Pharmacological properties of the drug Finasteride
A synthetic 4-azasteroid compound, a specific inhibitor of 5-α-reductase, an intracellular enzyme that converts testosterone into a more active androgen - dihydrotestosterone (DHT). In benign prostatic hyperplasia (BPH), its increase is caused by the conversion of testosterone to DHT in the prostate tissue. Finasteride is a highly effective drug that reduces the level of DHT in the blood and prostate gland. Finasteride has no affinity for androgen receptors. Finasteride treatment causes regression of prostatic hypertrophy, steadily increases the maximum urinary flow rate and improves clinical symptoms.
Finasteride (Propecia/Proscar)
Testosterone in men is produced primarily in the testicles and also in the adrenal glands. Most testosterone in the body is bound to sex hormone binding globulin (SHBG), a protein made in the liver that transports testosterone through the blood, prevents its metabolism, and prolongs its half-life. When freed from SHBG, free testosterone can enter cells throughout the body. In some tissues, particularly the scalp, skin and prostate, testosterone is converted to 5alpha-dihydrotestosterone (DHT) by the enzyme 5-alpha reductase. DHT is a more potent androgen than testosterone (with approximately 3-10 times more activity at the androgen receptor, the site of action of androgen hormones), so 5alpha reductase may be considered to enhance the androgenic effects of testosterone in the tissues where it is found. Finasteride, a 4-azasteroid and testosterone analogue, acts as a potent and specific, competitive inhibitor of one of the two subtypes of 5alpha reductase, in particular the type II isoenzyme. In other words, it binds to the enzyme and prevents the metabolism of endogenous substrates such as testosterone. 5α-reductases type I and II account for approximately one-third and two-thirds of systemic DHT production, respectively. Other 5a-reductase substrates include progesterone, androstenedione, epi-testosterone, cortisol, aldosterone, and deoxycorticosterone. The full physiological effect of their restoration is unknown, but it is likely related to their release or is itself physiological. In addition to acting as a catalyst in the rate-limiting testosterone reduction reaction, isoforms I and II of the 5alpha reductase enzyme reduce progesterone to dihydroprogesterone (DHP) and deoxycorticosterone to dihydrodeoxycorticostenor (DHDC). In vitro and animal models show that subsequent 3alpha reduction of DHT, DHP and DHDOC results in the creation of steroid metabolites that affect brain function by increasing GABA inhibition of gamma-aminobutyric acid. These neuroactive steroid derivatives increase GABA levels at GABA(A) receptors and have anticonvulsant, antidepressant, and anxiolytic effects, as well as influencing sexual and alcohol-related behavior. 5alpha-dihydrocortisol from intraocular fluid is synthesized in the lenses, and can itself participate in the production of intraocular fluid. Allopregnanolone and DGDOK are neurosteroids, and the latter may influence the susceptibility of animals to epilepsy. 5alpha-dihydroaldosterone is a potent antidiuretic, although it is different from aldosterone. Its formation in the kidneys is increased by limiting dietary salt, suggesting that sodium can be conserved by:
Substrate + NADPH + H + → 5alpha substrate + NADP +
5alpha-DHP is one of the main hormones in the bloodstream of women with normal cycles and pregnant women. By inhibiting 5-alpha reductase, Finasteride prevents the formation of testosterone in DHT by the type II isoenzyme, resulting in a decrease in serum DHT levels by approximately 65-70%, and an increase in DHT levels in the prostate to 85-90%, where it is dominant. expression of type II isoenzyme. Unlike dual inhibitors of both 5alpha-reductase isoenzymes, which can reduce DHT levels throughout the body by more than 99%, Finasteride is not able to completely suppress the production of DHT, since it cannot have a significant inhibitory effect on the type I 5alpha-reductase isoenzyme, having 100 times lower affinity for I compared to II. In addition to blocking the type II isoenzyme, Finasteride competitively inhibits the type II 5beta-reductase isoenzyme, but this is not thought to affect androgen metabolism. By blocking the production of DHT, Finasteride reduces androgen activity in the scalp. In the prostate, inhibition of 5alpha reductase reduces prostate volume, reducing the development of benign prostatic hyperplasia (BPH) and the risk of prostate cancer. Inhibition of 5alpha reductase also reduces epididymal weight, reduces motility, and alters the normal positioning of sperm within the epididymis. Cause of Mood-Related and Sexual Side Effects DHT and neuroactive steroids (NAS), such as allopregnanolone (ALLO) and tetrahydrodeoxyorthicosterone (THDOC), are potent positive allosteric modulators of the GABA receptor (affecting the same sites as euphoria-inducing substances and anxiolytic drugs such as benzodiazepines and alcohol) and are important endogenous neuroregulators with potent antidepressant and anxiolytic effects, as well as playing a positive role in sexual functioning. Their biosynthesis depends on both isoforms of 5alpha reductase. Finasteride reduces their formation in the body. This effect of Finasteride is the likely cause of the emotional and sexual side effects associated with the drug. Additionally, since it involves not only DHT but also NAS, it could potentially also explain the fact that mood and anxiety-related effects are seen in women as well as men.
