- Causes and symptoms of disorders
- Where is the spleen located and how is it examined?
- Is there life without a spleen?
The spleen is a little-studied organ whose functions are not fully understood by medicine. Many generally consider it unimportant and almost superfluous. But in fact, the spleen is part of the lymphatic system and performs important protective functions and participates in the formation of immunity. And to find the cause of many troubles, you need to do an MRI of the spleen.
Anatomy.
Also on topic:
HUMAN ANATOMY
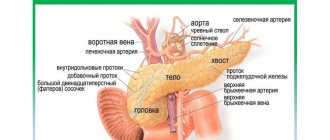
The spleen is made up of several types of tissue. Embryonically, it originates from the middle germ layer, the mesoderm. A certain number of original mesenchymal cells remain in the spleen throughout life, while the rest turn into lymphoid and reticuloendothelial cells. The pulp (pulp) of the spleen consists mainly of the latter, and lymphoid cells are grouped into lymphoid formations, the so-called. Malpighian bodies. The spleen has an abundant blood supply and its color is dull purple. In addition to the peritoneum (serous membrane), it is covered with a dense elastic fibrous capsule mixed with smooth muscle fibers. The capsule continues into the thickness of the organ in the form of crossbars - trabeculae, forming the skeleton of the spleen and dividing it into lobules. The spleen is directly connected to the portal vein system (carrying nutrient-rich blood from the gastrointestinal tract to the liver) and the systemic circulation.
Causes of development and risk groups
Malignant tumors develop as a result of mutations that occur in cells. These mutations affect genes that regulate cell reproduction, apoptosis (programmed cell death), and repair of damaged DNA. It is difficult to say why such mutations occurred in each specific case.
Some conditions increase your chance of developing cancer—they are called risk factors. If we talk about the most common type of spleen cancer - lymphoma, then the risk factors will be as follows:
- Age. Over the years, mutations accumulate in the cells of the human body and the likelihood of developing various types of cancer increases.
- Floor. Men get sick more often.
- Family history: if lymphoma has been diagnosed in close relatives.
- Immune system disorders: weakened immunity, autoimmune diseases.
- Exposure to the body of certain harmful substances.
- Some studies have found a link between chronic hepatitis C and B-cell non-Hodgkin lymphoma.
Physiology.
Galen considered the spleen an organ “full of mystery.” The ancient Greeks and Romans are known to have removed runners' spleens to increase their running speed. The functions of the spleen are not fully understood. For a long time it was considered an endocrine (without excretory ducts) gland. Since there is no reliable data on the secretory activity of the spleen, this theory had to be abandoned, although recently it has to some extent received a second life. The spleen is now credited with hormonal regulation of bone marrow function.
Also on topic:
COMPARATIVE ANATOMY
In the early stages of fetal development, the spleen serves as one of the hematopoietic organs. By the ninth month of intrauterine development, the formation of both erythrocytes and leukocytes of the granulocyte series is taken over by the bone marrow, and the spleen, starting from this period, produces lymphocytes and monocytes. In some blood diseases, however, foci of hematopoiesis reappear in the spleen, and in a number of mammals it functions as a hematopoietic organ throughout life.
In an adult, the spleen performs several functions. As part of the reticuloendothelial system, it phagocytizes (destroys) dead blood cells and platelets, and converts hemoglobin into bilirubin and hemosiderin. Because hemoglobin contains iron, the spleen is one of the richest iron reservoirs in the body. As a lymphoid organ, the spleen is a major source of circulating lymphocytes, especially in adolescence and young adults. In addition, it acts as a filter for bacteria, protozoa and foreign particles, and also produces antibodies; People without a spleen, especially young children, are very susceptible to many bacterial infections. Finally, as an organ involved in blood circulation, it serves as a reservoir of red blood cells, which in a critical situation are released back into the bloodstream.
Where is the spleen located and how is it examined?
