Biological therapy has taken a strong position in the treatment of immunoinflammatory diseases. This equally applies to inflammatory bowel diseases (IBD), in particular ulcerative colitis (UC). Among the wide range of biological drugs for the treatment of UC, only one has been registered so far - infliximab (Remicade®). In Russia, Remicade® has been used for UC since 2006, and some experience in its use has already been accumulated, although not as significant as abroad. In real clinical practice, doctors are often afraid to prescribe infliximab, do not have the opportunity to do so, or do not know the indications for its use. Therefore, we considered it appropriate to review in this article a number of fundamental studies assessing the effectiveness of infliximab in UC and share our own experience with the drug.
Goals of treatment for UC: • stopping the attack and inducing remission; • maintaining remission without the use of glucocorticosteroids (GCS); • prevention of complications and operations; • improving the quality of life.
The frequency of steroid-refractory and steroid-dependent forms of UC worldwide is 40–50%; For Russia, exact data is not yet available; for the Moscow region this value is about 40%. Some patients also do not respond to treatment with traditional immunosuppressants (azathioprine and cyclosporine). The most important goals of IBD treatment include overcoming treatment resistance and avoiding corticosteroids in steroid-dependent forms of the disease, maintaining remission without their use. This is what infliximab treatment aims to achieve. Indications for the use of this drug, according to the European Consensus (ECCO), are forms of IBD that are resistant to all types of basic therapy, including steroid dependence and steroid resistance [1]. At the expert council on the development of new standards for the use of infliximab in the EU and the USA, held in October 2005 (UC and CD: Treating to New Standards Expert Round Table), it was proposed to include not only steroid-refractory forms of Crohn's disease and UC in the indications for treatment , but also severe forms of these diseases (as a first-line drug).
Efficacy of infliximab
Systematic reviews evaluating infliximab in moderate to severe UC refractory to steroids and immunosuppressants indicate that the drug is effective in achieving clinical response, inducing clinical remission, accelerating colonic mucosal healing (CMH) and reducing the incidence of urgent colectomies [2, 3]. In randomized clinical trials, infliximab given as three infusions (baseline, weeks 2, and 6) was superior to standard therapy in inducing clinical and endoscopic remission and clinical response at week 8 [4–7]. In a double-blind, placebo-controlled study by G. Janerot et al., which included 45 patients with severe or moderate steroid-resistant UC, the rate of colectomy within three months after a single infliximab infusion was 2 times lower than in the standard therapy group [8].
The key to studying the effectiveness and safety of infliximab in UC were two randomized, double-blind, placebo-controlled studies: ACT I and ACT II. They were similar in design, differing only in the duration of active treatment and observation periods [4]. The ACT I study included 364 patients with severe or moderate UC (Mayo Activity Index score 6–12) treated with corticosteroids and/or thiopurines or refractory to oral administration of these drugs. The ACT II study also included 364 patients refractory to at least one of the basic drugs - corticosteroids (as monotherapy or in combination), aminosalicylates and thiopurines. In both studies, patients received infliximab infusions at a dose of 5 or 10 mg/kg as an induction course (baseline, weeks 2 and 6) and then regularly every 8 weeks or standard therapy: in ACT I for one year (last infusion - at the 46th week), in AST II the last infusion was carried out at the 22nd week. Outcomes were assessed at weeks 8, 30, and 54 in ACT I and at weeks 8 and 30 in ACT II.
Evaluation criteria: • rate of clinical response (decrease in Mayo index by at least three points compared to baseline and disappearance or significant decrease in visible blood in the stool); • frequency of achieving clinical remission (Mayo index no more than two points); • degree of healing of the somatic tissue (endoscopic index 0–1 points, which practically corresponds to endoscopic remission); • the possibility of refusing to take GCS by the 30th and 54th weeks in the presence of remission; • frequency of hospitalizations and proportion of hospitalized patients.
