in modern diabetology. Approximately 40–70% of all non-traumatic amputations are performed in patients with diabetes. During the first 5 years after amputation, up to 80% of patients who have undergone high amputation, i.e. amputation above the knee, die. The risk of developing DDS in patients with diabetes with more than 20 years of disease experience increases to 75%. Gangrenous lesions of the foot in 30–50% of cases result in amputation of the limb. As practice shows, patients with foot ulcers due to DFS are treated in hospital for 6–8 weeks. The average duration of further outpatient treatment for these patients is 4 months, and in 10% of patients it lasts more than 1 year. This is due to the fact that the course of the wound process in patients with diabetes has its own characteristics: a lower rate of epithelization, a tendency to generalize the infectious process, a negative effect on the reparative processes of chronic renal failure [5, 11, 13].
The pathophysiology of chronic wounds is complex and varied, but they all share common features: long-term inflammation that causes extensive tissue damage and impedes healing, and excessive scar tissue formation due to fibroproliferative disorders. The criterion for determining a chronic wound is the period of its existence, which, without signs of active healing, according to various sources, varies from 4 to 8 weeks. [1, 3, 17, 22]. As a result of unrecognized persistent infection or inadequate surgical debridement, acute wounds can develop into chronic wounds at any time. However, in the vast majority of cases, chronic wounds represent the last stage of advanced tissue destruction caused by vascular diseases of a venous, arterial or metabolic nature, or radiation injuries [20, 23].
At the end of the last century, 10 thousand methods of treating trophic ulcers were known [9]. Along with general treatment methods, there are chemical, physical, biological agents and methods of treatment. But, as a rule, these methods do not give the expected effect; many of them require long-term hospital treatment, and some require subsequent surgery to close the ulcer. Although even after the healing of a trophic ulcer has been achieved, the frequency of relapses is often, according to various sources, ranging from 7.3 to 56.2% [24]. Also N.I. Pirogov identified 3 stages of the wound process. Today, the staged approach to wound healing proposed by M.I. is most often used. Kuzin. Traditionally, the main element of local wound treatment is a dressing, which isolates the wound surface from harmful environmental influences and maintains an optimal condition for healing in the wound.
The discovery of antibiotics marked the beginning of a new era in the treatment of purulent diseases. These drugs have cured millions of people. However, in modern conditions, in many cases antibiotics are ineffective due to the increasing resistance of microorganisms to them [12]. In 1962, G. Winter published an article with observations about the preservation of a moist environment in a wound in animals, which, in his opinion, accelerated the epithelization of acute ulcers that did not penetrate the entire depth of the skin by 1.5 times [28]. Subsequently, the theory of wet wound healing was supported by a number of experiments, including on humans. At the end of the twentieth century. The theory of the “ideal dressing” arose, which did not take into account the peculiarities of the course of the wound process. In addition to the principle of moist healing, the management of chronic ulcers must comply with the conditions of thermal insulation, prevent the accumulation of excess exudate, and provide for regular debridement (surgical treatment).
Currently, staged treatment of venous trophic ulcers is generally accepted. In accordance with this concept, it is initially necessary to achieve closure or reduction in the area of the ulcerative defect, improve the condition of the surrounding tissues, and reduce pain and swelling syndromes. The use in practical medicine of scientific data concerning the pathogenesis of wound healing of chronic wounds obtained in recent years allows us to hope for improved treatment outcomes for patients with long-term non-healing wounds.
Modern dressings should keep the wound in a moderately moist state and protect it from trauma, but it must be remembered that excessive moisture causes the death of epithelial cells; when the wound dries, a crust forms, slowing down epithelization; the use of fat-based dressings can lead to the so-called greenhouse effect, when purulent discharge accumulates under the bandage, increasing the absorption of toxic products and the severity of intoxication. The goal of local exposure should be to restore the physiological mechanisms of the wound process, ensuring the delimitation of necrosis, cleansing of the wound surface, and activation of repair processes in the wound. In this case, local treatment does not determine the outcome of the ulcer, but can influence the duration of healing.
Currently, dressings are being developed and improved, not only new drugs, but also entire groups of them are appearing, which allows us to expand our capabilities for treating wounds. At this stage, medicine knows more than 2.5 thousand types of dressings, despite the fact that most of them repeat each other to one degree or another.
