Instructions for use of UROREC
Intraoperative floppy iris syndrome (ISID)
Patients taking or who have taken alpha-blockers during cataract surgery may experience intraoperative floppy iris syndrome, which can lead to complications during surgery. Patients scheduled for cataract surgery are not recommended to begin treatment with silodosin. Discontinuation of alpha-blocker treatment 1 to 2 weeks prior to such surgery is recommended, but the benefits and duration of discontinuation prior to cataract surgery have not yet been established.
In the preoperative assessment, surgeons and ophthalmologists should consider whether patients scheduled for surgery have taken or are taking silodosin to guide appropriate management and control of ISDR.
Orthostatic effect
As with the use of other alpha-blockers, a decrease in blood pressure and orthostatic hypotension may be observed during treatment with silodosin. Patients with a history of orthostatic hypotension are not recommended to take silodosin. At the first signs of orthostatic hypotension (dizziness, weakness), the patient should sit or lie down and remain in this position until the symptoms of orthostatic hypotension disappear.
Kidney failure
It is not recommended to use the drug for the treatment of patients with severe renal failure (creatinine clearance <30 ml/min).
Liver failure
Due to lack of data, the use of this drug in patients with severe hepatic impairment is not recommended.
Prostate carcinoma
Because BPH and prostate cancer have similar symptoms and can develop together, patients should be tested to rule out prostate cancer before being prescribed the drug.
Digital rectal examination and, if necessary, PSA determination should be performed before treatment and at regular intervals thereafter.
Treatment with Urorek leads to a decrease in the amount of seminal fluid released, which can affect male fertility. This effect disappears after stopping the drug. Before starting treatment, the patient should be informed about the occurrence of this effect, which temporarily affects male fertility.
Impact on the ability to drive vehicles and operate machinery
No studies have been conducted on the effects on the ability to operate vehicles and equipment. Patients should be informed about the possible manifestations of symptoms associated with orthostatic hypotension (for example, dizziness), and if they occur, refrain from driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reaction.
Urorek, 4 mg, capsules, 30 pcs.
Silodosin is extensively metabolized primarily through CYP3A4, alcohol dehydrogenase and UGT2B7. Silodosin is also a substrate for P-glycoprotein. Substances that inhibit or induce these enzymes and transporters may affect the plasma concentrations of silodosin and its active metabolite.
Alpha blockers
There is no sufficient information on the safety of co-administration of silodosin and other alpha-blockers. Therefore, simultaneous use of other alpha-adrenergic receptor antagonists is not recommended.
CYP3A4 inhibitors
In an interaction study, a 3.7-fold increase in maximum silodosin plasma concentration and a 3.1-fold increase in silodosin exposure (i.e., AUC) was found when coadministered with a potent CYP3A4 inhibitor (ketoconazole 400 mg). Concomitant use with strong CYP3A4 inhibitors (such as ketoconazole, itraconazole and ritonavir) is not recommended.
When silodosin was coadministered with a moderate CYP3A4 inhibitor, such as diltiazem, an approximately 30% increase in silodosin AUC was observed, but Cmax and elimination half-life were not affected. This change is not clinically important and no dose adjustment is required.
Phosphodiesterase (PDE) type 5 inhibitors
Only minimal pharmacodynamic interactions were observed between silodosin and the maximum dose of sildenafil or tadalafil. In a placebo-controlled study in 24 people aged 45-78 years receiving silodosin, co-administration of sildenafil 100 mg or tadalafil 20 mg caused a clinically non-significant mean reduction in systolic or diastolic blood pressure, as assessed by an orthostatic test (the difference between supine and diastolic heart rates). standing). In patients over 65 years of age, the mean decrease at different time points was between 5 and 15 mmHg. (systolic) and between 0 and 10 mm Hg. (diastolic). Positive results of orthostatic tests were only slightly more frequent during co-administration of drugs, however, symptomatic orthostasis or dizziness was not recorded. The condition of patients simultaneously receiving PDE-5 inhibitors and the drug should be monitored for the possible development of adverse reactions.
Antihypertensive drugs
In the clinical trial program, many patients received concomitant therapy with antihypertensive agents (mainly agents acting on the renin-angiotensin system, beta blockers, calcium antagonists and diuretics) without an increase in the incidence of orthostatic hypotension. However, precautions must be taken when using the drug simultaneously with antihypertensive drugs. Patients' condition should be monitored for possible development of adverse reactions.