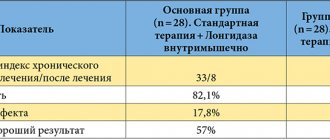
The results showed a significantly higher rate of clinical and endoscopic response (more than 60%), as well as remission, among patients receiving infliximab compared with the standard therapy group. As with Crohn's disease, the optimal dose of infliximab was 5 mg/kg/day. In the ACT I study, clinical response at week 8 was achieved in 69.6, 61.5 and 37.2% of patients in the 5, 10 mg/kg and standard therapy groups, respectively. The proportion of patients who achieved clinical remission in the same period was 38.8 in the corresponding groups; 32.0 and 14.9%, and the healing of the STC was noted in 62.0; 59.0 and 33.9% of patients. Almost identical results within the specified period were obtained in AST II (Table 1). A similar trend in achieving a clinical response was noted at week 30: 52.1; 50.8 and 29.8% of patients in these groups in AST I, 47.1; 60.0 and 26.0% – in AST II.
. Efficacy of infliximab (% of patients) in UC (AST I and II).
The ACT I and ACT II studies showed impressive results in terms of achieving endoscopic remission, significantly exceeding the effect in the standard therapy group. The most significant difference (2 or more times) in both studies was noted at week 8, somewhat less, but also significant, at week 30 (Table 1). All three indicators (clinical response, clinical remission and healing of the sore throat) in the groups receiving Remicade® maintained positive dynamics at week 54, more than 2 times higher than the indicators in the standard therapy group.
The proportions of patients reporting adverse events were similar in all three groups in ACT I and ACT II. Patients in the standard therapy group more often noted an increase in the severity of UC and anemia.
Based on the above studies, the following conclusions can be drawn: • infliximab is effective in the treatment of UC and is characterized by a high benefit/risk ratio; • infliximab is able to induce and maintain complete (clinical and endoscopic) remission in UC; • against the background of stable remission, infliximab allows one to stop taking corticosteroids in a significant number of patients.
One of the latest studies also confirmed the possibility of maintaining remission and complete refusal of GCS in 17 patients with steroid-dependent UC during two years of treatment with infliximab [9].
The action of infliximab develops quickly: within one week the onset of clinical effect can be observed. After 8–12 weeks, serum concentrations of the drug decrease, so repeat infusions every 8 weeks are recommended in patients with the most recalcitrant disease to maintain clinical response.
Infliximab (Remicade) in the treatment of refractory forms of Crohn's disease
The inflammatory process in CD is localized primarily in the intestines, although all parts of the gastrointestinal tract can be affected, including the esophagus, stomach, oral cavity, and tongue. The histological picture of CD is characterized by nonspecific immune inflammation involving all layers of the intestinal wall. The inflammation is regional in nature, in which zones of inflammatory infiltration alternate with relatively unchanged areas of the intestine. In the infiltration zone, deep ulcers-cracks are formed, which also penetrate through all layers of the intestinal wall. A pathognomonic histological feature is the presence of epithelioid granulomas consisting of giant multinucleated cells of the Pirogov-Langans type [1]. The clinical picture of the disease is dominated by diarrhea, constant localized abdominal pain corresponding to the site of the lesion, fever, and progressive anemia [2]. The nature of histological changes and the depth of damage to the intestinal wall determine the spectrum of complications of CD: strictures with subsequent development of intestinal obstruction, abdominal infiltrates, interorgan and external (intestinal-cutaneous) fistulas and abscesses. When the anorectal area is affected, paraproctitis, fistulas, rectal strictures and deep anal fissures are formed. Even with adequate treatment, in most patients the disease is progressive with the development of complications or the formation of continuous forms of the disease. The pathogenesis of granulomatous inflammation in CD is mediated by two main factors: firstly, a violation of cellular immunity and an imbalance of T-helper types 1 and 2 with predominant activation of T-helper type 1; secondly, with an imbalance of cytokines in the intestinal mucosa with a predominance of pro-inflammatory mediators, predominantly of macrophage origin, and a deficiency of anti-inflammatory regulatory cytokines [4,5,23,34]. In the normal mucosa, the content of cell subpopulations, and, accordingly, the ratio of pro-inflammatory and anti-inflammatory cytokines, is balanced, which ensures an adequate immune response to antigenic irritation [19,34]. The group of cytokines with a pro-inflammatory effect consists of intelekins (IL): IL-1, IL-2, IL-6, IL-8, etc., tumor necrosis factor-alpha (TNF-a), interferon-gamma (IF-g). Anti-inflammatory and regulatory cytokines include IL-10, IL-11, endogenous IL-1 receptor antagonists (IL-1ra), transforming growth factor-b (TGF-b) [21]. The pathogenetic mechanisms of inflammatory and autoimmune diseases are divided according to the