In recent years, the so-called method of active therapy for chronic wounds has been formulated, which included the topical application of growth factors and biosynthetic skin analogues. The use of fast-growing epidermal cell grafts can save the lives of many burn patients by providing early and permanent closure of the excised wound. There is also a revision of many ideas about methods of treatment and care of wounds and previously used classifications of local treatments for wounds of various etiologies. Effective and at the same time gentle wound care, maintaining the natural wound healing process, together with a caring attitude towards the patient, are recognized as the basic principles of wound treatment.
These requirements are met by a biosynthesized material – collagen. For more than half a century, this protein has been the subject of close attention of scientists in various specialties (biochemists, morphologists, physiologists and clinicians), which is explained by its important role in ensuring vital processes and pathology of connective tissue. In addition, purified collagen from cattle skin is used as an adjunct to hemostasis during vascular, abdominal and dental surgeries. Collagen fiber consists of groups of fibrils, which in turn consist of even thinner microfibrils, twisted into a triple helix. Such fibers have high tensile strength. Over time, collagen undergoes the aging process in the body, which is due to the formation of cross-links between its fibers. Cross-links are stable, prevent the natural breakdown of collagen fibers, reduce their flexibility, firmness and elasticity.
The technological aspect of studying collagen is of great practical importance. With the help of synthetic materials, it was not possible to solve the complex problems of reconstructive surgery: even relatively inert polymers, remaining a permanent foreign body in the body, maintained a chronic inflammatory reaction and changed their physical properties. Long-term functioning of synthetic prostheses was often impossible.
The most promising material for the manufacture of prostheses, which, acting as a temporary guiding frame for regeneration, would gradually be replaced by the body’s own tissues, is the biopolymer collagen, which has the positive qualities of synthetic polymers and tissue grafts, but is devoid of a number of their disadvantages [8, 16].
The main advantages of collagen as a new plastic material are the absence of toxic and carcinogenic properties, weak antigenicity, high mechanical strength and resistance to tissue enzymes, adjustable rate of lysis in the body, the ability to form complexes with biologically active substances (heparin, chondroitin sulfate, antibiotics, etc.) , stimulation of the regeneration of the body’s own tissues. Collagen belongs to a class of proteins called scleroproteins. A feature of proteins of this class is their phylogenetic relationship in different animal species and humans. The share of collagen in the total mass of body proteins is about 30% (6% of body weight), and it makes up the bulk of the dermis (up to 70% in terms of dry weight).
The term "collagen" is a collective term. It refers to both specific monomeric protein molecules and aggregates of these molecules that form fibrillar structures in the extracellular matrix of connective tissue. Most often, collagen is used in the production of films, sponges, and fibrous materials [19].
Collagen coatings create optimal conditions for wound healing, due to which the inflammation process occurs within physiologically acceptable limits, wound healing time is reduced, which is confirmed by the successful use of collagen for the treatment of burns and wounds. Collagen is able to reduce the activity of proteolytic enzymes (in particular, matrix metalloproteinases) in the wound, thereby stimulating the formation of granulation tissue. Modern collagen coatings are used in the form of sponges for the treatment of chronic wounds at the stage of exudation, granulation and epithelization in patients with purulent-necrotic lesions of the lower extremities, including in patients with DFS. Collagen dressings allow you to treat wounds of varying depths and degrees of complexity, as well as long-term non-healing wounds.
Collagen, thanks to the preserved fiber structure, not only stimulates the body’s natural healing process, but also promotes the rapid and effective growth of new full-fledged tissue at the site of the defect, acts as a matrix for the growth of new tissue: fibroblasts, blood and lymph vessels, nerve fibers from surrounding healthy tissue , introducing themselves into the collagen matrix, they spread strictly along it.
There are no contraindications to the use of these dressings. It should be noted that one more positive property is that collagen bandages do not stain linen, which creates certain convenience for patients and staff.