Digoxin
Co-administration of silodosin 8 mg once daily did not have a significant effect on plateau concentrations of digoxin, a P-glycoprotein substrate. No dose adjustment is required.
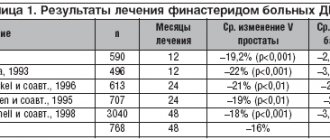
The problem of benign prostatic hyperplasia (BPH) and chronic abacterial prostatitis remains significant to this day. In 40–90% of men over 50 years of age, clinical manifestations are observed, united by the concept of “lower urinary tract symptoms” [1]. At the same time, a reliable relationship between the manifestations of BPH and the age of the patients is determined. Considering the prevalence of this symptomatology and the projected persistent increase in the population of men over 60 years of age, the relevance of this disease is significantly increasing [2-4].
The incidence of chronic prostatitis in the male population averages from 10 to 15%. Moreover, up to 95% of all patients have so-called abacterial prostatitis, in which modern cultural research methods do not allow identifying the bacterial agent causing the disease [5-7]. Taking into account the peculiarities of the pathogenesis of the disease, the question of choosing treatment tactics for this category of patients seems especially relevant today. Complete cure of chronic abacterial prostatitis currently appears to be an elusive goal, so symptomatic treatment, especially for category IIIB, is the most likely way to improve quality of life. The goal of treatment is to reduce irritative (urgency, increased frequency, nocturia) and obstructive (difficulty urinating, thinning urine stream, incomplete emptying of the bladder) symptoms.
Objective: to evaluate the effectiveness and safety of monotherapy with the new uroselective alpha-blocker Urorek® (active ingredient silodosin) in patients with a combination of BPH and chronic non-infectious prostatitis.
MATERIALS AND METHODS
The observation included men over 45 years of age with a diagnosis of BPH and proven chronic abacterial prostatitis, with the presence of irritative and obstructive symptoms (on the IPSS scale more than 8 points and lasting more than 6 months, on the NIH-CPSI scale more than 10 points and lasting more than 3 months), maximum volumetric urination rate from 5 to 15 ml/sec, urinary volume from 100 to 350 ml, residual urine volume less than 150 ml, prostate gland (PG) volume more than 25 cm3, serum PSA less than 4 ng/ml and absence growth of microflora in the culture of the 3rd portion of urine, pancreatic secretion and (or) ejaculate.
After the screening examination, 60 patients took part in the observation, who were randomly divided into two groups of 30 people each. The first group received Urorek® (silodosin) at a dosage of 8 mg once daily for three months. The second group of patients (control) received herbal remedies based on Serenoa repens. The duration of observation was 12 weeks. The groups of patients were statistically homogeneous (p>0.05) in terms of age, pancreatic volume, amount of residual urine, maximum urinary flow rate, severity of the inflammatory process in pancreatic secretions, symptom scores on the NIH scale, heart rate and blood pressure.
Methods for assessing effectiveness included studying the disease history, current complaints, digital rectal examination of the prostate gland, assessing disease symptoms using IPSS, quality of life (QoL), and NIH-CPSI scales. A bacteriological examination of the third portion of urine, pancreatic secretion and (or) ejaculate, as well as a general urine test and a Nechiporenko urine test in three portions were mandatory. All patients underwent uroflowmetry, ultrasound examination of the pancreas, including transrental ultrasound examination of the pancreas (power Doppler), as well as determination of the functional capacity of the bladder and the presence of residual urine. All patients underwent serum PSA determination.
The first stage was a transrectal ultrasound examination in B-mode: transverse scanning - from the base of the pancreas to its apex, and longitudinal scanning - from the center of the gland to the right and then the left parts of the gland. The size and volume of the gland, its contours and shape, and the echo structure of the pancreas were assessed. Then, color Doppler mapping of the pancreas and its study in power Doppler mode were performed, which made it possible to evaluate the vascular pattern of the pancreas and determine the degree of its severity in various parts of the gland. Transrectal Dopplerography ended with an analysis of blood flow characteristics in spectral Doppler mode, when in 2-3 zones of the prostate with the most pronounced blood flow its main indicators were determined, such as maximum and minimum linear velocities, pulsation index (PI) and resistance index (RI). We tried to select the indicated study areas for each patient in the area of the bladder neck and along the prostatic urethra.