The small organ is located behind the stomach in the left hypochondrium, so checking its condition is quite difficult. For research, palpation, x-rays, ultrasound and more advanced tomography methods are used. The advantage of MRI is that the diagnosis gives the most complete picture of the location, size, and condition of the organ tissues. In addition, this is the safest method that does not cause any harm to the body. You can undergo examination at least every day if the financial side of the issue is not an obstacle.
But it should be noted that stereotypes about tomography being undemocratic are greatly exaggerated. The price of an MRI of the spleen starts from 2,500 rubles (in Moscow, and even cheaper in the regions). On our website MRT-kliniki.ru you can find a list of all diagnostic centers, compare costs, choose clinics with preferential conditions or promotions and sign up for an examination with a discount of up to 50%.
Splenomegaly,
or enlargement of the spleen is a characteristic response of the organ to many pathological conditions. Splenomegaly may be associated with enlarged lymph nodes, ascites (fluid in the abdomen), jaundice, leukopenia (decreased white blood cell count), fever, enlarged liver, or severe anemia. It is observed in many cardiovascular diseases; for many infectious diseases - malaria, typhoid fever, smallpox, measles, syphilis, meningitis, scarlet fever, etc.; for blood diseases - leukemia, hemolytic jaundice, chronic hemolytic anemia, usually congenital. Sometimes an enlarged spleen occurs in Hodgkin's disease; It reaches enormous sizes in chronic myeloid leukemia. Metabolic disorders, especially fat metabolism, are also often accompanied by splenomegaly. Many liver diseases affect the condition of the spleen. This primarily concerns Banti syndrome, in which cirrhosis of the liver is accompanied by congestive splenomegaly and anemia. With a hereditary disease - Gaucher disease - there is a disorder of fat metabolism and splenomegaly.
Since splenomegaly is only a manifestation of some other disease, treatment should be aimed at the primary cause. Removal of the spleen is indicated in rare cases; sometimes it is performed in diseases associated with increased destruction of red blood cells or platelets, in particular in hemolytic jaundice, thrombocytopenic purpura, Banti syndrome, but even then an improvement in blood counts can be expected only in 30–60% of cases.
See also LYMPHATIC SYSTEM.
Human immune system
Ph.D. Goldinberg B.M., Vasyuk Ya.V.
City Center for Transfusiology of the health care institution "6th City Clinical Hospital", Minsk,
healthcare institution "7th city children's clinic", Minsk
HUMAN IMMUNE SYSTEM
Introduction
A group of organs that have a common origin, a common structural plan and perform a common function is called an organ system. Five of all ten organ systems are regulatory (control): nervous, circulatory, endocrine, lymphatic and immune. Let us clarify that the lymphatic organs and lymph nodes, of which there are about 600, are functionally part of the immune system, and the lymphatic system itself includes an extensive network of vessels that passes through almost all of our tissues, ensuring the movement of a fluid called lymph.
The word “immunity” comes from the Latin “immunis” (in English – immunity), which means “clean from anything”, immune to anything. The immune system appeared along with multicellular organisms and developed as an assistant to their survival. It unites organs and tissues that guarantee the body’s protection from genetically foreign cells and substances coming from the environment.
The immune system is represented at three levels: organ, cellular and molecular.
Organs of the human immune system
The immune system includes central and peripheral organs.
The central organs of the immune system are the red bone marrow and the thymus.
The bone marrow is the repository of stem cells from which blood cells are formed (Fig. 1). Depending on the situation, stem cells are transformed into immune B lymphocytes. If necessary, a certain part of B-lymphocytes turns into plasma cells, which are capable of producing antibodies.
Fig.1. Bone marrow contains stem cells
The thymus (or thymus gland) is one of the main organs of the immune system, located in a person behind the sternum below the collarbone, which is responsible for the formation of T-cells of the immune system in the lymphoid tissues of the body (Fig. 2).
Fig.2. Thymus
Peripheral organs include the spleen, tonsils and lymph nodes, which contain areas of maturation of immune cells.
Tonsils, which got their name because of their external resemblance to almonds, are a collection of lymphoid tissue in the upper part of the nasopharynx. A person has six tonsils: two palatine, two thoracic, and one each nasopharyngeal and lingual.