There are several foreign and domestic collagen dressings on the Russian market, but products from foreign manufacturers are highly expensive, so domestic developments are more accessible. One of the most famous and long-used collagen dressings is the Meturacol sponge. It was developed by scientists from the I.M. MMA. Sechenov in the 1980s. and its properties are not inferior to foreign analogues. Clinical trials of Meturacol were carried out at the Children's City Clinical Hospital No. 9 in Moscow (for the treatment of burn injuries in children injured in the disaster in Bashkiria in 1989), the Moscow Medical Dental Institute. M.A. Semashko (in the practice of treating purulent wounds), clinics of the Medical Center of the Administration of the President of the Russian Federation. This is a combination drug consisting of collagen from bovine split (a product obtained from the skin) of cattle, and methyluracil; It is a finely porous dry plate of white color with a slight specific odor. The drug has a porous structure with capillary activity, which promotes the constant absorption of wound secretions and necrotic cells, bacteria and fibrinous plaque, and accelerated formation of granulation tissue. Methyluracil, along with weakening exudative and alternative manifestations of the inflammatory process, accelerates tissue regeneration, healing of wounds and burns.
At the surgical department of the Doctor 2000 Medical Center, the experience of treating trophic ulcers using the Meturacol collagen sponge was analyzed. The planned tasks were:
- understand whether a constant moist environment is provided in the wound;
- ensure prevention of secondary infection;
- evaluate the possibility of rarely changing dressings (on average once every 3–6 days) and the painlessness of dressings;
- determine the timing of healing and rehabilitation of patients with chronic wounds.
The study included 32 patients of the surgical department of the Doctor 2000 Medical Center with chronic wounds of various etiologies: 17 men (53%) and 15 women (47%). Of these, 17 patients had VDS (53%), 11 had chronic venous insufficiency (CVI) (34%), 2 had erysipelas (7%), 1 patient had Martorell’s ulcer, 1 had a trophic ulcer due to chronic arterial insufficiency (Fig. 1).
The age of 21 patients (65.5%) ranged from 38 to 69 years, 11 patients (34%) were older than 69 years. The duration of existence of ulcers is from 3 months. up to 1.5 years. At the time of inclusion in the study, all patients had wound surfaces of a pale pink color, 8 had fibrin deposits and moderate serous-purulent discharge, 14 had areas of light brown, moist or partially rejected scab. All patients had perifocal inflammation of surrounding healthy tissues. There was no epithelialization.
Clinical assessment of treatment results when using the Meturacol collagen sponge was carried out on the basis of visual control of the course of the wound process, the amount and nature of discharge, and the timing of epithelization. The rate of ulcer closure was determined by calculating its area and depth in millimeters once every 10 days for 7 weeks. The study included wounds with an area of more than 30 mm2. The area of the wound and its changes during treatment were assessed quantitatively using transparent film at the beginning and after 3 weeks. treatment and at the end of the study. Clinical and laboratory studies were carried out before, during and after the course of treatment, which included de-escalation antibacterial therapy (1-2 drugs), taking into account the determination of the sensitivity of the microflora during a microbiological study of culture from the wound.
After treating the skin and cleaning the wound, the Meturacol collagen sponge was cut to the size of the wound and applied to it. It should have covered the entire surface of the wound defect (Fig. 2).
The collagen sponge Meturacol had a prolonged healing effect on the wound; a moist environment was created by the final application of the Krupoderm dressing. The collagen plate turned into a gel, the remnants of which were removed with a sodium chloride solution during subsequent dressings, which was painless for the patient. On wounds with little or no exudation, a Meturacol sponge was applied, after moistening it with a 0.9% sodium chloride solution.
Dressing was done once every 2–3 days, and if the wound was stable, the Meturacol sponge remained on the surface of the wound for up to 7 days. Against the background of antibacterial therapy and the use of Meturacol, in no case was there an increase in wound discharge under the dressing; by the second dressing, the phenomena of perifocal inflammation were stopped, and the amount of discharge decreased. With significantly exuding wounds in 4 patients (12%), dressings were changed daily until the wound discharge decreased; in other cases, due to the lack of discharge, dressings were performed once every 2–7 days. The period of epithelization was 21–57 days after the start of treatment. In 18 patients (56%), the wounds healed under a thin crust of exfoliated epidermis. In 3 cases (patients with CVI) (9.4%), the formation of a dry thin scab was noted under the dried bandage. In the remaining 11 (34%) patients, marginal epithelization was observed, the rejected fibrin layer and areas of thin wet scab were easily and painlessly removed during staged dressings. At subsequent dressings, melting and peeling of the remaining areas of the scab were noted.
