What diseases do staphylococci cause?
The content of the article
The main causative agents of purulent-septic diseases are staphylococci (from the Greek "staphyli" - clusters, "kokkos" - kernels, balls, which corresponds to the appearance of these microbes under a microscope) and streptococci (from the Greek "streptos" - chain).
We owe to these microorganisms such diseases as boils, carbuncles, pyoderma, abscesses, phlegmons, panaritiums and other suppurations of the skin. When the mucous membranes are damaged, conjunctivitis, stomatitis, otitis, and enteritis develop.
Severe forms of the disease are pneumonia, osteomyelitis (bone tissue lesions) and septic - generalized inflammatory processes in internal organs when staphylococci and other microorganisms circulate in the blood (blood poisoning) are especially dangerous.
After purulent infections, various complications arise, including those that can lead to a chronic course of the disease and disability (myocarditis, heart defects, thrombophlebitis, peritonitis - inflammation of the peritoneum).
Losses associated with loss of ability to work only from staphylococcal diseases exceed those caused by all infectious diseases, with the exception of influenza. The number of newborns and adults who die from staph infections is more than twice the number who die from other contagious diseases.
Causes of staphylococcus
The cause of the development of almost all staphylococcal diseases is a violation of the integrity of the skin or mucous membranes, as well as the consumption of contaminated food. The level of harm also depends on the strain of the bacterium, as well as the functioning of the immune system. The stronger the immune system, the less harm staphylococci can cause to human health. Thus, we can conclude that in most cases, staphylococcus disease requires a combination of 2 factors - infection inside and disruption of the normal functioning of the immune system.
How is staphylococcus transmitted? Let's look at the most popular ways of contracting staphylococcal infections.
How can staphylococcus enter the body?
Airborne path. During the season of respiratory diseases, frequent stay in places with large crowds of people also increases the risk of infection, not only staphylococcal, but also many other types of infection, incl. viral, fungal. Sneezing, coughing - such symptoms serve as a kind of beacons, from which healthy people, if possible, need to stay away.
Airborne dust path. Household and street dust contains a large number of different microscopic particles - plant pollen, exfoliated skin particles, hair of various animals, dust mites, particles of various materials (fabric, paper), and all this is usually seasoned with various infections - viruses, bacteria, fungi. Staphylococcus, streptococcus and other types of infection are very often found in dust, and when we breathe such air, it does not have the best effect on our health.
Contact and household path. Typically, infection occurs through sharing personal hygiene items and bed linen, especially if one of the family members is sick. The risk of infection increases when the skin and mucous membranes are injured.
Fecal-oral (nutritional) route. Infection occurs when eating food with dirty hands, i.e. - failure to comply with personal hygiene rules. It is also worth noting that infection through nutritional means is also a common cause of diseases such as botulism, hepatitis and other complex infectious diseases.
Medical path. Infection with staphylococcus occurs through contact with insufficiently clean medical instruments, both during surgical interventions and during certain types of diagnostics, which imply a violation of the integrity of the skin or mucous membranes. This is usually due to the treatment of instruments with a product to which the staphylococcus has developed resistance.
How can staphylococcus seriously harm human health, or what weakens the immune system?
Presence of chronic diseases. Most diseases indicate a weakened immune system. If pathological processes are already occurring in the body, it is more difficult for it to protect itself from other diseases. Therefore, any disease increases the risk of a secondary infection, and staphylococcal one of them.
The most common diseases and pathological conditions in which staphylococcus often attacks the patient are: hypothermia, acute respiratory infections, ARVI, influenza, sore throat, pharyngitis, laryngitis, tracheitis, bronchitis, pneumonia, diabetes mellitus, HIV infection, tuberculosis, endocrine diseases and others systems, as well as other chronic diseases.
In addition, the risk of infection with staphylococcus increases:
- Bad habits: smoking, drinking alcohol, using drugs;
- Stress, lack of healthy sleep;
- Sedentary lifestyle;
- Eating unhealthy and unhealthy foods;
- Hypovitaminosis (vitamin deficiency);
- Abuse of certain medications - vasoconstrictors (violate the integrity of the nasal mucosa), antibiotics;
- Violations of the integrity of the skin, mucous membranes of the nasal cavity and mouth.
- Insufficient ventilation of rooms in which a person often stays (work, home);
- Work in enterprises with high air pollution, especially without protective equipment (masks).
Antibiotic resistance of staphylococci
The more we attack, for example, pathogens of purulent-septic infections with antibiotics, the more often antibiotic-resistant microorganisms appear.
Under the influence of antibiotics, the inhibitory effect on staphylococci of other microbes - common inhabitants of our body - is disrupted. Moreover, sometimes antibiotics simply stimulate the development of staphylococci and streptococci. Therefore, without medical prescription, the independent use of antibiotics (and other antibacterial agents) in the event of purulent skin lesions should be excluded.
Any treatment for staphylococcal skin pathologies should be prescribed by a dermatologist! Diseases of other organs are treated by appropriate specialists - gynecologists, urologists, therapists.