The largest of them are the palatine tonsils, or tonsils, which can be easily examined independently in the mirror if you open your mouth wide enough (Fig. 3).
Rice. 3. Palatine tonsils
The spleen is the largest lymphoid organ (Fig. 4). In addition, it can accumulate some blood. In emergency situations, the spleen is able to send its reserves into the general bloodstream. This allows you to improve the quality and speed of the body's immune reactions. The spleen cleanses the blood of bacteria and processes all kinds of harmful substances. It completely destroys endotoxins, as well as the remains of dead cells from burns, injuries or other tissue damage. In people who are left without a spleen for any reason, their immunity deteriorates.
Rice. 4. Spleen
Lymph nodes are small round-shaped formations (Fig. 5), located in the chest cavity (bronchopulmonary, bronchotracheal) and abdominal cavity (Peyer's patches, appendix and others), perithoracic on the surface of the chest, on the neck and on the limbs. The lymph node is one of the barriers to infections and cancer cells, playing the role of a kind of customs (Fig. 5). It produces lymphocytes - special cells that take an active part in the destruction of harmful substances.
Rice. 5. Lymph node
The central organs of the immune system are responsible for the formation and maturation of cells, and the peripheral organs provide protection, that is, the immune response. The peripheral and central organs of the immune system perform their work only together, and if any one of these organs fails, the body will lose its protective barrier.
Components of the immune system
Modern immunology distinguishes between two interacting components of the immune system - innate and acquired types of immunity , which ensure the development of an immune response to genetically foreign substances (entities).
Innate (species) immunity is a hereditarily fixed system of protecting the human body from pathogenic and non-pathogenic microorganisms, as well as products of tissue decay. Innate immune cells recognize pathogens by molecular markers specific to them—the so-called “pathogenicity patterns.” These markers do not allow one to accurately determine whether a pathogen belongs to one species or another, but only signal that the immune system has encountered troublemakers: a stranger or one’s own, but who has become a traitor to the body (Fig. 6).
Fig.6. Innate immunity: the main thing is calm!
Innate immunity at the cellular level is represented by:
- monocytes are the precursors of macrophages (cells that devour foreign particles). They are formed in the bone marrow, then enter the blood, but quickly leave it, turning into tissue macrophages and dendritic cells (Fig. 7);
Fig.7. Monocyte
- macrophages and dendritic cells are located in the skin and mucous membranes. They have mobility and are transported through the blood and lymph. They absorb (phagocytose) the pathogen, and within themselves, using the contents of the vacuoles, dissolve it. Dendritic cells branch like a tree. Thanks to the antenna branches, they work as communicators between the innate and acquired types of immunity (Fig. 8);
Fig.8. dendritic cell and
and macrophage
- blood cells containing granules (granulocytes) in the cytoplasm: neutrophils, eosinophils and basophils (Fig. 9);
Fig.9. Granulocytes
Neutrophils are the most numerous immune cells in human blood. They circulate in the blood for only 8-10 hours and spend most of their lives traveling through body tissues. When they encounter a pathogen, they capture it and digest it, after which they usually die themselves. Granules containing antibiotic substances are released from the destroyed neutrophils.
Granules of eosinophils and basophils provide chemical defense of the body against large pathogens, for example, parasitic worms, fungi, and extracellular bacteria. However, with excessive activity they can also participate in the development of an allergic reaction;
- natural killer cells or NK cells (English Natural killer cells, NK cells) are a type of cytotoxic lymphocytes involved in the functioning of innate immunity. They destroy cells infected with bacteria and viruses, as well as tumor cells, at high speed.