dominant subclass of activated T-lymphocytes in the lesion and, accordingly, according to the profile of the cytokines they produce [4]. Thus, rheumatoid arthritis and CD are associated with a type 1 T-helper immune response, in which the main mediators are IL-2 and IF-g [23]. The commonality of disorders of the immune mechanisms in these diseases determines the general approaches to their treatment. Among the inflammatory cytokines, one of the most active is TNF-a. It is considered key in the process of inflammation in rheumatoid arthritis, other autoimmune diseases and CD. This cytokine exists in two forms: in a transmembrane form and in the form of a soluble trimer (Fig. 1) [4,36]. TNF-a is synthesized by different types of cells: macrophages, T-helper type 1, endothelial cells, but monocytes/macrophages are certainly its main source. Under normal physiological conditions, TNF functions as an immunoregulatory mediator and ensures the growth, proliferation and differentiation of different types of cells, activates neutrophils, T and B lymphocytes, lyses tumor cells and cells infected with bacteria and viruses, and participates in the regulation of apoptosis. Under pathological conditions, TNF-a behaves as an active proinflammatory agent. Biological reactions associated with its excess production and pathological effects include [4,34,36]: • activation of neutrophils, T and B lymphocytes, including Th1 and the synthesis of the corresponding pro-inflammatory mediators – IL-2, IF-g; • activation of macrophages and induction of synthesis of IL-1 and IL-6; • stimulation of inflammatory reactions and symptoms of endotoxemia - fever, weight loss, leukocytosis, sepsis; • formation of acute-phase inflammatory proteins by the liver (C-reactive protein, seromucoid, a-1-antitrypsin); • induction of synthesis of free oxygen radicals; • increased expression of adhesion molecules on leukocytes and endothelial cells; • increased vascular permeability and migration of leukocytes from the vascular bed to the site of inflammation; • inhibition of apoptosis of inflammatory cells; • induction of HLA class II expression on neutrophils; • stimulation of osteoporosis and osteomalacia. TNF-a plays a dominant role in the formation of granulomas in CD [36]. Granuloma consists of epithelioid and giant multinucleated cells, which are formed from T cells (CD4+) and monocytes/macrophages. The interaction between them is regulated by cytokines: TNF-a and IF-g and IL-1b. The presence of TNF-a is a prerequisite for the formation of granuloma. Basic pathogenetic therapy for CD includes three groups of drugs: corticosteroid hormones, 5-aminosalicylic acid (5-ASA) drugs and immunosuppressants [2]. 5-ASA (sulfasalazine, mesalazine) is used to treat mild CD, but even in these cases, therapy is not always sufficient. Corticosteroid hormones (prednisolone and its methylated analogues, hydrocortisone, budesonide) have been and remain the most widely used drugs for the treatment of CD with severe and moderate course of the disease. The mechanism of action of corticosteroids and aminosalicylates is to inhibit the synthesis of inflammatory mediators, including arachidonic acid metabolites (leukotrienes, platelet activating factor) and the vast majority of cytokines of macrophage origin [30,36]. Despite a wide range of anti-inflammatory and immunosuppressive effects, steroid hormones often do not produce positive results, and long-term use of the drugs is fraught with the development of serious side effects. If there is no response to treatment with hormones, reserve drugs are prescribed - immunosuppressants (azathioprine, methotrexate) [26,27,33]. The use of immunosuppressants, unfortunately, does not completely solve the problem of treating refractory forms of CD. The rate of achieving clinical effect and entering remission for both azathioprine and methotrexate ranges from 40–70% [12,14,26,27]. In addition, the use of immunosuppressants is limited to a certain extent by a wide range of side effects characteristic of cytostatics (nausea, vomiting, diarrhea, leukopenia, agranulocytosis, thrombocytopenia, anemia, hepatotoxicity, etc.). Side effects develop with a frequency of 6–20%. The effect of azathioprine and methotrexate develops slowly, improvement may be noticeable no earlier than after 3–4 weeks; a period of 3–4 months is required to obtain the maximum effect of azathioprine. (slightly less for methotrexate), which is why immunosuppressants cannot be used in acute situations; they are used to treat chronic, sluggish active forms of CD. Thus, treatment with immunosuppressants is limited by side effects, slow onset of action and lack of effectiveness. In practice, an alternative treatment method for patients with resistant CD is surgical treatment. The development of refractoriness to treatment is observed in the population of patients with CD in an average of 35% and leads to severe complications, surgical interventions and disability in young working age people [2]. Studies by V. Moun et al show that the risk of relapse during the first year after diagnosis is 47% for CD [16]. Among patients with CD treated with glucocorticosteroids, only 44% experience stable remission during the first year, while 36% develop steroid dependence and 20% develop steroid