Special instructions for the use of Finasteride
With a large volume of residual urine and/or with a sharply reduced urine outflow, it is necessary to strictly monitor the patient’s condition, as obstructive uropathy may develop. So far, no clinically beneficial effect has been found from treating prostate cancer patients with finasteride. Patients with BPH and elevated PSA levels were followed in controlled clinical trials with serial PSA testing and prostate biopsy. In these studies, finasteride did not affect the detection rate of prostate cancer. The overall incidence of prostate cancer was not significantly different between patients receiving finasteride or placebo. Before starting and periodically during treatment with finasteride, it is recommended to conduct a digital rectal examination, as well as other types of studies to exclude the presence of prostate cancer. PSA level determination is also used in medical practice to detect prostate cancer. As a rule, a PSA level above 10 ng/ml indicates the need for further laboratory tests and, possibly, a biopsy; Further laboratory tests are also indicated at PSA levels of 4–10 ng/ml. There was significant similarity in PSA levels between men with and without diagnosed prostate cancer. Therefore, in men with BPH, PSA levels in the normal range do not exclude the presence of prostate cancer, regardless of finasteride treatment. A PSA level exceeding 4 ng/ml does not exclude the presence of prostate cancer. Finasteride reduces PSA concentrations by approximately 50% in patients with BPH, even in the presence of prostate cancer. It should be taken into account that in patients with BPH taking finasteride, a reduced serum PSA level does not exclude the simultaneous presence of a malignant tumor. This decline in PSA levels can be predicted with reasonable confidence over a fairly wide range of values, although it may vary in individual cases. According to clinical studies, when determining PSA in patients taking finasteride, the values obtained should be doubled compared to normal values in men. This correction allows us to maintain the sensitivity and specificity of the method and its diagnostic value for detecting prostate cancer. If there is a persistent increase in PSA levels in patients who have previously been prescribed finasteride, this indicator should be carefully analyzed, without excluding the possibility that the patient did not take finasteride. The concentration of PSA in the blood plasma depends on the age of the patient and the size of the prostate gland; in turn, the volume of the prostate gland depends on the age of the patient. When interpreting the results of PSA laboratory tests, it should be taken into account that, as a rule, PSA levels are usually reduced in patients taking finasteride. In most patients, a rapid decrease in PSA levels is observed already during the first months of treatment, after which the PSA level stabilizes to a new initial value, which is 2 times less than that before treatment. Thus, when treated with finasteride for 6 months or more, the PSA level should be doubled to compare with normal values in men. Due to the ability of 5-α-reductase inhibitors to inhibit the conversion of testosterone to DHT, such drugs (including finasteride) may cause disturbances in the development of the external genitalia of the male fetus and should therefore be avoided by pregnant women or women who may become pregnant. finasteride. Not intended for use in pediatrics.