Fig. 10. Natural killer
Natural killers act with the help of aggressive substances perforin and granzyme, which, like gimlets, “bite” and destroy the affected cell that has become their target (Fig. 11)
Fig. 11. Penetration of perforin and granzyme into a cancer cell and its destruction
The molecular (humoral) factors of innate immunity are (Fig. 12):
- proteins that bind metal ions (iron, zinc) necessary for the life and reproduction of pathogens - lactoferrin, calprotectin, membrane protein and others;
- enzymes that generate oxidizing agents - oxygen and nitric oxide:
- enzymes capable of breaking down the cell membrane of pathogens - lysozyme, chitinase, phospholipase A2;
- proteins and peptides that violate the integrity of the cell membrane of a microorganism - complement, eosinophilic protein, defensins and others.
Fig. 12. Humoral factors of innate immunity
The complement system is a multicomponent, self-assembled system of more than 20 serum proteins that are normally inactive.
After activation, the biological effects of complement appear: the formation of a membrane attack complex for the lysis of pathogens, the release of inflammatory mediators to attract phagocytes and enhance their absorption capacity.
Cytokines are a system of low molecular weight proteins of the body, synthesized mainly by active cells of the immune and hematopoietic systems, regulating intercellular interactions (a “universal” language for all cells), presented in Fig. 13 and 14.
Rice. 13. Cytokines: IL – interleukins, of which there are currently 34 varieties;
Rice. 14. Multidirectional action of cytokines using the example of interferon gamma
As a result of activation of humoral and cellular factors of innate immunity, a basic reaction of infectious inflammation is formed within several hours after the introduction of a pathogen into the internal environment of the body (Fig. 15)
Rice. 15. Infectious inflammation of tissue at the site of foreign body insertion for the purpose of its removal
Acquired immunity (or adaptive – from the French adapter “ to adapt ” ) is formed individually during life under the influence of antigenic stimulation and, in turn, is divided into natural and artificial (Fig. 16).
Fig. 16. Adaptive
immunity
Natural immunity is formed when encountering a pathogen, as a result of which protective immune factors are produced in the body (active natural immunity), or they come ready-made from maternal orgasm during fetal development or during breastfeeding (passive natural immunity).
Artificial immunity is created by administering vaccines or toxoids that stimulate the production of antibodies against specific pathogens or their poisons. At the same time, for preventive purposes, the process of the patient’s immune system’s reaction to the pathogen is reproduced, but in an asymptomatic or mild clinical form, while maintaining their protective immune power for several months, years, or even for life (artificial active immunity). When it is necessary to quickly and for a short time protect a patient from the real risk of encountering a pathogen during an epidemic or neutralize a pathogen that has already entered his body, immunoglobulins (antibodies) are used both in purified form and in dosed volumes of plasma or serum obtained from the blood of a donor ( person or animal). The use of ready-made antibodies forms passive artificial immunity that lasts 2-3 weeks.
Adaptive immunity is based on three main processes:
- recognition of antigens (usually foreign to the body) using receptors;
- removal (elimination) of recognized foreign agents (Fig. 17);
- the formation of an immunological memory of contact with an antigen, allowing for faster and more efficient removal of this antigen when it is recognized again.
Fig. 17. Options for the response of the immune system to organ or tissue transplantation, the occurrence of malignant neoplasms and infections
The immunocompetent cells of adaptive immunity are lymphocytes that live in the human body from several months to several years. According to their functions, cells are divided into T-lymphocytes - 80% and B-lymphocytes - 20%.
The fact that the T lymphocyte recognizes only foreign antigens, and not molecules from its own body, is a consequence of a process called selection, which occurs in the thymus, where T cells complete their development. The essence of selection is this: the cells surrounding the young, or naive, lymphocyte show (present) to it the peptides of their own proteins. The lymphocyte that recognizes these protein fragments too well or too poorly is destroyed. The surviving cells (and this is less than 1% of all T-lymphocyte precursors that came to the thymus) have an intermediate affinity for the antigen, therefore, they, as a rule, do not consider their own cells to be targets for attack, but have the ability to react to a suitable foreign peptide.
To activate a T-lymphocyte, it needs to receive special signals from the receptors of the leukocyte antigen system and a cocktail of many pro-inflammatory cytokines.
Using special reagents, markers of surface proteins of leukocytes of a certain type are determined, which are called clusters of differentiation (CD). Currently, 350 CD antigens and their subtypes are known (Table 1).