Why is staphylococcal infection dangerous?
Staphylococcus is especially dangerous in association with viruses and fungi, as well as with concomitant childhood droplet infections that reduce the overall reactivity of the body. It is not without reason that in many diseases local or general complications caused by staphylococci are observed.
Thus, during influenza epidemics, staphylococcal pneumonia is not uncommon, which most often turns out to be the cause of a child’s long-term illness and is the most common prerequisite for death.
Staphylococcal and streptococcal toxins have pronounced sensitizing properties, causing allergies and toxic damage to the heart muscle, kidneys and other important organs. Unlike a number of pathogens of childhood infections, the site of action of which is limited to certain areas of body tissue, staphylococcus and streptococcus are “omnivorous”.
They can cause inflammation of the gallbladder (cholecystitis), toxic dyspepsia and gastroenteritis, inflammation of the joints (arthritis), and genitourinary tract (urethritis and endometritis). In newborns, staphylococcal and other purulent inflammatory phenomena begin with the umbilical wound or other skin lesions that are invisible at first glance (scratches, abrasions).
It must be emphasized that up to 90% of cases of sepsis in young children are associated with staphylococcus.
Treatment of the disease
Staphylococcal infections are difficult to treat, especially in children. The earlier the disease is detected, the greater the likelihood that the child will soon become healthy.
Therapy includes the following activities:
- Local treatment. This is the treatment of rashes and pustules.
- Antibiotics. Staphylococcus is very adaptable and difficult to treat with antibiotics. However, penicillin drugs are often prescribed.
- Lubricating, rinsing - this treatment is effective for staphylococcus in the nose and throat.
- Vitamin and mineral complexes.
- Immunomodulators.
- Blood or plasma transfusions. Prescribed in severe cases, with sepsis.
- Operation. In cases of severe chronic tonsillitis, the tonsils are removed. The operation is also prescribed for multiple skin lesions with profuse inflammation and exudate.
Staphylococcus in pregnant women is a threat to the life of newborns
The source of staphylococcal infection in maternity hospitals, children's hospitals and at home can be purulent lesions of the skin and mucous membranes of adults - the mother, others, and staff. Therefore, it is impossible to separate contagious skin diseases from other human infectious diseases.
Under normal conditions, the skin has protective properties in the fight against microbes, in particular, its secretions contain antibacterial substances. However, nutritional disorders, general diseases, and microtraumas of the skin can contribute to the development of pathogenic microflora on the child’s skin.
Active immunization of pregnant women with staphylococcal toxoid, as practice has shown, does not have a harmful effect on either the course of pregnancy or the development of the fetus, while at the same time reducing the incidence of purulent infections in mothers and newborns by 3-5 times.
At the same time, the mother develops active and the child develops passive immunity to pathogens of such infections as otitis media, pneumonia, skin suppuration, tonsillitis (tonsillitis), laryngotracheitis, sinusitis and other inflammatory diseases of the nasopharynx.
Degrees of staphylococcus
To determine the exact treatment regimen, doctors divided the course of staphylococcal disease into 4 conventional degrees. This is due to the fact that different types of infection, as well as their pathological activity at different times and under different conditions, differ. In addition, this approach to diagnosis distinguishes between a staphylococcal infection and which group it belongs to - a completely pathogenic effect on the body, opportunistic and saprophytes, which practically do not cause any harm to humans.
Degrees of staphylococcus
Staphylococcus stage 1. Localization of infection for collection for diagnosis - nasopharynx and oropharynx, skin, genitourinary system. Clinical manifestations are absent or minimal. With a healthy immune system, drug therapy is not required.
Staphylococcus stage 2. Clinical manifestations (symptoms) are minimal or absent. If there are complaints, a thorough diagnosis is carried out for the presence of other types of infection. If it is determined that another type of bacteria is present in the body, antibacterial therapy is prescribed privately.
Staphylococcus 3 degrees. The patient has complaints. In most cases, antibiotic therapy is necessary, unless the attending physician considers the use of antibiotics to be unjustified. Treatment of stage 3 staphylococcus is usually aimed primarily at strengthening the immune system. If within 2 months the body does not recover, an individual treatment regimen for the infection is developed, incl. using antibacterial agents.
Staphylococcus stage 4. Therapy is aimed at strengthening the immune system, eliminating hypovitaminosis, and intestinal dysbiosis. Before using antibacterial therapy, a thorough diagnosis is carried out to determine the reaction of a particular type of staphylococcus to the drug.
Staphylococcal diseases in young people and children
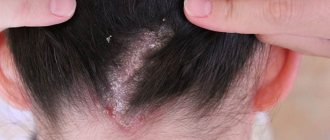
Pustular skin diseases are called pyodermatitis, which, depending on the cause of occurrence, are divided into strepto and staphyloderma. Their mixed form is often observed - streptostaphyloderma.
Streptoderma most often affects children and young men and is usually localized around natural openings - the nose, mouth, ears, that is, in areas subject to irritation by the discharge of these cavities (saliva, mucus). These lesions are very contagious and quickly spread to healthy areas of the skin. Streptoderma is transmitted through toys, diapers, clothes, and underwear.