Finasteride in the treatment of patients with prostate hyperplasia
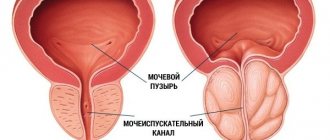
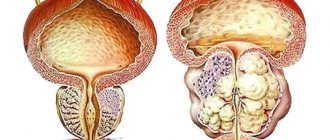
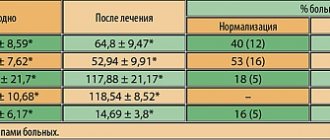
The clinical manifestations of the disease are determined by the degree of disturbance of the outflow of urine. The symptom complex of the disease includes obstructive (difficult, sluggish stream, straining urination, feeling of incomplete emptying of the bladder) and irritative (frequent urge to urinate during the day and night, imperative urge to urinate, which may be accompanied by urinary incontinence) symptoms, which are combined into symptoms lower urinary tract (LUTS). Although symptoms of impaired urinary outflow (weak stream, intermittent urination) are much less common than irritative symptoms (frequent urination in small portions, nocturia, urgency, urinary incontinence), they are dominant among the symptoms that reduce the quality of life of patients [2]. About 25% of men over 50 years of age have lower urinary tract symptoms (LUTS), the severity of which is usually determined by the score of the International Prostate Symptom Score (IPSS), which was developed to objectify the clinical manifestations of BPH [6,7]. Clinical studies show that LUTS are prevalent and progressively worsen with age (4,8). Today, the average life expectancy of a man in Europe and North America is 72 years and continues to grow [8]. The growth dynamics are such that from 1980 to 2050 the population aged over 65 years should double and reach 15% of the entire human population. As a consequence, we can expect an increase in the number of potential patients suffering from prostatic hyperplasia. In this regard, the diagnosis and treatment of BPH is not only a serious medical, but also a major social problem, which emphasizes the importance of timely treatment of BPH [2]. Currently, medical and surgical methods for treating BPH are used, which are quite effective. As a result of the use of drug therapy, the number of surgical interventions for prostate hyperplasia has significantly decreased. For example, in the United States, the number of transurethral and open adenomectomies decreased from 250,000 in 1987 to 88,000 in 2000, despite the fact that the total number of patients suffering from BPH has increased as a result of the aging population [5]. Similar trends can be traced in other countries, including Russia. The main directions of conservative treatment of BPH are: • reducing the severity of symptoms, • improving the quality of life, • preventing the progression of the disease (in particular, preventing such a serious complication as acute urinary retention and the need for surgical treatment) [9]. Today, as is known, there are 3 groups of drugs used in the treatment of patients with prostate hyperplasia: β1-blockers, 5β-reductase inhibitors and plant extracts [2]. One of the most widely used and studied drugs is finasteride (Prosteride, etc.), a synthetic 5a-reductase inhibitor. One of the central provisions of the theory of the pathogenesis of BPH concerns the role of the enzyme 5a-reductase and dihydrotestosterone (DHT). The basis for the development of this concept was the observation of cases of pseudohermaphroditism caused by the congenital absence of 5a reductase. With normal serum testosterone levels, these men showed a significant decrease in DHT levels, accompanied by underdevelopment or virtual absence of the prostate gland. Subsequently, they did not experience the occurrence of adenoma and prostate cancer (PCa). According to its chemical structure, finasteride belongs to 4-azasteroids and is a powerful competitive inhibitor of 5a-reductase, mainly type 2. The drug blocks the conversion of testosterone into a more active androgen - 5a-DHT at the level of the prostate gland. Treatment with finasteride (Prosteride) reduces prostate volume, serum DHT levels decrease by 70–75%, and PSA levels by approximately 50%. The mechanism of action of finasteride on the pathogenesis of BPH through growth factors has been established: the specific effect of the drug on the TGFb signaling system, which has an inhibitory effect on the proliferation of prostate tissue, leads to glandular atrophy and stimulates the process of cell death (apoptosis), as well as a significant decrease in intraprostatic levels of DHT and EGF (mainly in the periurethral zone) and a decrease in the presence of bFGF in the stroma of the prostate gland of patients with BPH [F. Sciarra et al., 1997; F. Torruba et al., 1997; S. Saez et al., 1999]. A series of morphological studies have demonstrated that in men, finasteride leads to atrophic processes in the glandular and stromal tissues of the prostate gland, and in the first case, changes are detected after 3, and in the second, after 6 months of treatment [M. El-Demiry, E. Ishak, 1997]. According to L. Marks et al. (1997) and M. Feneley et al. (1997), finasteride therapy reduces the proportion of epithelial tissue in the prostate gland and the epithelial-stromal