In the 32 patients in this study and in other patients who received a total of approximately 700 Meturacol collagen sponges, no cases of allergic manifestations or intolerance were recorded. The results of observation of clinical cases during the study suggest the safety of the impact on bacterial contamination of wounds in the 2nd and 3rd phases of the wound process when using the Meturacol collagen sponge.
conclusions
The study showed the possibility of using Meturacol collagen dressings in the 2nd and 3rd phases of the wound process, the absence of allergic reactions.
It can be stated that Meturacol collagen sponges:
- simple and easy to use by both doctors and patients;
- provide a constant moist environment in the wound;
- economically profitable (3 times cheaper than imported analogues, do not require frequent changes);
- the painlessness of changing dressings, the absence of side effects, and the effectiveness of use are noted;
- in some cases, the healing time of ulcerative defects is reduced.
In conclusion, we give an example of treatment of patient B., 76 years old, with Meturacol collagen sponges. Diagnosis: type 2 diabetes, insulin-requiring, DFS, trophic ulcer of the left foot (Fig. 3–5).
Manufacturers of hemostatic sponges
Popular manufacturers of hemostatic agents used by dentists in our country are domestic companies. Let's consider them further:
- Alvostaz compress from Omega-Dent: available in the form of cubes, flagella, powders and solutions. The compresses are numbered - “1” with iodoform, “2” with metronidazole and chlorhexidine, “3” with neomycin and chloramphenicol,
- "Gemasept" with gentamicin from the Russian Research Institute of Hematology and Transfusiology of the Federal Medical and Biological Agency",
- “Collagen hemostatic sponge” with silver and boric acid from “Zelenaya Dubrava”,
- “Hemostatic sponge” with furatsilin and boric acid from “Belkozin”.
Indications for use
A hemostatic sponge can be useful after tooth extraction, especially wisdom teeth. Because the “eights” often do not erupt completely, and the dentist has to perform a mini-operation to get to them and remove them along with the roots. When a tooth is removed, a hemostatic agent is placed 3-4 months before implantation to completely eliminate the possibility of complications and problems with the formation of new bone in the socket.
Also, hemostatic sponges or powders are used during operations on the gums1 - plastic surgery, curettage of gum pockets (removal of deep “deposits” of tartar), and when cutting off dead areas of the jaw bone due to osteomyelitis. In the latter case, bone blocks and materials for regeneration of bone substance are additionally planted. In general, the doctor always assesses the patient’s condition in advance - asks about diseases and other conditions, and about the medications taken. If there is a risk of bleeding or poor regeneration, then rest assured, a hemostatic sponge will definitely be placed in the hole.
These sponges are used for bone augmentation
It is important to know! A few days after tooth extraction, it may be necessary to apply a hemostatic sponge if the dentist sees a tendency to worsen. For example, a clot has fallen out or the gums/bone have become inflamed.
How to use the drug correctly
How to use the sponge? The hemostatic sponge is placed into the socket of the extracted tooth only by the dentist. Home use is not recommended due to the risk of infection deep into the tissues. The application procedure is given step by step below:
- tooth extraction or treatment of the hole a few days after removal: in the latter case, purulent masses are removed, irrigation is carried out with an antiseptic,
- a piece of the hemostatic plate is cut off with sterile scissors or a hemostatic cube is taken with tweezers,
- the drug is placed in the hole and pressed with a flat instrument: if the hemostatic agent is pre-saturated with thrombin, the hemostatic effect is enhanced,
- if necessary, an additional piece of hemostatic is applied,
- if necessary, a small tampon is placed on top of the hole (it will be removed the next day),
- suturing the gums - a seam in the shape of the letter “P”: also, if necessary, in most cases you can do without stitches.
A piece of the hemostatic plate is cut with sterile scissors.
Application
Hemostatic agents are used locally to pack the wound when necessary:
- Stop parenchymal and capillary bleeding;
- Close the gallbladder bed after surgery;
- Stop bleeding in the bone marrow canals;
- With tamponade of the sinuses of the dura mater of the brain;
- To strengthen the suture of the intestinal anastomosis;
- To strengthen the suture of the vascular anastomosis;
- Get rid of alveolar bleeding in the tooth extraction cavity.