Table 1. Main identification CD markers of cells
| Cluster designation | Cells |
| CD 10, CD34 | Lymphoid stem cell |
| CD3 | T lymphocyte |
| CD4 | T helper |
| CD8 | T-killer |
| CD19, CD72, CD79, etc. | B lymphocyte |
| CD16/ CD56 | NK cells |
| CD14, CD64 | Monocyte/macrophage |
T lymphocytes recognize cells carrying foreign antigens and destroy them after direct contact (attack), and also perform the function of regulating the immune response.
T lymphocytes have subtypes (Fig. 18):
Rice. 18. Subtypes of T-lymphocytes and their functions
- Killer T cells (also called CD8+ T lymphocytes), which, like an NK cell (natural killer), secrete the proteins perforin and granzyme, which leads to lysis of the target cell;
- T-helpers (from English helper - assistant). They are also referred to as Th cells, CD4+ T lymphocytes. Activated T helper cells produce chemokines and cytokines involved in the immune process (Fig. 19);
Rice. 19. Activation of different subpopulations of T-helper cells by cytokines
- Suppressor T cells (Ts) inhibit (suppress) B cell responses and block helper T cells. Moreover, these cells do not sabotage immune processes and do not harm health. They simply regulate the strength of the immune response, which allows the immune system to respond to stimuli with restraint and with moderate force (putting out a fire, not a fire);
- T-regulatory cells (Tr1) influence the formation of granular leukocytes (granulocytes), which we have already introduced as macrophages.
The ratio of CD4/CD8 cells is called the immunoregulatory index (IRI). If the patient’s IRI is increased (more than 2.2), this indicates excessive activity of T-helper cells and a weakening of the regulatory function of T-killer cells. At this rate, immune cells can destroy the body’s own tissues. Increased IRI is most often observed in patients with autoimmune diseases (systemic lupus erythematosus, scleroderma, rheumatoid arthritis, etc.). The cause of excessive activity of T-helper cells can also be a tumor of the thymus gland. With this pathology, an excessive number of lymphocytes are produced. High rates of IRI are observed in acute lymphoblastic leukemia. This severe oncological disease is accompanied by an uncontrolled increase in the number of immature lymphocytes.
If the immunoregulatory index is reduced (less than 1.6), then this indicates a serious deterioration in the functioning of the immune system. Low levels of IRI indicate that the function of protective cells in the body is weakened, and regulation by T-killers is excessive. This is usually observed in the following pathologies accompanied by immunodeficiency: infectious diseases (including HIV infection); congenital immunodeficiency; any protracted and chronic diseases; bone marrow tumors.
B lymphocytes are responsible for the humoral component of immunity - the production of antibodies. After an antigenic stimulus, the B lymphocyte turns into a lymphoblast, a cell capable of dividing. Some lymphoblasts differentiate into memory B lymphocytes, the other part turns into plasma cells that produce antibodies.
B lymphocytes carry the B cell receptor on their surface. Upon contact with an antigen, these cells are activated and turn into a special cell subtype - plasma cells , which live up to three weeks and have the unique ability to secrete thousands of antibodies .
The antibody has an affinity for the antigen it recognizes and, as it were, “sticks” to it. This allows antibodies to envelop (opsonize) cells and viral particles coated with antigen molecules, attracting macrophages and other immune cells to destroy the pathogen. Antibodies are also able to activate a special cascade of immunological reactions called the complement system, which leads to perforation of the pathogen’s cell membrane and its death.
Rice. 20. Antibody production and pathogen marking
There are several classes of antibodies (immunoglobulins). The first to appear after antigenic irritation, causing agglutination of bacteria and neutralization of viruses, are immunoglobulins M (IgM). Immunoglobulins G (IgG) are involved in long-term immunity.
Table 2 provides an interpretation of laboratory tests for the presence of a pathogen at the molecular level and using tests for immunoglobulins M and G.