ratio by approximately 50%, mainly in the transition zone. Finasteride has been used in clinical practice since the late 80s - early 90s of the 20th century. Clinic of Urology MMA named after I.M. Sechenova has experience with long-term use of finasteride, which was first tested in a two-year, multicenter, placebo-controlled, double-blind study in 1992–1994. We analyzed our own experience of long-term treatment of patients with prostatic hyperplasia with finasteride in the urological clinic of the Moscow Medical Academy in the period from 1995 to 2004. The analyzed data were based on the results of 5 years of treatment with finasteride in 56 patients suffering from BPH. The average age of the patients was 63.8 years (from 59 to 81 years). Patients took finasteride 5 mg once daily. Control examinations were carried out in patients after 2, 6, 12, 24 months, 3, 4 years and 5 years. We carried out a quantitative assessment of complaints from patients with prostatic hyperplasia using the IPSS questionnaire. The initial mean IPSS total score was 14.6 points. A statistically significant decrease in the average IPSS score was noted after 6 months of treatment and amounted to 13%. An even more pronounced (22%) decrease in the average IPSS value was recorded after 12 months of therapy. Over the next 4 years, the effect of treatment remained: the average IPSS score slowly decreased and by the end of the fifth year of treatment it was 10.65 (–27% of the original). The quality of life of patients was assessed based on the results of the answer to the question about the degree of distress (BS) caused by symptoms of impaired urination, contained in the IPSS-BS questionnaire. Analysis of the BS indicator values showed a significant decrease after 6 months of treatment, which indicated an improvement in the quality of life of patients and, accordingly, the subjective effectiveness of treatment. The average value of the BS quality of life index was initially 3.8 points - mostly unsatisfactory, and after 60 months of treatment - 1.9 - mostly satisfactory. A persistent improvement in quality of life over 5 years of treatment with finasteride was recorded in 73.2% of patients. As criteria for objective assessment of the effectiveness of drug treatment with finasteride, we used the change in the maximum volumetric flow rate of urination (Qmax) according to uroflowmetry and the volume of the hyperplastic prostate gland according to transrectal ultrasonography before, during and after the end of therapy. The initial mean Qmax value was 9.4 ml/s. The average Qmax value increased statistically significantly after six months of finasteride therapy and amounted to 11.4 (+2) ml/s. The most significant increase in the indicator was observed at the end of the 12th month of treatment (12.5 ml/s; +3.1 ml/s). By the end of the five-year period, the average Qmax increased slightly and amounted to 12.7 (+3.3 ml/s), which objectively indicated the continued effectiveness of finasteride with long-term use. According to transrectal ultrasonography, the average value of the initial volume of the hyperplastic volume of the prostate gland was 51.8 cm3. During the first 6 months of treatment with finasteride, there was a decrease in the enlarged prostate gland by an average of 9%; after 12 months of therapy, the most significant decrease in the average prostate volume was noted to 40.7 cm3 (-23%), over the next four years of treatment there was a further decrease in volume hyperplastic prostate gland, as a result of which the average prostate volume decreased to 37.9 cm3 (-26.7% of the original). The degree of reduction in the volume of the hyperplastic prostate was more pronounced in patients with an initial volume of more than 40 cm3, which is probably due to the predominance of the glandular component in large-sized hyperplasia. Drug treatment with finasteride led to a significant decrease in the average volume of the hyperplastic prostate gland, with this trend maintaining throughout the 60 months of observation. Thus, treatment with finasteride for 5 years was effective both according to subjective assessment (IPSS-BS) and objective parameters (Qmax, prostate volume) [1]. The safety of long-term treatment with finasteride was assessed based on the frequency and severity of adverse reactions and changes in blood and urine tests. Changes in sexual function were assessed using the Brief Sexual Function Inventory (BSFI). Of the 56 patients taking finasteride, 6 (10.7%) patients were diagnosed with 7 adverse events. The majority of adverse events were due to the effect of finasteride on sexual function - 8.9% of our patients experienced erectile dysfunction and/or decreased libido. Within 60 months of finasteride use, 13 (23.2%) of 56 patients discontinued treatment. Due to side effects, 4 (7.1%) patients stopped taking the drug: 3 patients developed sexual dysfunction, and one patient developed gynecomastia after 3 years of continuous use of finasteride. All three patients with sexual dysfunction who refused further treatment with finasteride were under 65 years of age, and maintaining sexual activity was important to them. After discontinuation of finasteride, all 3 patients experienced recovery of sexual function. 