A fairly common streptococcal skin lesion in newborns is impetigo (from the Latin “impetus” - sudden, attacking). With this disease, flat cavities with a flabby folded covering are formed, filled with serous-purulent contents - phlyctenas.
Merging, they form continuous foci with tortuous inflammatory outlines. Exposed parts of the body are predominantly affected, especially the skin of the face. Nearby lymph nodes often swell, and as the infection spreads, phenomena of general intoxication are observed.
Another form of purulent lesions is neonatal pemphigus. This type of pyoderma is characterized by the formation of large blisters that reach the pigeon egg and are filled with purulent contents. When they rupture, a bare bright red surface (erosion) is formed, which is easily subject to additional infection, resulting in a sharp deterioration in the child’s condition.
Streptococcal pemphigus is extremely contagious, contagious and, in the absence of proper preventive measures, can cause massive outbreaks of pyoderma in maternity hospitals and children's hospitals.
When a child moves home from the maternity hospital, a vesiculopustular form of pyoderma often occurs in places that are most often irritated by sweat (on the back of the head, neck, forehead, groin). Small bubbles with transparent contents (vesicles) the size of a pinhead appear. Then the contents of the vesicles become purulent (pustule) and are surrounded by a halo of hyperemia.
After two to three days, the pustules burst or undergo reverse development and superficial crusts form in their place. Despite the relative ease of this infection, its complications can be very insidious. They are expressed in widespread and severe phlegmon, occurring with significant swelling and subsequent necrosis (melting) of the subcutaneous tissue.
In infants with low nutrition, in improperly bottle-fed children, as well as in older children with weak protective reactions (due to tuberculosis or other types of intoxication), streptococcal infection is not limited to superficial skin lesions, but invades deeper tissues, forming deep ulcers - ecthyma .
They occur on the most frequently injured parts of the body (lower back, lower legs) and are characterized by a sluggish, long-lasting course. After they are healed, scars remain.
Speaking about streptococcal pyoderma in children, we cannot ignore erysipelas, which especially often affects the face (hence the name “erysipelas”). The skin of the face becomes red and swollen. The disease is accompanied by an increase in temperature and sometimes a severe general condition requiring emergency treatment measures.
The so-called “zaeda” is also known - streptococcal phlyctena (a type of impetigo) in the corners of the mouth, which opens and forms a long-term non-healing wound, constantly renewed in connection with facial movements of the facial muscles and food intake. With insufficient sanitary control, seizures can cause real outbreaks in children's groups.
Pemphigus of newborns can also be caused by staphylococci. In the most severe form of this disease, serous fluid accumulates in large areas under the stratum corneum of the skin and detachment of the skin occurs to such an extent that the child gives the impression of being “scalded.”
With staphyloderma, hair follicles are often affected, for example on the head, which leads to a small pustular rash, which disappears without a trace after treatment.
Staphylococci cause not only local, limited skin lesions, but also sore throat, severe pneumonia, inflammatory foci in various organs and tissues (liver, spleen, kidneys), resulting from the spread of microbes through the blood and lymphatic tracts. A generalized staphyloccal infection - sepsis - poses a mortal threat.
Staphylococci can cause real epidemic outbreaks in maternity hospitals and children's hospitals. Due to them, the vast majority of skin and septic lesions in newborns and purulent diseases in mothers occur. Postpartum sepsis, mastitis, pneumonia, meningitis, inflammation of the birth canal, conjunctivitis are a real disaster. Of course, they are especially dangerous for women in labor and newborns.
How does infection occur?
Transmission of pathogens of purulent-septic infections occurs through the air (aerogenously), for example, when shaking bed linen. In parallel, transmission occurs through household items, as well as through food and water. In the latter cases, foodborne illnesses occur.
With regard to nosocomial cases of infection, the most dangerous are the so-called “hospital” strains of Staphylococcus aureus, resistant to external influences and antibiotics.
Staphylococci, being permanent inhabitants of the human body and home, naturally concentrate where there is an increased density of people, where there is less air ventilation and more opportunities for the reproduction and spread of microbes.
These are the conditions that are often created in hospital wards, where staphylococcus spreads with linen, by shaking beds, with dust, and by talking and coughing of people in close proximity. Therefore, such simple measures as ventilating rooms, wet cleaning with disinfectants, using clean linen, timely change of bedding, ultraviolet irradiation of the air or simply access to sunlight significantly reduce the concentration of microbes and the possibility of their dispersion.
Thorough sanitation is necessary - examination and treatment of people with signs of staphylococcal and other similar diseases. In addition to the purulent lesions described above, children may experience specific infectious skin diseases, including fungal ones.
ONLINE REGISTRATION at the DIANA clinic
You can sign up by calling the toll-free phone number 8-800-707-15-60 or filling out the contact form. In this case, we will contact you ourselves.
If you find an error, please select a piece of text and press Ctrl+Enter
Staphylococcus intermedius is a coagulase-positive staphylococcus, the predominant representative of the microflora of the skin and mucous membranes of dogs and the most common causative agent of staphylococcal infections in small animal veterinary medicine over the past 35 years.