Table 2. Interpretation of laboratory tests for the presence of a pathogen at the molecular level
| Result of molecular research | Antibody test | Interpretation | |
| IgM | IgG | ||
| Positive | Negative | Negative | Acute infection |
| Positive | Positive | Negative | Acute infection |
| Positive | Positive | Positive | Infected patient |
| Positive | Negative | Positive | Infected or re-infected patient |
| Negative | Positive | Negative | Early stages of infection. More research needed |
| Negative | Positive | Positive | Infection. More research needed |
| Negative | Negative | Positive | Post-infectious period |
| Negative | Negative | Negative | Uninfected patient |
Innate and acquired types of immunity have points of contact, which represent two triads (Fig. 21)
Rice. 21. Two triads combining innate and acquired types of immunity
The development of an adaptive immune response takes quite a long time (from several days to two weeks), and in order for the body to defend itself against an already familiar infection faster, so-called memory cells . They, like veterans, are present in the body in small quantities, and if a pathogen familiar to them appears, they are reactivated, quickly divide and come out as an entire army to defend the borders (Fig. 22).
Fig.22. Memory T cells quickly form a secondary immune response
Immunological tolerance
Immunological tolerance (tolerance, arereactivity) is understood as the absence of an immune response to a specific antigen. The list of antigens to which tolerance can develop is practically indistinguishable from the set of antigens against which a specific immune response develops (Fig. 23).
Rice. 23. Immune tolerance
Tolerance mechanisms are necessary because the immune system produces a huge variety of antigen-specific receptors, and some of them are specific to the body's own antigens; Tolerance prevents unwanted reactions against one’s own organs and tissues, also for the normal course of pregnancy.
Immune system disorders in humans
Immune system disorders can be divided into three categories: immunodeficiencies, autoimmune diseases, and hypersensitivity reactions.
Immunodeficiencies
Immunodeficiency is a decrease in the quantitative indicators and/or functional activity of the main components of the immune system, leading to a disruption of the body’s defense against pathogenic microorganisms and manifested by an increased incidence of infectious diseases.
Primary immunodeficiencies (PIDs) are hereditary diseases caused by defects in genes that control the immune response. In general, PIDs manifest themselves already in early childhood, but sometimes only by the age of 30-40.
- symptoms that may be signs of primary immunodeficiencies:
- 4 or more cases of otitis during the year;
- 2 or more sinusitis during the year;
- low effectiveness of antibiotics for two or more months of use;
- 2 or more cases of pneumonia during the year;
- the child’s inability to gain weight and grow normally;
- frequent and deep abscesses of the skin and internal organs
- constant candidiasis of the oral cavity and skin;
- the need for intravenous antibiotics to resolve the infection;
- two or more systemic infections, including sepsis;
- hereditary predisposition.
According to development mechanisms, there are 4 main groups of PIDs (Table 3):
- Group 1 – predominantly humoral or B-cell PIDs;
- Group 2 – combined PID (all T-cell immunodeficiencies have impaired B-cell function);
- Group 3 – PID caused by defects in phagocytosis;
- Group 4 – PID caused by defects in the complement system.
Table 3. Some primary immunodeficiencies
| Pathology | Symptoms | Diagnostics | Treatment |
| Defects in antibody formation | |||
| Agammaglobulinemia | Frequent bacterial infections | Deficiency or complete absence of B lymphocytes | Antibiotics, lifelong administration of IgG |
| General variable immune deficiency | Frequent respiratory infections, otitis | Defects of T- and B-lymphocytes | Antibiotics, lifelong administration of IgG |
| Combined PIDs | |||
| Ataxia-telangiectasia (Louis-Bar syndrome) | Abnormal motor function, muscle weakness, speech impairment | Deficiency of T- and B-lymphocytes | Symptomatic |
| PID caused by defects in phagocytosis | |||
| Chronic granulomatous disease | Frequent pneumonia, purulent infections | Genetic defect | Lifelong antibacterial and antifungal therapy, interferon gamma |
| PID caused by defects in the complement system | |||
| Hereditary angioedema | Swelling of the lips and eyelids without itching. Swelling of the larynx, nose, tongue is life-threatening | Low concentration of C1 esterase inhibitor | Administration of C1 esterase inhibitor concentrate |
As follows from Table 3, the main and often the only treatment method for most patients with primary B-cell immunodeficiencies are immunoglobulins. These are medicines obtained from human blood plasma. They are designed to replace protective antibodies missing in the immune system in order to prevent or stop the development of severe infectious diseases. Today, a doctor’s arsenal includes immunoglobulins that differ in the concentration of the active substance (5 and 10%), as well as in the method of administration (intravenous and subcutaneous).