Before use, the package should be opened with sterile scissors and gloves. Then, remove the gelatin plastic and apply it to the bleeding site, pressing lightly for 1-2 minutes. It can also be used after soaking it in saline or antibiotic.
Is it necessary to remove the hemostatic agent?
Patients often wonder what to do with a hemostatic sponge after it has been placed in the socket of an extracted tooth? How long should I keep it on, and will it dissolve on its own? The rules of conduct are as follows: carefully but thoroughly observe oral hygiene, and also take medications prescribed by the dentist. There is no need to specially remove the hemostatic agent - after all, it consists of components that will completely dissolve during wound healing. And instead of a hemostatic agent, its own tissues form in the hole.
“When my wisdom tooth was removed, they stuffed it into the wound like a piece of foam rubber. I then saw the houses through the seams. I was also surprised - what is it? And at the next appointment, my dentist told me that this is a special sponge that absorbs blood and protects against infection. And when it resolved, I didn’t even notice)) It probably completely disappeared in a couple of weeks.”
Ekaterina R., review from irecommend.com
Dental sponge
Dental sponge "Stimul Oss" with chlorhexidine based on hydroxyapatite and collagen is used for filling bone defects and intraosseous implantation, for contour osteoplasty, in the surgical treatment of periodontitis, periodontal disease, etc.
The dental hemostatic sponge “Stimul Oss” is unique. The collagen sponge, in contact with the wound surface, stimulates the growth of connective tissue, i.e. wound healing. Designed to optimize reparative osteogenesis, prevent jaw atrophy after removal of teeth, cysts, benign tumors, sequestration, as well as increasing bone volume (contour osteoplasty) in order to eliminate its deformation and prepare the mouth for prosthetics. It is also used for intraosseous implantation of supports for dentures and surgical treatment of periodontitis and periodontal disease. Dental sponge “Stimul Oss” is a dry porous material with a base in the shape of a circle or square, soft elastic consistency, white color, diameter 11 mm, welding temperature in water is not less than 45.0 ° C; pH of aqueous extract 5.0–7.5; sorption activity no more than 10 minutes; mass fraction of moisture no more than 18.0% Contains: hydroxyapatite, chlorhexidine, formaldehyde. Hydroxylapatite, which is part of the drug, has a stimulating effect on the activity of reparative osteogenesis in the socket of the extracted tooth. Chlorhexidine is introduced to increase antibacterial activity, and the dosage is selected so that it does not reduce the osteoinductive properties of the entire product as a whole. Indications for use:
- After tooth extraction in order to reduce atrophy of the alveolar edge of the jaw.
- After tooth extraction for the purpose of osteoreparation, which allows earlier intraosseous implantation of supports for dentures.
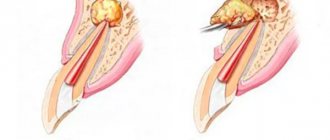
- During resection of the apex of the tooth root for chronic periodontitis, cystogranulomas.
- After removal of jaw cysts (odontogenic and non-odontogenic) and removal of benign jaw tumors (fibroma, adenoma, etc.)
- During sequestrectomy for destructive osteomyelitis of the jaws.
- For radical flap operations for periodontal disease and for filling the periodontal pocket after its curettage for local periodontitis in order to restore the walls of the alveoli.
- When implanting denture supports (to achieve osseointegration).
- For alveoplasty with atrophy of the alveolar process.
- To raise the floor of the maxillary sinus in order to create conditions for intraosseous implantation.
- For contour plastic surgery (hernioplasty, in particular for dentofacial deformities).
The warranty shelf life is 3 years from the date of manufacture. Packaging: double bag made of polyethylene film, combined material or other materials approved for packaging medical products. The product is sterilized upon release by gamma rays at a dose of 1.5 MRAD. Contraindication:
- Polyallergy.
- Allergy to chlorine-containing substances.
- Allergy to collagen-containing drugs.