Although microbiological studies have shown variability in the biochemical properties of different strains of S. intermedius isolated from animals, routine phenotypic testing provides an acceptable level of diagnostic accuracy for clinical purposes. However, three recent developments have changed our understanding of S. intermedius, and veterinary microbiologists are faced with the challenge of correctly identifying small animal pathogenic staphylococci. First, the increasing prevalence of methicillin-resistant strains of Staphylococcus aureus in small animal veterinary practice and human medicine requires accurate species identification. Second, the use of molecular techniques to analyze isolated staphylococcal strains led to a revision of the taxonomy, and strains isolated from dogs previously classified as S. intermedius were reclassified as S. pseudintermedius. Thirdly, the rapid emergence of methicillin and multi-antibiotic resistant Staphylococcus pseudintermedius (MRSP) strains is a major concern in veterinary practice around the world, including in the UK. This article discusses the basis of recent changes in the taxonomy of the genus Staphylococcus, examining its key features and implications for laboratory diagnostics and small animal veterinary medicine.
STAPHYLOCOCCUS. INTRODUCTION
Most small animal veterinarians encounter staphylococcal skin and wound infections on a daily basis [34]. Most staphylococci are facultative anaerobes, catalase-negative, nonmotile gram-positive microorganisms with cell walls containing teichoic acid and peptidoglycans, as well as 30-40% guanine and cytosine, visible under the microscope in the form of clumps [54]. The main pathogenic species produces coagulase, an enzyme that coagulates plasma by converting fibrinogen into fibrin. The role of coagulase-negative staphylococci as pathogenic microorganisms is relatively small; These are mainly causative agents of hospital infections in weakened animals.
54 species and 24 subspecies have been described, of which Staphylococcus aureus and Staphylococcus intermedius are of greatest importance in veterinary medicine. However, following recent taxonomic changes described below, all strains isolated from dogs (and possibly cats) previously classified as S. intermedius should now be classified as Staphylococcus pseudintermedius. In this review, the term “S. pseudintermedius" refers to strains classified in older sources as S. intermedius (this refers to strains isolated from dogs and cats).
ECOLOGY
Staphylococci, coagulase-positive (Co+) and coagulase-negative (Co-), are normal inhabitants of the skin and mucous membranes of animals and humans. There has been a tendency towards preferential colonization of the skin and mucous membranes of mammals and birds by certain types of staphylococci. Staphylococci that enter the environment from skin and fur can remain viable for several months [53, 79]. In humans, more than 80% of hospital-acquired S. aureus infections are caused by endogenous strains residing in the patient's nasal cavity [78]. Similarly, Pinchbeck and others [58] showed that more than 94% of S. pseudintermedius strains isolated from lesions of dogs with pyoderma were genetically identical to strains found on the normal integument of the same dog.
In dogs, the predominant species is S. pseudin-termedius, which is isolated from the skin and mucous membranes of healthy dogs in 20-90% of cases [2, 14, 17, 28, 32]. The frequency of isolation of individual Co+ staphylococci from the skin and fur of healthy cats is approximately 10% for S. aureus and 45% for S. pse-udintermedius [1, 12, 41].
LATEST REVISION OF THE TAXONOMIC CLASSIFICATION “S. INTERMEDIUM
In a 1992 review of taxonomic classification, Noble cites a 1962 paper entitled “An introduction to chaos: or the classification of micrococci and staphylococci”; At the time of writing this work, only three species of staphylococci were known, namely S. aureus, S. epidermidis and Staphylococcus saprophyticus. The advent of molecular biological methods became the basis for an extensive revision of the classification of staphylococci. Currently, the genus includes 45 species and 24 subspecies, which can be classified into 11 clusters based on the results of 16S RNA gene sequencing and 4 clusters based on the results of gap gene sequencing [24].
From the point of view of veterinary dermatology, it can be stated that the chaos has not gone away, but has only slightly changed its form due to phenotypic intra- and interspecific variability among microorganisms related to S. intermedius. Staphylococcus inter-medius was first described by Hajek [30], who isolated staphylococci, with biochemical properties located “between” S. aureus and S. epidermidis (intermediate strains - intermedius), from pigeons, dogs, minks and horses. It soon became apparent that the majority of Co+ staphylococcal strains isolated from dogs actually belonged to S. intermedius and not S. aureus, as in the previous classification. However, it was later shown that the significant phenotypic variability among S. intermedius, noted by Hajek [30] and some time later by Devriese and van de Kerckove [18], reflects significant genotypic variability [4, 10, 49]. Staphylococcus pse-udintermedius was first described in 2005 after molecular analysis of strains isolated from cats, dogs, horses and parrots. Phenotypic properties were similar to those of S. intermedius and Staphylococcus delphini, species first isolated from dolphins in 1988 [19, 77]. In 2007, two scientific groups published the results of a detailed phylogenetic analysis of collections of strains of “S. intermedius" from Japan [65] and Europe [4], which were very similar; the authors showed that all their strains from dogs, cats, and humans belonged to the species S. pseudintermedius. Most strains isolated from wild pigeons were S. intermedius, and most strains from horses and domestic pigeons were S. delphini. While detailed biochemical testing can differentiate S. intermedius from S. pseudintermedius and S. delphini, the only reliable way to differentiate the latter two species is through molecular testing, such as sequencing of the thermonuclease (nuc) gene or heat shock protein (hsp60) gene [65 ], or restriction by endonuclease MboI of a fragment of the pta gene [5, 67]. The results of these molecular studies confirm the correctness of the term “S. intermedius group”, which includes at least three closely related species: S. intermedias, S. delphini and S. pseudintermedius [4, 24, 67, 70].