The safety study of silodosin was assessed by measuring blood pressure and heart rate, 6-channel ECG, general and biochemical blood tests.
Exclusion criteria from observation were ongoing anti-inflammatory therapy for chronic prostatitis and (or) BPH in the last 3-6 months, the presence of a malignant disease of the urinary system, neurogenic dysfunction, diverticula and bladder stones, urethral stricture, sclerosis of the bladder neck, as well as genitourinary tract infections in the phase of active inflammation and severe concomitant diseases, clinically significant renal and liver failure. A history of surgical procedures on the pelvic organs and systematic use of drugs that affect bladder function and urination were contraindications for inclusion of patients in the observation groups.
All patients were examined three times: 1st time at the time of inclusion, 2nd time – 6 weeks after the start of the study, 3rd time – after 12 weeks.
The average age of patients with BPH in combination with chronic non-infectious prostatitis was 56 years. The duration of the disease varied from 6 to 12 years. At the time of inclusion, patients complained of frequent and difficult urination, sluggish stream, a feeling of incomplete emptying of the bladder, nocturia from 1 to 5 times.
RESULTS
According to the results of the control examination after 3 months, no negative dynamics in the cardiovascular system were observed. Urorek® (silodosin) was well tolerated by patients. During the examination, no side effects associated with taking the drug were noted.
Rice. 1. Characteristics of the IPSS clinical symptom index according to the results of the study
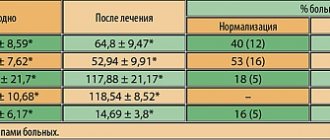
In the group of patients taking Urorek® (silodosin), there was a decrease in the IPSS clinical symptom index from 14 to 6.8 points (Fig. 1) and NIH-CPSI (from 18.4 to 12.3 points), which significantly exceeded the indicators in control group (from 13 to 11.2 and from 17.1 to 16.8 points, respectively). The quality of life index (QoL) in the first group increased by 2 points (from 1.9 to 3.9), while in the second group the change in this parameter was not pronounced (from 3.3 to 3.6). The dynamics of the decrease in scores based on comparison of visual analogue pain scale indicators turned out to be the same in patients of both groups.
There were no statistically significant results on the questionnaire and pancreatic volume in patients of both groups 1 and 2.
A significant increase in maximum urinary flow rate was observed in patients treated with silodosin. Thus, the maximum urine flow rate, studied during uroflowmetry, in the first group increased from 12.4 to 18.2 ml/sec, in the control group from 13.5 to 14.5 ml/sec (Fig. 2). The volume of urination in the first group increased from 174 ml to 216 ml, in the control group from 191 ml to 209 ml. The volume of residual urine in the first group decreased from 57 ml to 38 ml, in the control group - from 52 ml to 45 ml. The dynamics of leukocyte levels in the 3rd portion of urine and pancreatic secretion were without significant changes in all patients.
Studying blood flow parameters in spectral Doppler mode revealed its improvement in the prostate gland after completion of treatment in 16 (53.3%) patients receiving therapy with Urorek® (silodosin) (Fig. 3, 4). There was an increase in resistance index (RI) as a measure of peripheral vascular resistance: from 0.48 to 0.72 (p
Rice. 2. Change in maximum urine flow rate in patients taking Urorec® (silodosin) and in the control group
Rice. 3. Transrectal ultrasound of a patient treated with Urorek® (silodosin): areas of increased echogenicity in the prostate gland of varying intensity and size
Rice. 4. Same patient. Transrectal Doppler ultrasound after treatment with Urorek® (silodosin). Improving prostate blood flow parameters in spectral Doppler mode
Side effects in the group receiving therapy with Urorek® (silodosin) (3.3% of patients) consisted of general weakness that arose on the first day of administration and spontaneously resolved by the end of the second day. There was no need to discontinue the therapy received by the patients. All patients of group 1 completed treatment. Neither in the first nor in the second group were there any statistically significant changes in systolic and diastolic blood pressure and heart rate.