8 (14.3%) of 56 patients refused further treatment due to lack of effect. Only 1 (1.8%) patient experienced acute urinary retention during treatment, which required surgical treatment. During treatment with finasteride, no clinically significant changes in laboratory parameters were noted. Our results indicate a relatively low incidence of adverse events and the safety of long-term use of finasteride in patients with prostate hyperplasia [1]. The effectiveness and safety of long-term finasteride therapy have also been established in several large studies, including placebo-controlled ones. Data from studies of the effectiveness of the drug are presented in Table 1. According to all the studies presented, during continuous treatment for up to 4 years, finasteride allowed a statistically significant reduction in the severity of urinary disorders (reduce the total IPSS score) compared to placebo, and improve the quality of life of patients (reduce the BS) and reduce the volume of the prostate gland (according to transrectal ultrasound). Thus, studies indicate a long-lasting effect and the safety of long-term treatment of patients with BPH with finasteride. Data on the effectiveness of finasteride as a chemoprevention agent for prostate cancer (PCa) are also interesting and important. The effect on testosterone metabolism in the prostate gland has long attracted the attention of specialists to finasteride as a potential means of cancer chemoprevention [I. Thompson et al., 1995]. It was possible to evaluate such capabilities of the drug only after the completion of a large-scale study conducted under the auspices of the US National Cancer Institute and called PCPT. It involved 18,882 men over 50 years of age, with no changes suspicious for prostate cancer on examination per rectum, a PSA level of less than 3 ng/ml, and an IPSS score of less than 20 points. All patients underwent a prostate biopsy at the initial stage of the study, based on the results of which cases of prostate cancer and high-grade dysplasia (prostatic intraepithelial neoplasia - PIN) were excluded. For 3 months, all study participants received placebo, after which they were randomized into two approximately equal groups, one of which (n=9423) received finasteride 5 mg/day, and the other (n=9459) received placebo. Control biopsies were performed during the program “as indicated” (if the PSA level exceeded 4 ng/ml) and in all patients upon its completion. The study lasted 7 years. As a result, prostate cancer was diagnosed in 18.4% of patients taking finasteride, and in 24.4% taking placebo, i.e. the relative risk of developing the disease decreased by 24.8% (p<0.001). The vast majority of patients were diagnosed with a localized T1–2 tumor (97.7–98.4%). At the same time, when treated with finasteride compared to placebo, high Gleason grade tumors (G 7–10) were detected significantly more often – 37 and 22.2%, respectively. Based on the results of the PCPT study, the following conclusions were drawn: 1) finasteride prevents or slows down the development of prostate cancer; 2) a significant decrease in the incidence of prostate cancer was recorded (by approximately 25%) in the finasteride group compared to placebo; 3) a reduction in the risk of developing prostate cancer was demonstrated by the results of a biopsy at the end of the study and by the results of an “indicated” biopsy; 4) this result was obtained in all groups of patients, regardless of age, race, PSA level, or family history of prostate cancer. Turning to the results of PCPT, it should be noted that the study operated only on patients with “pure” BPH and did not answer the question of the nature of the effect of finasteride in pre-existing prostatic epithelial dysplasia (PED). A.V. Sivkov et al. A pilot study of the effect of finasteride on prostate tissue in patients with BPH and PIN was conducted. All program participants underwent a multifocal prostate biopsy before and after using the drug for at least 6 months [A. Sivkov et al., 2004]. The result revealed a significant decrease in the proliferative activity of the epithelium in 95% of patients, the frequency of epithelial forms of BPH in 76%, as well as a statistically significant increase in the frequency of stromal forms of BPH and the stromal-epithelial ratio by approximately 2 times (from 2.21 to 4.75). PCa was diagnosed in only 6.7% of patients and had a Gleason grade of 6–8. At the same time, a significant decrease in the incidence of epithelial metaplasia from 67 to 15%, the frequency of detection of high-grade PIN from 73 to 13%, and a decrease in the area occupied by the inflammatory reaction were registered. The latter fact is also important for chemoprophylaxis of prostate cancer in the light of data on the influence of chronic inflammation