All these observations indicate that strains with traditional phenotypic characteristics of “S. intermedius" should be identified as S. pseudintermedius if isolated from dogs. Strains with such properties isolated from other species are best identified as “bacteria of the S. intermedius group” unless assessed by molecular biological methods [33]. Although molecular biological techniques have clarified the taxonomy of S. intermedius group bacteria, phenotypic classification in diagnostic laboratories is still in a state of chaos due to differences in the expression of biochemical properties both within the same species and between species belonging to the S. intermedius group. For example, S. pseudintermedius in culture has been reported to produce acetoin (Voges-Proskauer reaction) when analyzed using the API STAPH kit (Bio Merrier) [19]. In contrast, according to Sasaki and others [65], 28 of 83 S. pseudintermedius strains identified by molecular biological methods do not produce acetoin when tested by standard methods, and testing using the API STAPH kit does not show acetoin production. In addition, this culture is reported to not produce an agglutination factor when tested with rabbit plasma, while, according to Cox and others [13], 55 of 105 strains of “S. intermedius" (and probably S. pseudintermedius) expressed an agglutination factor, which is consistent with the generally accepted view held by many veterinary microbiologists. The previously described data regarding S. intermedius need to be re-evaluated, since some strains previously classified as S. intermedius may very likely belong to S. pseudin-termedius or S. delphini [20].
ANTIBIOTIC RESISTANCE
In the past, most S. pseudintermedius infections in dogs were successfully treated with antibiotics given empirically or based on antibiotic susceptibility testing because of multidrug resistance, i.e. resistance to at least three classes of antimicrobials in addition to β-lactams [11] , at that time it was extremely rare, at least in Europe [27,29, 43, 56, 60]. In the UK, a study of more than 1200 strains of staphylococci isolated in clinical settings from 1987 to 1995 showed no resistance to cephalexin, amoxiclav, oxacillin/methicillin and enrofloxacin [43]. In fact, resistance to any of the first generation cephalosporins has not been reliably confirmed. In Europe, cephalexin resistance was first described in S. pseudintermedius isolated from dogs in a specialized dermatological veterinary clinic in Germany in 2005; resistance to methicillin and several other antibacterial drugs was simultaneously discovered [45].
METHICILLIN-RESISTANT STRAINS OF S. PSEUDINTERMEDIUS (MRSP)
Methicillin, a semisynthetic antibiotic from the penicillin group, was introduced in 1959 to combat β-lactamase-forming staphylococci that are resistant to penicillin. Shortly thereafter, methicillin-resistant S. aureus (MRSA) strains were isolated, primarily in hospital settings. Methicillin resistance is based on the expression of the mecA gene, which encodes a modified penicillin-binding cell wall protein (PBP2a), the low affinity of which for p-lactam antibiotics makes penicillins and cephalosporins ineffective. The mecA gene is located in the staphylococcal chromosomal cassette mec (SCCmec), a large mobile genetic element, with additional genetic determinants often conferring additional resistance to other antibiotics used in clinical practice.
The insertion of the SCCmec element into the genome of S. pseudintermedius strains led to a sharp increase in the prevalence of methicillin-resistant strains across Europe, mainly in the period from 2005 to 2006 [45, 57, 62, 66]. A report from a study of S. intermedius in North America reported that between 2003 and 2004, 57 of 336 strains isolated (17%) were methicillin resistant [50], while between 1995 and 1998 only one strain of staphylococci out of 25 was resistant to methicillin [26]. In Europe, 23% of S. pseudintermedius strains isolated in a dermatology clinic in northern Germany in 2006 were resistant to methicillin [45]. The frequency of isolation of methicillin-resistant staphylococci (n = 61, 7.4% MRSP) was more than 4 times higher than MRSA (n = 15, 18.75% of S. aureus strains isolated), according to the review, 901 Co+ strain of staphylococci isolated from dogs in Germany in 2007 [62]. In a review of 590 samples from dogs submitted to a veterinary diagnostic laboratory in Italy over a two-month period in 2008, MRSP accounted for 10 of the 48 group 5. intermedius strains isolated (21%); all of these methicillin-resistant strains were also resistant to fluoroquinolones, gentamicin, lincosamides, tetracyclines, and potentiated [15], reflecting the acquisition of additional resistance-conferring genes.