resistance [10,17]. An analysis of the natural history of CD shows that most patients have a relapsing course with varying durations of remissions, and in 10–15% of patients during treatment the disease is continuous with constant, more or less pronounced activity [18]. Regardless of the nature of the course of CD, in approximately 60% of patients, due to the development of complications, there is a need for surgical treatment, especially in cases of damage to the colon and ileum [15]. The lack of effect from corticosteroid and immunosuppressive therapy, even without complications, is also an indication for surgery - resection of the affected area. There is, however, no guarantee that after the operation a relapse will not occur in the anastomotic area or in any other place in the gastrointestinal tract, because the natural course of CD is not interrupted after surgery. The postoperative recurrence rate is 20–40% within 5 years after resection and at least 1/3 of patients require reoperation [25]. The imperfection of existing treatment methods and the need to reduce the number of operations highlight the search for new approaches to therapy. All new possibilities take into account modern ideas about the pathogenesis of CD and the participation of cytokines in this process and are aimed at optimizing the treatment of refractory forms of IBD and overcoming steroid resistance. Progress in the treatment of CD lies in the development of a fundamentally new “biological” treatment strategy. This strategy is based on the concept of the leading role of inflammatory cytokines (TNF-a, IL-1, etc.) in the pathogenesis of intestinal inflammation and the possible blockade of their biological effects by specific inhibitors or anti-inflammatory cytokines [23,31,36]. Theoretically, any links in the inflammatory cascade are selective targets and points of application for biological drugs. There are quite a lot of biological methods today, but only some of them have already entered clinical practice. Within the framework of a biological strategy, the most promising today is the strategy of inhibiting tumor necrosis factor, since this cytokine is, if not the main one, then one of the leading ones in the development of granulomatous inflammation in CD [34,36]. The use of antibodies to TNF-a is a viable way to limit granulomatous inflammation by binding soluble TNF trimers and, indirectly, inhibiting CD4+ activation. Currently, the recombinant drug infliximab (Remicade), which is a chimeric monoclonal mouse antibody to TNF-a combined with human immunoglobulin G1 (25% mouse protein and 75% human immunoglobulin), has entered clinical practice. Infliximab specifically binds human TNF-a, and has a very high affinity for soluble TNF-trimer, but also blocks membrane-bound TNF (Fig. 1). The drug causes lysis of cells in the inflammatory infiltrate and enhances apoptosis of activated T lymphocytes [11,23,34]. Remicade was registered in Russia in 2001 for two main indications - rheumatoid arthritis and CD, which are similar in the mechanisms of inflammation. In CD, infliximab is used in cases of disease that are resistant to the action of steroid hormones and immunosuppressants; it is active in both moderate and severe forms of CD [26,27,28]. The drug has a prolonged effect. In uncomplicated CD, a single intravenous injection is sufficient to achieve remission. In the presence of fistulas, 3 infusions are recommended at an interval of 2 weeks [20]. The optimal dose, according to controlled studies, is 5 mg/kg body weight; increasing the dose to 10 mg/kg does not lead to an increase in the frequency of clinical response [23]. The effect of infliximab develops quickly; after 2 weeks, the onset of clinical effect can be observed. Its duration of action is up to 30 weeks after a single infusion, but after 8–12 weeks the serum concentration of the drug decreases, so repeated infusions every 8 weeks are recommended to maintain clinical response and remission [23]. In controlled multicenter trials, the response rate to infliximab in steroid-resistant forms of CD ranges from 50–82%, the rate of clinical remission is 25–48% (Fig. 2) [13,20,35]. The largest clinical trial (ACCENT I) showed that in CD, the frequency of maintaining remission as a result of Remicade therapy for 30 weeks after a single dose is 39–44% and does not depend on the initial dose of the drug - 5 or 10 mg/kg. Compared with placebo, there were 3 times more patients in the groups receiving Remicade who continued to have CD remission at week 54 (Fig. 3). In addition, the ACCENT I trial showed that infliximab therapy is safe, well tolerated, and eliminates corticosteroids (Fig. 4) [13]. By week 22, steroids were discontinued in all patients receiving infliximab, and maintenance therapy with Remicade increased the possibility of remission by 2.2 times at weeks 30 and 54 and allowed the discontinuation of GCS. Infliximab significantly reduces the index of histological activity in patients with CD in the form of ileocolitis, while the pathological expression of HLA DR disappears and the content of TNF and adhesion molecules in colon biopsy specimens decreases [7,8]. One study showed that under the influence of infliximab, a decrease in endoscopic activity correlates with the clinical activity