on tumor development [M. Lucia, K. Torkko, 2004]. In addition, the drug can be recommended for patients with persistent chronic prostatitis [A.V. Sivkov et al., 2004] [3]. To date, the most comprehensive and up-to-date study on the treatment of BPH remains the MTOPS (Medical Treatment of Prostatic Symptoms) study. This was a double-blind, placebo-controlled, randomized study examining the effectiveness of therapy with doxazosin, finasteride, or their combination in preventing and slowing the progression of prostatic hyperplasia, which included an examination of 3047 patients (average follow-up period - 4.5 years). The results showed that both doxazosin and finasteride and their combination were significantly effective against lower urinary tract symptoms. The risk of clinical progression of the disease when taking doxazosin was significantly reduced by 39% (p<0.001), by 34% when taking finasteride (p<0.002) and by 66% when using a combination of doxazosin and finasteride (p<0.002) compared with placebo [11 ]. A number of authors [G. Crea, G. Sanfilippo, G. Anastasi, C. Magno, C. Vizzini, A. Inferrera] studied the possibility of using finasteride in preparation for endoscopic treatment of benign prostatic hyperplasia. 30 patients of the main group took finasteride at a dose of 5 mg/day as preoperative preventive treatment. within 8–10 weeks. As a result, it was noted that in the group of patients who received finasteride in the preoperative period (average age 69 years), the amount of blood loss, determined by the difference in hemoglobin levels before and after surgery, was minimal. Patients in this group did not require blood transfusion. The decrease in hemoglobin level 24 hours after surgery in the main group averaged 0.9%. In the control group (mean age 67 years), 4 (12%) patients underwent blood transfusion. The decrease in hemoglobin averaged 2.36%. Patients in both groups were observed for 3 months after surgery. In two (6.6%) patients from the control group a week after discharge, an acute urination delay developed, due to the accumulation of blood clots. The results of the study were statistically reliable (p <0.001) [12]. Thus, the use of finsteride (protoride, etc.) in the treatment of benign prostate hyperplasia allows you to effectively affect the symptoms of the lower urinary tract both when used as monotherapy and in combination with A -dreno -blocking. At the same time, the data obtained in recent years make it appropriate for the purpose of finsteride as a means of preventing the development of prostate cancer.
Literature 1. Alyaev Yu.G., Vinarov A.Z., Lokshin K.L., Spivak L.G. Choice of treatment method for patients with prostatic hyperplasia, Kostroma, 2005. 2. Lopatkin N.A. Benign prostatic hyperplasia. Ed. ON THE. Lopatkina, Moscow, 1999. 3. A.V. Sivkov, V.N. Oshchepkov. Finasteride: 20 years of clinical practice in the treatment of patients with prostate adenoma // Consillium Medicum Volume 08/N 4/2006 4. Berry SJ, Coffey DS, Walsh PC, et al. The development or human benign prostatic hyperplasia with age // J. Urol. 1984. 132. 474–79. 5. Health Care Financing Administration: BESS Data, Washington, DC, 2000. 6. Girman CJ, Chute CG, Panser LA, et al. The prevalence of prostatism: a population–based survey of urinary symptoms // J. Urol. 1993. 150. P. 85–9. 7. Garraway WM, Collins GN, Lee RJ High prevalence of benign prostatic hypertrophy in the community // Lancet 1991. 338. R. 469–71. 8. Logie JW, Clifford GM et al. Lower urinary tract symptoms suggestive of benign prostatic obstruction – Triumph: the role of general practice databases // Eur. Urol. 2001; Vol. 39. (SBppl 3). P.42–7. 9. F. Montorsi, I. Moncada, Safety and tolerance of treatment for BPH // Eur. Urol. Appl. 2006. 5. P. 1005. 10. Novara G., Galfanoa., Gardi M., Ficcara V., Boccon Gibonl., Artibani W. Critical review of guidelines for BPH diagnosis and treatment strategy. Eur Urol Suppl 2006;5: 418–29. 11. McConnell JD, Roehrborn CG, Bautista OM, Andriole GL Jr ed. The long–term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. J Urol, 2006 Jan; 175(1):217–20. 12. G. Sanfilippo, G. Crea, G. Anastasi, C. Magno, C. Vizzini, A. Inferrera, The use of finasteride in preparation for endoscopic treatment of benign prostatic hyperplasia // Consillium Medicum Volume 09/N 4/2007.
Drug interactions Finasteride
Clinically significant interactions with other drugs have not been noted. Finasteride does not appear to have a significant effect on the cytochrome P450-related enzyme system involved in the metabolism of certain drugs. Clinical trials have examined combinations with propranolol, digoxin, glyburide, warfarin, theophylline and antipyrine. In clinical studies, finasteride has also been used in combination with ACE inhibitors, α- and β-adrenergic blockers, calcium channel blockers, nitrates, diuretics, H2-histamine receptor antagonists, HMG-CoA reductase inhibitors, NSAIDs, quinolones and benzodiazepines. However, no clinically significant negative reactions were found.