Prevalence data in the UK do not appear to have been published in peer-reviewed journals, although a commercial laboratory in Devon, UK, recently reported that MRSP accounted for 14% of the 125 Co+ strains isolated in a 12-month period up to July 2008. [68]. These strains were resistant to more antibiotics than MRSA strains isolated during the same period.
MOLECULAR TYPING METHODS FOR STUDYING MRSP EPIZOOTOLOGY
A number of molecular methods have been developed for efficient and accurate clonal typing of S. pseudintermedius. These methods showed pronounced genetic diversity among methicillin-sensitive S. pseudintermedius strains [63]. The multilocus sequencing-typing method makes it possible to type isolated strains by sequencing internal fragments of numerous constitutive genes (five in the case of S. pseudintermedius at present). When examining each constitutional gene, the different sequences present in bacteria of certain species are assigned to specific alleles, and the alleles at each of the five loci of each strain determine the allelic sequence type (TS) profile; profiles can be easily compared with those stored in online databases. Typing of protein A of S. aureus includes amplification, sequencing and analysis of various regions of the X gene of protein A. The method of pulsed gel electrophoresis consists of hydrolysis of genomic DNA with SmaI endonuclease and subsequent electrophoretic separation of DNA fragments in an agarose gel. Insertion of the SCCmec element into the chromosome of susceptible species causes the emergence of methicillin-resistant staphylococcal lines. SCCmec typing identifies the types of recombinase genes (recombinase genes) along with the gene class :taec and associated regulatory sequences. In contrast to the observed genetic variability of MSSP, a study of MRSP strains showed that a single clone predominates in dogs and cats in Europe, particularly with the sequence type ST71 (MLST)-J(PGFE)-t02(spa)-II-III(SCCmec). This clone has been isolated in Germany, Switzerland, the Netherlands, Denmark, Sweden and Italy and, sporadically, in North America and Hong Kong [4, 7, 37, 57, 63]. One predominant clone, in particular ST68-C-t06-V, has also been found in North America [57, 63]. The current absence of methicillin-sensitive ST71 5. pseudintermedius strains does not support the simultaneous and rapid acquisition of SCCmec by a widespread and successfully evolving 5. pseudintermedius lineage, but rather indicates the rapid expansion of this particular clone. These molecular epidemiological data suggest that when microorganisms are isolated and infection is detected in animals, careful hygienic measures are required to limit further spread.
ZOOANTHROPONOTIC POTENTIAL.
Staphylococcus pseudintermedius is rarely found on human skin, although the incidence of its transmission among people who regularly come into contact with dogs is increasing [25, 29, 32]. Of the 3397 strains isolated from general hospital patients, 3357 were S. aureus and only two were S. pseudintermedius [48], although cases of S. pseudintermedius being misidentified as S. aureus have been reported in medical laboratories for which the latter type was more common [39, 59, 67, 72, 76]. A study of 56 healthy volunteers did not show the presence of S. pseudinter-medius on the nasal mucosa, although in 89% this organism was detected in saliva and dental plaque [55]. In a study by veterinary college staff, the rate of oropharyngeal isolation was less than 1.5% [44, 71], but more recent studies of dog owners show higher numbers. A study of the external nasal passages of 16 owners of dogs with atopic dermatitis and 13 employees of a veterinary clinic, constantly in contact with dogs, for staphylococci revealed one permanent and four temporary carriers of Staphylococcus “intermedius” [32]; strains isolated from humans generally matched strains isolated from dogs with whom they were in contact [25]. In a study of 242 dog and cat owners in Ontario, S. pseudintermedius was isolated from nine people, and indistinguishable strains were isolated from dogs in four of nine carriage cases [31]. Guardabassi and others [29] showed that carriage of S. pseudintermedius on the nasal mucosa was more common among owners of dogs with deep pyoderma (7 of 13) than in people without daily contact with dogs (1 of 13), and that in 6 Of 13 owners, strains were found identical to those isolated from their dogs according to the results of electrophoresis.
The emergence of MRSP strains has led to the identification of carriage in humans in contact with dogs, as well as sporadic cases of infection in humans [8, 23, 39, 69]. MRSP were isolated from five dogs and one cat with infected surgical wounds in a laboratory in the Netherlands [74]. In further studies, MRSPs with the same pattern of resistance were isolated from the nasal cavity of the veterinarian and 3 of 6 assistants, from 4 of 22 environmental samples, and from the nasal cavity of a healthy dog belonging to a staff member who was regularly present at the clinic. MRSP and MRSA were isolated from 3 and 8 of 34 samples, respectively, collected from veterinarians at a university veterinary hospital in Japan, with all 36 samples from non-clinical laboratory staff testing negative [36]. A review of 171 veterinary dermatology clinic staff and their animals in North America found MRSP in nine individuals and MRSA in six [51]. The corresponding MRSP strains were isolated from animals kept in a house where three human carriers lived. In another North American study, nasal mucosal carriage of MRSP was found in 2 of 15 owners of MRSP-infected dogs with the same SCCmec type and antibiotic susceptibility pattern; when repeated sampling two months after treatment, these strains were absent, suggesting temporary carriage of MRSP in humans [21]. In a similar study from the Netherlands, human nasal mucosal samples were positive in 2 of 45 cases, while MRSPs were isolated from about a third of samples from exposed dogs and cats and 44% of environmental samples. environment [75]. These observations clearly indicate the possibility of transmission of staphylococci from humans to dogs and vice versa. Although human contact with S. pseudintermedíus from a dog is likely to result in only transient non-obvious carriage, in rare cases an infection may develop that is difficult to treat in the case of MRSP [69]. In addition, canine MRSP strains should be considered as a potential source of transmission of SCCmec and possibly other mobile determinants of antibiotic resistance of staphylococci living on human skin and mucous membranes [29].