index (Best index) and a histological decrease in the inflammatory infiltrate [9]. A decrease in histological activity was also shown in studies with labeled technetium in the number of leukocytes migrating to the inflammation zone [32]. Long-term treatment with infliximab for 44 weeks was effective and well-tolerated in relieving CD symptoms and achieving remission in patients who did not respond to conventional treatment [33]. The experience of treating 500 patients with refractory CD at the Mayo Clinic also demonstrated high clinical efficacy and good tolerability of infliximab [21]. Successful results were obtained in patients with CD with the development of inflammation in the area of the ileoanal reservoir and with perianal localization of CD [22,24]. The side effects of infliximab were studied in several placebo-controlled studies that included 771 patients with rheumatoid arthritis and CD [29]. Reactions associated with direct administration of the drug and observed within the first 2 hours after injection (fever, headache, urticarial rash, shortness of breath, hypo- or hypertension) were observed in 17% of cases versus 7% when using placebo. During 27 weeks of observation after the last injection, 26% of patients receiving infliximab versus 16% receiving placebo had various infections (most often respiratory) and purulent-inflammatory processes, which, in most cases, were not serious. Less than 2% of patients required discontinuation of the drug due to the development of adverse reactions. According to S. Hanauer et al., various infections that required additional treatment developed in 29% of patients, and post-transfusion reactions were noted in 12% [13]. The greatest danger is the reactivation of old foci of tuberculosis under the influence of infliximab. Patients (especially those with a history of tuberculosis) before starting treatment should be thoroughly examined x-ray and using tuberculin tests, and, if necessary, consulted with a phthisiatrician. Approximately 13% of patients receiving infliximab developed antibodies to it (HACA - Human antihimeric antibodies), but the presence of these antibodies did not reduce the effectiveness of the drug [23]. The multicenter, randomized, placebo-controlled studies ACT I and ACT II, which assessed the effectiveness and safety of Remicade in active ulcerative colitis (UC), have been completed. They included 364 patients with moderate to severe UC who did not respond to at least one drug of standard basic therapy. Remicade was administered at a dosage of 5 or 10 mg, and results were assessed at weeks 22 and 46 of treatment. By week 8, 30 to 40% of patients receiving Remicade achieved remission and more than 60% achieved endoscopic healing, with improvements maintained until the end of the study. This indication is currently in the process of registration in the European Union. The Russian experience of using Remicade for CD is based on the results of phase IV clinical trials conducted in three research centers (State Research Center of Coloproctology, MONIKI, Lipetsk Regional Hospital) [6]. The study included 23 patients with CD who did not respond to treatment with corticosteroids, with localization of the process in the ileum and colon (21 patients) and with combined lesions of the colon and upper gastrointestinal tract (2 patients). In 1 patient, the disease was complicated by the formation of a rectovaginal fistula. The Best index in all patients corresponded to a severe or moderate course (220–450 points). The results of the study showed that Remicade effectively relieves refractory exacerbations of CD; a clinical effect was achieved in 12 patients (52%). Two patients were operated on, the rest of the effect was moderate. Over the next year, after a single infusion, remission was preserved in 35% of patients. In general, the results obtained correspond to the data of international controlled research. Side effects were recorded in one case. Our own experience of removal of remicide also indicates its effectiveness in refractory forms of BC [3]. We observed 8 BC patients (colitis or ileocolite), resisted by steroids with an activity index of 220–400. 4 weeks after the introduction of remicide, positive dynamics were noted in 5 of 8 patients (62.5%) - a decrease or disappearance of pain, a decrease in the frequency of the stool. After 8 weeks, abdominal pain was preserved in 3 patients, a chair 2-3 times a day in 4 patients. The activity index decreased by the end of 8 weeks by 50–86 points in 5 patients, by less than 50 points in 2 patients, 1 patient was operated on due to the inefficiency of treatment. Thus, the vast majority of patients for 8 weeks have achieved positive dynamics. After 16 and 24 weeks, in 5 patients, the activity index decreased to 150 or less, they also have a remission supported by 5 -ASK during the year. Side effects were not noted in any case. Currently, a large number of “biological” treatment methods are being developed, based on the effect on various cytokines. All new areas in the treatment of BC are apparently promising, but so far they have only theoretically and have not received practical development. The only rather effective and safe method in the refractory course of BC and already having clinical confirmation is infliximab.