CONCLUSIONS
The use of the drug Urorek® (silodosin) for three months at a daily dose of 8 mg led to a decrease in symptoms and an improvement in quality of life, a decrease in the volume of the prostate gland and an increase in the maximum volumetric flow rate of urine. Thus, according to the results of the study, Urorek® (silodosin) is an effective drug for long-term and continuous treatment of patients with BPH in combination with chronic non-infectious prostatitis.
The therapeutic properties of the drug Urorek® (silodosin) allow effective and targeted therapy aimed at reducing irritative and obstructive symptoms characteristic of manifestations of benign prostatic hyperplasia in combination with chronic abacterial prostatitis. The effectiveness of the drug Urorek® (silodosin) in patients with BPH and chronic bacterial prostatitis can be explained by a decrease in the severity of urethroprostatic reflux by reducing the turbulence of urine flow and eliminating the retention of pancreatic secretions due to the expansion of the excretory ducts of the pancreatic acini. This direct effect probably contributes to a more complete and rapid sanitation of the pancreas from bacterial flora.
The most pronounced clinical effect was observed among patients who, according to transrectal Doppler ultrasound, showed an improvement in blood circulation in the pancreas. Transrectal Doppler sonography is a study that allows us to purposefully select the most adequate drug therapy for each patient with chronic prostatitis and BPH and evaluate its effectiveness.
In summary, the results of using the drug Urorek® (silodosin) can be expressed as follows:
- Effective treatment of two diseases simultaneously: – benign prostatic hyperplasia – chronic prostatitis.
- Safety: – minimal risk of lowering blood pressure – no effect on sexual function – no effect on PSA levels (does not interfere with cancer diagnosis)
- Ease of use: – no dose selection required (1 capsule once a day) – no adjustment of existing treatment required (possibility of combination with other medications)
Summary:
The purpose of the study was to evaluate the effectiveness and safety of the new uroselective alpha-blocker silodosin (Urorek®) in monotherapy for patients with a combination of benign prostatic hyperplasia (BPH) and chronic non-infectious prostatitis.
Materials and methods. 60 patients took part in the observation and were divided into two groups of 30 people each. The first group received Urorek® (silodosin) at a dosage of 8 mg once daily for three months. The second group of patients (control) received herbal remedies based on Serenoa repens. The groups of patients were statistically homogeneous in terms of age, prostate volume, amount of residual urine, maximum urination rate, severity of the inflammatory process in prostate secretions, symptom scores on the NIH scale, heart rate and blood pressure.
Results. The use of the drug Urorek® (silodosin) for 3 months at a daily dose of 8 mg led to a decrease in symptoms and an improvement in quality of life, a decrease in the volume of the prostate gland and an increase in the maximum volumetric flow rate of urine. The most pronounced clinical effect was observed among patients who, according to transrectal Doppler ultrasound, showed an improvement in blood circulation in the prostate gland. Side effects in the group receiving therapy with Urorek® (silodosin) consisted of general weakness that arose on the first day of administration and spontaneously resolved by the end of the second day. There was no need to discontinue the therapy received by the patients.
Conclusion. Urorek® (silodosin) is an effective drug for long-term and continuous treatment of patients with BPH in combination with chronic non-infectious prostatitis.
LITERATURE
1. Berry SL, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. // J Urol. 1984. Vol.132. P. 474-479.
2. Benign prostatic hyperplasia [ed. ON THE. Lopatkin]. M. 1999. – 216 p.
3. WHO. Aging and health. Global movement for active aging. – Geneva, 1999.
4. Apolikhin O.I., Kakorina E.P., Sivkov A.V., Beshliev D.A., Solntseva T.V., Komarova V.A. The state of urological morbidity in the Russian Federation according to official statistics. // Urology. 2008. No. 3 P. 3-9.
5. Sivkov A.V. Diagnosis and treatment of benign prostatic hyperplasia. // Consilium Medicum. 2002. (Urology Appendix). pp. 9–18.
6. Oesterling JE. The origin and development of benign prostatic hyperplasia. An age-dependent process. // J Androl. 1991. Vol. 12. P. 348–355.
7. Jepsen JV, Bruskewitz RS. Comprehensive patient evaluation for benign prostatic hyperplasia. //Urol. 1998. Vol. 51, Suppl. 4A. P. 13–18.
| Attached file | Size |
| 767.8 kb |
‹ Epidemiological analysis of the incidence of urolithiasis in the adult population of the Irkutsk region Up