PID can appear at any age. Depending on this, the patient has unique problems that require certain types of support throughout his life (Table 4).
Table 4. Need for types of support for patients with PID in different age groups
| Age, years | Types of support | |||
| families | doctor | psychologist | society | |
| 0-14 | +++ | + | + | +++ |
| 14-18 | +++ | + | +++ | +++ |
| 18-65 | + | ++ | + | +++ |
| Over 65 | + | ++ | ++ | +++ |
Between the ages of 0 and 14 years, parental care is required to prevent infections and during the treatment period. May require: home training; providing psychological assistance; social support in purchasing medicines.
In adolescence (14-18 years), additional needs may arise for continued continuous education, vocational guidance, establishing relationships with peers, and organizing leisure time.
At the age of 18 to 65 years, patients more often experience infectious complications, and with them the costs of purchasing medications that are not subject to replenishment, as well as problems with employment.
In old age (over 65 years), there are needs for material, social and psychological support for a patient with PID.
Autoimmune pathology
Damage to the body's own organs and tissues by the immune system is called an autoimmune process . About 5% of humanity suffers from diseases of this type. The patient’s body develops hostilities reminiscent of a civil war: “friends against friends” go on the attack. There are no winners in this struggle - only suffering.
The selection of T lymphocytes in the thymus, as well as the removal of autoreactive cells in the periphery (central and peripheral immunological tolerance), which we discussed earlier, cannot completely rid the body of autoreactive T lymphocytes. As for B lymphocytes, the question of how strictly their selection is carried out still remains open. Therefore, in the body of every person there are necessarily many autoreactive lymphocytes, which, if an autoimmune reaction develops, can damage their own organs and tissues.
As an analogue, we can cite the Janissary infantry created by the Turks in the 14th century, which recruited Christian youths aged 8-16 who fought against their relatives.
T-cell autoimmune aggression has been well studied in rheumatoid arthritis, type 1 diabetes, multiple sclerosis and many other diseases.
The same Janissary cells, which do not remember their kinship, can be traced among B-lymphocytes:
- autoantibodies can cause cell death by activating the complement system on their surface or attracting macrophages;
- Receptors on the cell surface can become targets for antibodies.
For example, due to a breakdown of immunological tolerance, B-lymphocytes that produce antibodies are activated. This leads to a marked increase in the production of thyroid hormones (T4 and T3), as well as an increase in the size of the thyroid gland (hypertrophy). The pathology is called Graves' disease.
Another example would be myasthenia gravis, which is characterized by skeletal muscle weakness due to the formation of autoantibodies against structures responsible for cholinergic transmission and muscle fiber contraction;
- autoantibodies, together with soluble antigens, can form immune complexes that settle in various organs and tissues (for example, in the renal glomeruli, joints, on the vascular endothelium), disrupting their function and causing inflammatory processes.
Typically, an autoimmune disease occurs suddenly, and it is impossible to determine exactly what caused it. It is believed that almost any stressful situation can serve as a trigger, be it an infection, injury or hypothermia. A significant contribution to the likelihood of an autoimmune disease is made by both a person’s lifestyle and genetic predisposition - the presence of a certain variant of a gene.