References 1. Aruin L.O., Kapuller L.L., Isakov V.I. Morphological diagnosis of diseases of the stomach and intestines. M., Triad-Kh. – 1998, 496 p. 2. Belousova E.A. Ulcerative colitis and Crohn's disease // M., Triad, 130 p. 3. Morozova N.A., Belousova E.A., Nikitina N.V., Zlatkina A.R. Experience with the use of Remicade (infliximab) in patients with Crohn's disease. // Gastroentnrology of St. Petersburg, 2004, No. 9, materials of the 6th International Slavic-Bantian Scientific Forum. 4. Nasonov E.L. Immunity disorders in autoimmune diseases // Russian Journal of Gastroenterology, Hepatology, Coloproctology, 1999, Vol.IX, No. 4, Appendix 7, pp. 43–48 5. Nasonov E.L. Tumor necrosis factor is a new target for anti-inflammatory therapy of rheumatoid arthritis // RMJ, 2000, vol. 8, no. 17, pp. 718–722. 6. Khalif I.L. Remicade: treatment of disease in the third millennium (Oral communication, Russian Gastroenterological Week, 2004) 7. Baert FJ, D'Haens G., Peeters M., Hiele MI et all. Tumor necrosis factor–a antibody (infliximab) therapy profoundly down–regulates the inflammation of Crohn’s ileocolitis.// Gastroenterology.– 1999.– v. 116.– p. 22–28 8. Chey WY, Hussain A, Ryan C, Potter GD et all. Infliximab for refractory ulcerative colitis // Am. J. Gastroenterol.–2001.– v. 96.– p.1860–1866 9. D'Haens G., van Deventer SJH, van Hogezand R., Chalmers D. et all. Endoscopic and histological healing with Infliximab anti–tumor necrosis factor antibodies in Crohn's disease: a European multidisciplinary trial. // Gastroenterology.– 1999.– v. 116.– p. 1029–1034 10. Faubion WA, Loftus EV, Harmsen WS, Zinsmeister AR et all. The natural history of corticosteroid therapy for inflammatory bowel disease: a population based study // Gastroenterology. –2001.– v. 121.– p. 255–260 11. Feagan BG Infliximab in the treatment of Crohn's disease.// Scand. J. Gastroenterol. 2000.–v.14.–suppl. C.–6B 12. Feagan BG, Fedorak RN, Irvine EJ, Wild G. et all. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease // N. Engl. J. Med.– 2000.– v.342.– p. 1627–1632 13. Hanauer SB, Lichtenstein GR, Colombel JF et all. Maintenance infliximab (Remicade) is safe, effective and steroid–sparing in Crohn's disease: preliminary results from the Accent I trial // Gastroenterolgy.–2001.–v.120.–Suppl.1.–p.99 14. Lemann M. , Zenjari T., Cosnes J., Mesnard B. Methotrexate in Crohn's disease: long–term efficacy and toxicity.– m. J. Gastroenterol. 2000.–v. 95.– p.1730–1734 15. Mechjian HS, Switz DM, Watts HD, Deren JJ et all. National Cooperative Crohn's disease Study. Factors determining recurrence of Crohn's disease after surgery.//Gastroenterology.– 1979.–v.77.–(4 Part 2).– p.907–913 16. Moum B., Ecbom A., Vatn MH et all. Clinical course during the 1st year after diagnosis of ulcerative colitis and Crohn's disease. Results of a large prospective population–based study in southeastern. Norway 1990–93 // Scand. J. Gastroenterol..– 1997.– v. 32.– p. 105–112 17. Munkholm P., Langholz E., Davidsen M., Binder V. Frequency of glucocorticoid resistance and dependency in Crohn's disease //Gut.–1994.– v.35.– p. 360–362 18. Munkholm P., Langholz E., Davidsen M., Binder V. Disease activity courses in a regional cohort of Crohn's disease patients.// Scand.J.Gasrtroenterol. –1995.– v.30.–p.699–706 19. Papadakis KA, Targan SR Role of cytokines in the pathogenesis of inflammatory bowel disease // Ann. Rev. Med.