Instructions for use: After removing a tooth, cyst shell, sequestrum, benign tumor or other pathological tissue, blood clots are removed from the resulting bone defect with a stream of saline solution, a curettage spoon or a gauze swab. After treating the outer surface of the outer package - container with an antiseptic solution (furacilin, chlorhexidine, etc.), it is opened with sterile scissors and the small packaging bag containing a dental sponge is removed with sterile tweezers, the small package is opened with sterile scissors, the GSK-X-GA block is removed with tweezers -50 and inject it into the bone tissue defect. The number of blocks used is determined by the volume of the bone defect. Having ensured hemostasis, the edges of the soft tissue wound over the bone defect are brought together with sutures. After its removal, the tooth socket is filled with 1–2 GSK-Kh-GA-50 blocks to 1/4 of its depth. The edges above the hole are brought together with sutures or applied for 20–30 minutes. gauze swab. The patient is not recommended to eat for 4 hours. With contour osteoplasty and alveoplasty, the periosteum is peeled off from a small soft tissue incision. The resulting cavity (tunnel) is tightly filled with GSK-Kh-GA-50 blocks. The edges of the soft tissue wound are brought together with sutures. STORAGE CONDITIONS "Stimul Oss" in packaging of the enterprise is stored in protected from light, closed, clean warehouses without foreign odors. Manufacturer: JSC Luzhsky, Russia Price: 48.00 rub.
Contraindications and side effects
The only contraindication to the use of a hemostatic sponge after tooth extraction is an individual allergy to any of the components of the drug. In particular, for antibiotics that are used to impregnate the hemostatic agent. Therefore, be sure to tell your dentist if you are allergic and to what medications. Hemostatic agents should also be used with extreme caution in children.
There are no side effects, because There are no toxic substances or components that affect perception in hemostatics. But if itching, inflammation, or swelling appears, feel free to contact your dentist. These manifestations can be caused either by a newly manifested allergy or by incipient complications - if, for example, the treatment was carried out poorly or the patient did not follow the doctor’s instructions.
1Skulean A. Periodontal regeneration, 2012.
Hemostatic sponge - instructions for use
This medication is intended for external use to pack an open wound. The dry substance-solution is applied over the open wound, then wait a few minutes. During this time, the hemostatic sponge fills with blood and the bleeding stops. Its edges fit tightly to the wound, but for greater reliability it is better to use a second sponge on top of the first. When the hemorrhage has stopped, the treatment agent is fixed with a U-shaped suture and a bandage is applied. To enhance the effect, the sponge must be moistened with thrombin solution.
If you use a hemostatic sponge with Ambien, the rules for use are somewhat different. The contents of the bottle are intended for tamponing the cavity of an open wound, and the product itself must be held with a surgical instrument and a gauze swab for 5 minutes. You can leave a layer of gauze in the wound for a short period of time, but it must be removed the next day. A hemostatic sponge after tooth extraction is used exactly according to this principle. The attending physician will advise you on the correct choice of prescription and intensive therapy regimen.
- Tiberal - instructions and indications for use in suppositories or tablets, composition and side effects of the drug
- How is grapefruit beneficial or harmful to the body? Useful properties and contraindications of grapefruit
- Crochet crop top
Side effects
Not all patients are allowed to stop bleeding using a hemostatic sponge, since side effects may occur in the form of allergic, local reactions on the skin. This is itching, burning, redness, increased swelling of the dermis. Therefore, if the body is hypersensitive to the active substances, it is better not to use the drug after surgery and during intensive care. In addition, doctors do not exclude the risk of secondary infection. Detailed instructions for using the hemostatic sponge do not report other side effects.
Contraindications
If the surface of the dermis is damaged, not all patients are allowed to use this inexpensive drug, since there are medical restrictions. For example, in case of arterial bleeding from large vessels after resection, it is better not to use a hemostatic sponge. This drug should be prescribed to a child with caution, but it is strictly prohibited if the body is hypersensitive to the active components. So dissolving the product in the cavity of an open wound does not help all patients, as stated in the detailed instructions.
Storage conditions
The sponge must be stored in a dry place, since with high humidity this medicine will soon become unusable. The instructions say that such a local antiseptic should not fall into the hands of children or be used for other purposes. Self-medication is possible, especially if you immediately need to stop heavy bleeding. There is an expiration date written on the packaging, which is also important not to violate, otherwise you may not get the desired result. The family first aid kit is the best place to store a hemostatic sponge.