Hypersensitivity
Hypersensitivity refers to an excessive immune response to an antigen. Hypersensitivity reactions are divided into several types depending on their duration and the mechanisms underlying them:
- Type I hypersensitivity involves immediate anaphylactic reactions, often associated with allergies. This type of reaction can range from mild discomfort to death. The basis of type I hypersensitivity is immunoglobulin E (IgE), which causes degranulation of basophils and mast cells;
- Type II hypersensitivity is characterized by the presence of antibodies that recognize its own proteins and mark the cells that express them for destruction. Type II hypersensitivity is also called antibody-dependent or cytotoxic hypersensitivity and is based on immunoglobulins G (IgG) and (IgM);
- type III hypersensitivity is caused by immune complexes consisting of antigens, complement proteins, IgG and IgM;
- Type IV hypersensitivity, also known as delayed hypersensitivity, develops over 2-3 days. Type IV hypersensitivity reactions are observed in many autoimmune and infectious diseases and are based on T cells, monocytes and macrophages.
Effective methods of influencing the immune system:
- Regular vaccination in terms of speed and quality of reaction exceeds the natural process of developing immunity to a specific infection;
- a balanced diet that ensures the maintenance of normal metabolism;
- regular physical activity ensuring the physiological functioning of all body systems and maintaining optimal body weight;
- giving up bad habits that lead to addiction (alcohol, nicotine, drugs, toxic, computer);
- daily routine, especially the influence of circadian rhythms (day and night): during wakefulness, the number of T-killer and NK cells peaks, as well as the concentration of anti-inflammatory substances such as cortisol and catecholamines; During sleep, the formation of memory T cells reaches its peak.
Speculative methods around immunity:
- taking immunostimulants is not clinically justified. If you constantly stimulate the production of leukocytes with drugs, the immune system will begin to lose its direct functions. This is when the moment of serious problems with immunity begins. Natural adaptogens do not affect the immune system at all: Schisandra chinensis, ginseng, eleutherococcus, radiola rosea. They act as amplifiers of RNA and protein synthesis (the basis of human cells), activate metabolic enzymes and the work of the endocrine and vegetative systems;
- Taking vitamins is clearly overrated. Vitamin D has a positive effect on the immune system, which stimulates the formation of T-killer cells. All other groups of vitamins do not directly participate in the functioning of the immune system;
- bath procedures and sauna do not affect the immune system;
- folk remedies such as honey and garlic have a mild bactericidal but not immunogenic effect.
Conclusion
The immune system is represented by three levels: organ, cellular and molecular with complex interactions between them.
Modern immunology distinguishes two interacting components of the immune system - innate and acquired (adaptive) types of immunity, which ensure the development of an immune response to genetically foreign substances, which are microorganisms, malignant tumor cells, transplanted organs and tissues.
Adaptive immunity is based on three main processes: recognition of antigens, their removal (elimination) and the formation of immunological memory.
Failures in the structure of the immune system lead to the development of immunodeficiencies, autoimmune diseases or hypersensitivity reactions.
Immunodeficiency at the genetic level (primary) or acquired (secondary) can appear at any age and lead to increased infectious morbidity. In recent years, replacement therapy has become available to prolong the lives of these patients. To improve their quality of life, it is necessary not only to provide expensive treatment, but also to organize support from family, psychologists and social institutions.
Autoimmune diseases and hypersensitivity are the body’s inability to resist a raging immune system that has confused its own and someone else’s.
Unfortunately, medicine has not yet learned to cure any of the diseases of the immune system, but only to use replacement therapy.
Effective preventive methods of influencing the immune system are vaccination and a healthy lifestyle. No one has yet been able to buy immunity at the pharmacy.
Survival prognosis
The prognosis depends on the type, stage, and degree of aggressiveness of the tumor. In particular, with marginal zone cell lymphoma of the spleen , the prognosis is influenced by factors such as the patient's age, leukocytosis, lymphocytosis, leukopenia, thrombocytopenia, anemia, damage to the red bone marrow and other organs, the use of chemotherapy and monoclonal antibodies.
One of the researchers divided patients into risk groups and calculated their five-year survival (% of patients alive within 5 years from the date of diagnosis of the disease):
- low risk - 88%;
- average risk - 73%;
- high risk - 50%.