–2000.– v.51.– p.289–298 20. Present DH, Rutgeerts P., Targan S., Hanauer SB et all. Infliximab for the treatment of fistulas in patients with Crohn's disease.– N. Engl. J. Med. – 1999. – v. 340.– 1398–1405 21. Ricart E., Panaccione R., Loftus E., Tremain W. Infliximab for Crohn's disease in clinical practice at the Mayo Clinic: the first 100 patients.// Am. J. Gastroenterol.– 2001.– v.96.– p. 722–729 22. Ricart E., Panaccione R., Loftus E., Tremain W. Successful management of Crohn's disease of the ileoanal pouch with infliximab // Gastroenterology.– 1999.– v. 117.– p. 429–432 23. Rutgeerts P. A critical assessment of new therapies in inflammatory bowel diseases.// J. Gastroenterol Hepatol.–2002.– v.17. suppl. – S177 QR 24. Rutgeerts P. Management of perianal Crohn’s disease // Scand. J. Gastroenterol.– 2000.– v. 14, (Suppl. C).– 7C. 25. Rutgeerts P., Geboes K., Vantrappen G., Beyls J. et all. Predictability of postoperative course of Crohn's disease // Gastroenterology. 1990.– v. 99. – p. 956–963 26. Sanborn WJ Steroid–dependent Crohn's disease. // Scand. J. Gastroenterol..– 2000.– v. 14.– (Suppl. C).– 17C. 27. Sands BE Medical therapy of steroid–resistant Crohn's disease. // Scand. J. Gastroenterol.– 2000.– v. 14.– (Suppl. C).– 33C. 28. Sands BE, Tremaine WJ, Sanborn WJ, Rutgeerts P. et all. Infliximab in the treatment of severe steroid–refractory ulcerative colitis: a pilot study // IBD.– 2001.–v.7.– p.83–88 29. Schaible TF Long–tern safety of infliximab // Scand. J. Gastroenterol.– 2000.– v. 14.– (Suppl. C).– 29 C. 30. Schreiber S. Aspects of the immunology of inflammatory bowel diseases. // Recent Advances in the Pathophysiology of Gastrointestinal and Liver Diseases. –Nantes.– July 1997.– p.133–171. 31. Schreiber S, Campieri M, Colombel JF, van Deventer SJH et all. Use of anti-tumor necrosis factor agents in inflammatory bowel diseases. European guidelines for 2001–2003. //Int. J. Colorectal Dis.–2001.– v.16.– No. 1.– p. 1–11 32. Schluter U., Ledeboer M., Arndt J., Griffioen G. et all. Very rapid anti–inflammatory effect of infliximab (Remicade) in Crohn's disease as assessed by 99M TC–WBC–scintigraphy // 8th UEGW, Brussels, Nov. 2000. 33. Steinhart H. Steroid resistant and steroid dependent Crohn's disease. // IBD, salicylates and other relevant therapies–Proceeding of the International IBD Symposium.– London.– 1999.– p.83–90 34. Targan SR Biology of inflammation in Crohn’s disease: mechanism of action of anti–TNF–a therapy . // Scand. J. Gastroenterol.– 2000.– v. 14.– (Suppl. C).– 13C. 35. Targan SR, Van Deventer SJH, et all. A short–term study of chimeric monoclonal antibody cA2 to tumor necrosis factor a for Crohn’s disease.// N.Engl.J.Med. –1997.–v.337.–p.1029–1035 36. Van Deventer SJH Tumor necrosis factor and Crohn's disease.– Gut. –1997. – 40 (4) . – p.443–448
Clinical and economic aspects
Early disease onset, hospitalization, and surgery determine the economic costs of UC. The purpose of the study by RD Cohen et al. (2010) conducted a systematic review of the literature on the cost of managing patients with UC in Western countries. Annual medical costs per patient with UC range from $6200–$11,500 in the United States and €8,900–€10,400 in Europe, with hospitalization costs accounting for 40–55% of total direct medical costs. Indirect costs account for 30% of total costs in the United States and 54–68% in Europe [10].
