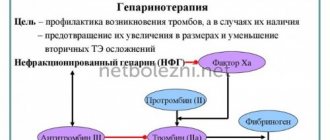
Anticoagulant therapy is a set of therapeutic measures aimed at inhibiting blood clotting activity and preventing the formation of blood clots. The use of anticoagulants makes it possible to have a targeted effect on different parts of the blood coagulation process. This makes it possible to use them in a number of serious pathological conditions.
CELT phlebologists are well acquainted with the principles of anticoagulant therapy and have been successfully applying them in practice for several years. They carry out an individual selection of appropriate drugs, preventing the development of such dangerous conditions as thrombosis and blockage of blood vessels. Their efforts reduce the risk of heart attacks and strokes.
Our doctors
Drozdov Sergey Alexandrovich
Cardiovascular surgeon, phlebologist, Doctor of Medical Sciences
47 years of experience
Make an appointment
Malakhov Yuri Stanislavovich
Doctor - cardiovascular surgeon, phlebologist, Honored Doctor of the Russian Federation, Doctor of Medical Sciences, doctor of the highest category
Experience 36 years
Make an appointment
Indications and contraindications for anticoagulant therapy
Anticoagulants are substances that inhibit blood clotting activity. They have a wide spectrum of action, minimizing exacerbations of diseases of the cardiovascular system in the form of strokes and heart attacks. By starting treatment with anticoagulants in a timely manner, it is possible to prevent the formation of blood clots in blood vessels with their subsequent blockage and the development of a number of serious complications.
Usually blood clots are tightly fixed to the walls of blood veins, but under the influence of blood flow, elevated temperature, blood pressure or physical stress they can come off. This situation arises suddenly, and a person’s life in it depends on how correctly and quickly medical care is provided to him. The consequences can be the most tragic: from stroke and heart attack to pulmonary embolism and thrombosis of the leg veins. Correct use of anticoagulants can eliminate these complications.
| Indications for anticoagulant therapy: | Contraindications to the use of anticoagulants: |
| Taking anticoagulants is not recommended for pregnant women, as it can cause disturbances in the embryonic development of the fetus and bleeding during the gestational period. It is also not recommended for patients suffering from:
|
Factor Xa inhibitors
According to its mechanism of action, rivaroxaban is an oral direct inhibitor of factor Xa that binds reversibly to it [22]. Rivaroxaban has high bioavailability (60–80%) and reaches peak plasma concentrations approximately 3 hours after administration [23]. There is evidence that rivaroxaban can bind not only to factor Xa, but also to Va (prothrombinase complex). This is one of the clinical advantages of the drug compared to heparin, which has a molecular weight that is too large to block factor Xa as part of the prothrombinase complex [22]. Unlike dabigatran, the absorption of this drug is not affected by the pH of the intestinal environment [24]. The half-life of rivaroxaban is approximately 5–9 hours in patients with normal renal and hepatic function [23, 25]. Higher levels of rivaroxaban may be determined in patients with impaired renal and hepatic function, since one third of the drug is excreted by the kidneys and about two thirds is metabolized in the liver, primarily through the cytochrome P450 system [26].
The primary outcome of the phase III clinical trials that led to the approval of rivaroxaban was a clinical trial program that evaluated the effectiveness of rivaroxaban compared with enoxaparin in 1000 patients undergoing knee surgery or total hip replacement. The drug was approved for use in Canada (September 2008), Europe (EMEA, October 2008), Australia (November 2008) and Russia (December 2008). In March 2009, a panel of experts from the FDA (Food and Drug Administration) recommended rivaroxaban for clinical use, albeit with adjustments to the need for more detailed information about the safety of the drug. The currently approved dosage of rivaroxaban for the prevention of VTE in patients undergoing hip or knee surgery is 10 mg as a single dose 6 to 10 hours after surgery, then daily for 5 weeks after hip surgery and two weeks after knee surgery.
Currently, approval for rivaroxaban dosage guidelines is based on doses used in the double-blind, randomized clinical trials RECORD-1, -2, -3, and -4 (Table 2). In particular, RECORD-1 [27] and RECORD-2 [28] studied rivaroxaban in patients undergoing hip replacement, and RECORD-3 [29] and RECORD-4 [30] studied patients after knee surgery. The RECORD-1 and RECORD-2 studies showed superiority of rivaroxaban compared with enoxaparin 40 mg administered as a single dose. Superiority over enoxaparin, including at dosages of 30 mg 2 times / day (North American doses of enoxaparin), is shown in RECORD-3 and RECORD-4. Rivaroxaban was overall superior to enoxaparin in all of these trials, despite a small, non-significant increase in bleeding.
In the cardiovascular clinic, rivaroxaban is still in phase III clinical trials - for the prevention of thromboembolic events in patients with non-valvular atrial fibrillation (ROCKET-AF), for secondary prevention of VTE (EINSTEIN), for primary prevention of VTE in patients with acute illness ( MAGELLAN) and for the secondary prevention of adverse cardiovascular events in patients with acute coronary syndrome (ATLAS ACS 2 TIMI 51).
In the MAGELLAN trial, rivaroxaban 10 mg was administered for 31–39 days, similar to the doses used in RECORD, compared with enoxaparin 40 mg as a single subcutaneous dose for 6–14 days in 8000 hospitalized patients (Identifier: NCT00571649). The ROCKET-AF trial is examining rivaroxoban 20 mg/day versus warfarin with a target INR range of 2–3 for thromboembolic prophylaxis in 14,000 patients with nonvalvular atrial fibrillation (ID: NCT00403767). Rivaroxaban was found to have an additive effect compared with standard treatment with acetylsalicylic acid or the combination of acetylsalicylic acid with clopidogrel for the prevention of adverse cardiovascular events in patients with recent acute coronary syndrome - ATLAS ACS 2 TIMI 51 phase III clinical trial (Identifier: NCT00809965). This drug is taken 2.5 or 5.0 mg 2 times a day. This dose is regulated by the phase IIb clinical trial ATLAS ACS TIMI 46, which showed not only a trend towards a reduction in the risk of adverse cardiovascular events, but also a dose-dependent increase in clinically significant bleeding [31].