IMPORTANCE FOR LABORATORY IDENTIFICATION
Species differentiation of pathogenic staphylococci is more complex than standard microbiological tests suggest [59, 73]. Before the emergence of MRSA as a canine pathogen, species identification of Co+ staphylococci isolated from dogs was of minimal clinical significance. However, accurate species identification and differentiation of MRSA and MRSP is now necessary due to the significant difference in the zoonotic potential of the two species, and the breakpoints in in vitro susceptibility testing may differ. Initial identification of staphylococci with genus accuracy is possible by colony morphology (smooth, convex, slightly shiny, white to yellow, 1 to 2 mm in diameter after 24 hours of incubation on blood or other nutrient agar at 37 °C [6]) ; microscopy (Gram-positive cocci) and the formation of catalase (this feature allows them to be differentiated from streptococci and enterococci; reviewed by Freney and others [22]).
The formation of DNase and coagulase are important indicators of the pathogenicity of staphylococci. Coagulase expression by three Co+ strains often isolated from small animals (S. pseudintermedius, S. aureus and S. schleiferi ssp. coagulans) can be detected by tube reaction (free coagulase) or glass reaction (bound coagulase or agglutination factor) [ 6]. Although the test tube method with rabbit plasma is considered the "gold standard", the reaction on glass is faster, easier and cheaper. However, even this basic method does not produce uniform results. Research using standard methods shows that only 11-89% of S. intermedius strains give a positive reaction, as opposed to 100% in the test tube method [22, 40], and some Co+ strains may be erroneously classified as Co- due to the lack of sensitivity of the method [12 , 13].
Differentiation between members of the S. intermedius group currently requires molecular biological methods (for example, multilocus sequencing or MboI restriction of the pta gene fragment) [5, 65, 67], however, differentiation of MRSA and MRSP is possible on the basis of a precisely selected series phenotypic parameters. Biochemical properties, especially the ability to ferment sugars, help differentiate Co+ strains. Biochemical properties of S. aureus, “S. intermedius” and S. schleiferi spp. coagulans are briefly described in standard microbiology textbooks [6, 40, 46], and S. pseudintermedius in more recent publications [19, 65]; The methods used in the authors' laboratory are presented in the table.
However, it is well known that none of these methods is 100% accurate, so it is advisable to use several methods in parallel [47, 61]. 21% of the 133 MRSA strains isolated by the authors from animals did not have the classic golden pigmentation (see figure). The Voges-Proskauer reaction is a cost-effective and time-efficient method, although some variability has been noted in its use [65]; this method should give a positive reaction with S. aureus and a negative reaction with the S. intermedius group [6]. The basis for suspicion of MRSP is the typical pattern of antibiotic sensitivity. In the UK, these strains are usually resistant to antimicrobials often used to treat pyoderma in dogs (potentiated sulfonamides, lincomycin, clindamycin, co-amoxiclav, cephalexin, enrofloxacin, marbofloxacin), while MRSA strains isolated from small animals most often sensitive to potentiated sulfonamides, tetracyclines and sometimes clindamycin. Detection of the mecA gene by polymerase chain reaction or its product (PBP2a) by latex agglutination is often practiced in medical laboratories, but not in veterinary laboratories. The molecular typing methods described in the taxonomy section above are not yet widely used in commercial veterinary microbiology laboratories, although for the reasons described below they are essential for accurately differentiating MRSP from MRSA.
Table.
TREATMENT OF INFECTIONS CAUSED BY MRSP
Although methicillin resistance of staphylococci is not always associated with multidrug resistance, most MRSP strains described in the literature are resistant to most drugs used in veterinary medicine. The MRSP clone, predominant in Europe, is generally resistant to β-lactam antibiotics, aminoglycosides, macrolides, lincosamides, tetracyclines, chloramphenicol, trimethoprim and fluoroquinolones and sensitive only to amikacin, fusidic acid, rifampicin, vancomycin, teicoplanin and linezolid , however, none of these latter drugs are licensed for the systemic treatment of small animals [16, 57]. In 2009, the World Health Organization's Advisory Group on Integrated Surveillance of Antibiotic Resistance (AGISAR) released a standard document to help formulate and prioritize risk assessments and risk management strategies associated with antibiotic resistance. According to their classification, antimicrobials are divided into critical, very important and important based on their role as the only therapeutic agent or one of the few alternatives for the treatment of serious diseases in humans, or diseases caused by microorganisms transmitted from other sources (non-human), or microorganisms capable of acquiring resistance determinants from other sources [81].