The ACT I and ACT II studies analyzed the frequency of hospitalizations in patients with UC. A total of 728 people were involved in the two studies, of which 484 patients received Remicade® at different doses (5 and 10 mg/kg/day). The overall rate of hospitalizations within 30 weeks, including readmissions, was half as high in the group receiving Remicade® compared to the standard therapy group (9 vs. 18%). The proportion of hospitalized patients was 8 and 14%, respectively [11]. Since inpatient treatment is the most expensive part of the management of patients with UC; it can be concluded that the use of Remicade provides significant economic benefits for national health care systems.
results
- The analysis included 100 patients who were randomized to infliximab and standard therapy in a 1:1 ratio.
- It was shown that at week 10, a greater number of patients in the infliximab group, compared with standard therapy, achieved clinical remission (59% vs. 34%, respectively, p=0.021) and endoscopic remission (59% vs. 17%, respectively, p= 0.001).
- At week 52, there were no significant differences between groups in terms of clinical remission (p=0.421). Moreover, 41% of patients treated with infliximab had clinical remission on azithromycin monotherapy without the need to intensify therapy, compared with 15% of those in the standard therapy group (p=0.004).
Own experience of using Remicade for UC
The Department of Gastroenterology of MONIKI has experience in using Remicade in patients with IBD: 20 patients with Crohn's disease, 40 with UC, of which 3 patients after subtotal resection of the colon with activity in the stump of the sigmoid and rectum.
The objectives of this study: • to evaluate the effectiveness of an induction course of therapy with infliximab in patients with UC refractory to other treatment methods; • assessment of the possibility of achieving and maintaining clinical and endoscopic remission of UC during infliximab therapy; • maintaining remission of UC without corticosteroids during infliximab therapy.
The objectives of the study were formulated to compare the effectiveness of biological therapy with infliximab in Russia (using the example of a population of patients in the Moscow region), Europe and other countries.
Material and methods
The open prospective study included 37 unoperated patients with UC who were observed from January 2008 to December 2010. The diagnosis of UC was confirmed endoscopically and histologically. All patients were examined for the presence of latent forms of tuberculosis.
The clinical characteristics of the patients are presented in Table. 2. Steroid resistance developed in 5 patients with the acute form (first attack) of the disease, the remaining 32 patients developed steroid dependence. The initial severity of the UC attack was assessed using the activity index of the Truelove and Witts criteria [12–14]. The effectiveness of therapy was monitored using the Mayo index. Before infliximab therapy, GCS as monotherapy (prednisolone or methylprednisolone) was used in 29 patients. Of these, 5 people with an acute severe form of the disease first received 250–500 mg of methylprednisolone intravenously, followed by a dose reduction and switching to oral prednisolone at the rate of 1 mg/kg body weight per day; 24 patients with another attack of a recurrent form of UC received oral prednisolone at the same dose - 1 mg/kg body weight.
A combination of oral corticosteroids at a dose of 30–40 mg and azathioprine at a dose of 100–150 mg (at least 2 mg/kg) was used in 4 patients with a steroid-dependent continuous form of UC. A combination of azathioprine at the same dose and 5-aminosalicylic acid was administered to 4 patients with a steroid-dependent form of UC and severe side effects of corticosteroids. Thus, before the prescription of Remicade, a total of 33 patients received GCS, and 8 patients received azathioprine AZA.
Patients were prescribed infliximab (Remicade®) according to the DLO program of the Moscow region at the rate of 5 mg/kg body weight per day as an induction course (3 infusions at baseline, 2nd and 6th weeks) and then every 8 weeks. The effectiveness of treatment was monitored using the Mayo index. 35 of 37 patients received an induction course (at least 3 infusions). Its effectiveness was assessed by analogy with AST I and II studies at 8 weeks after the start of treatment (2 weeks after the 3rd infusion). In table Table 3 shows the number of Remicade infusions in the UC patients we observed, up to and including December 2010.
Number of infliximab infusions in patients with UC.