Amikacin and rifampicin are listed as critical drugs for the treatment of mycobacterial infections in humans, while vancomycin, teicoplanin, and linezolid are listed as critical drugs for the treatment of infections caused by multidrug-resistant MRSA and enterococci. Fusidic acid is the only antibiotic that may be effective against European strains of MRSP isolated from dogs that is not classified as critical; it is on the list of very important drugs for the treatment of MRSA.
Sometimes superficial skin infections and some wound infections respond well to topical treatments in combination with correction of the underlying cause or after removal of foreign material such as sutures or implants. Loeffler and others [45] described resolution of superficial pyoderma caused by MRSP in five of seven dogs after topical fusidic acid and chlorhexidine preparations and ethyl lactate-containing shampoo.
Treatment of deep pyoderma or other serious infections may require off-label use of systemic antibacterial drugs, although there is no evidence of optimal dosage and frequency of use.
These drugs are often not enough. Amikacin is not absorbed from the digestive tract and must be administered by injection. Rifampicin can be given orally, but there is a risk of developing resistance, especially when used as monotherapy [38], and the risk of hepatotoxicity requires regular blood chemistry monitoring. Selected MRSP strains with resistance to rifampicin have been described [57]. While 54 of 57 MRSP strains from North America were susceptible [50], among 25 European strains, only 30–40% were susceptible [15, 16]. This geographic variation highlights the importance of drug selection for MRSP based on in vitro susceptibility testing of individual strains.
Cases of resistance of MRSP to vancomycin, teicoplanin and linezolid are still unknown [57, 62], although Perreten and others have expressed doubts about the appropriate use of these antibiotics in animals. In view of the status of these drugs in medicine as reserve drugs for the treatment of MRSA bacteremia, and the active study of the use of antibiotics in veterinary medicine by the European Medicines Agency (EMEA), the authors are of the opinion that the use of vancomycin, teicoplanin and linezolid in veterinary medicine is not justified.
The medical literature recommends topical treatment to kill the organism as an adjunct to treatment for MRSA infection, although data on its effectiveness is inconsistent. Topical forms of antibiotics, such as mupirocin, fusidic acid or chlorhexidine, are applied to surfaces where microorganisms live to kill MRSA so that less resistant staphylococci can take their place. The results of studies using such treatment of the nasal/anal mucosa in dogs with MRSP have not yet been described, although topical application of fusidic acid to the nasal and anal mucosa has been shown to reduce the carriage of S. pseudintermedius on the skin of healthy Beagles [64]. ]. According to published data, systemic antibiotic therapy with cefpodoxime does not lead to the removal of sensitive Co+ staphylococci from carrier zones [35]; In addition, third generation cephalosporins are critically important antibiotics in medicine.
PREVENTION AND CONTROL OF STAPHYLOCOCCAL INFECTIONS CAUSED BY POLYRESISTANT STRAINS
The spread of 5. pseudintermedius from the skin of dogs and cats is responsible for the frequent detection of this microorganism in the environment of veterinary clinics [74]. If an infection is detected or suspected, or
Carriage of MRSP in animals requires careful precautions to avoid hospital-acquired infections and further spread of this multidrug-resistant bacterium [42]. These measures should include personal hygiene (hand washing, use of masks, gowns and gloves during surgical procedures) and environmental hygiene (regular cleaning and disinfection of all surfaces in the clinic to kill MRSA [52] and other contagious microorganisms [9] as recommended).
To date, no strains resistant to detergents and disinfectants have been found among multidrug-resistant staphylococci; It has been shown that disinfectants common in veterinary practice have an inhibitory effect on MRSP even at low concentrations [3].
CONCLUSIONS
Strains isolated from dogs that were previously classified as S. intermedins should now be classified as S. psendintermedins. The term “S. intermedins group” should be used to refer to strains isolated from other hosts in the absence of molecular testing. The rapid emergence and widespread spread of MRSP in Europe and North America, as predicted by Waller [80], poses a significant challenge to both veterinary and human medicine. Due to the prevalence of staphylococcal infections in dogs, MRSP can significantly impair the ability to effectively treat common skin and soft tissue infections in animals. The phenotypic variability of the S. intermedins group and the need to accurately differentiate between MRSP and MRSA poses a challenge for veterinary diagnostic laboratories, which may require revision and updating of routine laboratory practices. Veterinary practices should implement the strict infection control measures recommended when MRSA is detected to prevent further spread of MRSP, which is a major animal pathogen and is a potential zoonotic microorganism and an emerging reservoir of resistance genes. The emergence of MRSP highlights the importance of prudent use of antibiotics in veterinary practice.
Text of a scientific paper on the topic “What happened to Staphylococcus intermedius? Revision of taxonomic classification and development of drug multidrug resistance.” Authors: R. Bond, A. Loeffler
Article from the website cyberleninka.ru