The EINSTEIN-DVT study analyzed the effects of rivaroxaban at a dose of 15 mg twice daily for 3 weeks, then 20 mg once daily for up to 12 months. The study included 2900 patients with acute DVT but without PE; the effects of rivaroxaban were compared with those of warfarin (www.clinicaltrials. identifier: NCT00440193).
The EINSTEIN-PE study, identical in design to EINSTEINDVT, included 3300 patients with PE (identifier: NCT00439777). Final results from both EINSTEIN-DVT and EINSTEIN-PE have not been reported (as of June 2010), but results from the preliminary EINSTEIN-EXTENSION trial (ID: NCT00439725), in which patients received rivaroxaban 20 mg/day compared with placebo were presented in 2009 at the annual meeting of the American Society of Hematology [32]. According to the results of a study of 1197 patients with an average treatment duration of 190 days, recurrent VTE was recorded in 7.1% of cases in the placebo group and in 1.3% of patients receiving rivaroxaban (p < 0.001) [32]. There was no significant difference in the incidence of major bleeding, while clinically significant minor bleeding was observed in 1.2% of cases in the placebo group in 5.4% of patients receiving thromboprophylaxis with rivaroxaban [32].
Types of anticoagulants
Depending on the mechanism of action, it is customary to distinguish two types of anticoagulants: direct and indirect effects. The former reduce the activity of the thrombin enzyme, the latter increase the production of prothrombin. It is worth noting that anticoagulants of indirect action have a number of disadvantages that are absent in analogs of direct action. This determines the popularity of the latter, although they became available for treatment relatively recently: about ten years ago.
| Direct anticoagulants | Anticoagulants of indirect action |
The use of direct anticoagulants has a number of advantages:
Direct anticoagulants are represented by products containing heparin and hirudin. They are available as solutions for intramuscular and intravenous administration, ointments and gels | The use of indirect anticoagulants is based on the accumulation effect, which provokes an increase in the production of prothrombin. It can last from four to fourteen days. Indirect anticoagulants are used to prevent thromboembolism. They are available in the form of capsules and tablets (for example: Warfarin, Phenilin, others). Their disadvantages are as follows:
|
Direct and indirect effects of medications
Medicines affect thrombin production. A list of popular drugs with direct spectrum of action based on heparin is presented:
- Ardeparin;
- Klivarin;
- Longiparin;
- Nadroparin;
- Sandoparin.
Heparin derivatives reduce the rate of blood clotting.
The first indirect anticoagulant was the drug Dicumarol; today there are more than a hundred varieties. The effect of medications is based on slowing down the function of vitamin K, on which individual coagulation factors depend. Uncontrolled use of this subgroup of drugs increases the risk of hemorrhagic complications.
Indirect coagulants are divided into two main types:
- Based on indandione - a list of known representatives of the subgroup is presented by Phenindione, Diphenindione, Anisindione. In practice, only Phenilin is used; other medications cause allergic reactions and do not show a stable effect on the anticoagulant system of the blood.
- Based on coumarin, the main component is isolated from plants in the laboratory and is used to combat the increased formation of blood clots. Treatment of the pathology is carried out with Warfarin, Marevan, Sinkumar, Neodicoumarin and other anticoagulants.
Therapy with this group of drugs requires constant medical supervision due to the risk of adverse reactions.
Complications of anticoagulant therapy
Taking anticoagulants is associated with a number of complications. They can be excluded if you strictly follow the instructions of the treating phlebologist, follow the dose and regularly monitor blood coagulation function. When anticoagulants are administered by injection, a hematoma may appear in the injection area. This phenomenon occurs due to the dilution of blood in the blood capillaries and its leakage through the vascular walls.
While taking anticoagulants, the patient needs to be especially attentive to his condition and contact his doctor if the following clinical manifestations appear:
- Painful symptoms affecting the lumbar region;
- Feeling of weakness and numbness in the legs;
- Recurrent pain and discomfort in the upper abdomen;
- Any disturbances in the functioning of the genitourinary system;
- Regular sudden hemorrhages.
If you want to be sure that treatment with anticoagulants will give the desired result and go without complications, contact the specialists of the phlebology department of CELT. You can make an appointment with them online or by contacting our operators.
Make an appointment through the application or by calling +7 +7 We work every day:
- Monday—Friday: 8.00—20.00
- Saturday: 8.00–18.00
- Sunday is a day off
The nearest metro and MCC stations to the clinic:
- Highway of Enthusiasts or Perovo
- Partisan
- Enthusiast Highway
Driving directions
Antiplatelet drugs (drugs that disrupt primary hemostasis)
Acetylsalicylic acid (ASA)
Egyptian papyri that date back to approximately 1550 BC. e., mention the use of a decoction of white willow leaves for many diseases. Hippocrates prescribed willow bark extract for headaches and fever. Willow is the first source of aspirin. Acetylsalicylic acid, the active ingredient in Aspirin, was synthesized from willow bark by Edward Stone in 1897. The age of medicine is shorter than the age of man and many of them quickly become obsolete. But Aspirin not only has not become obsolete over the last hundred years, it has also demonstrated new qualities. It seems to cure everything from colds to strokes.
Acetylsalicylic acid (ASA) has been the gold standard of antiplatelet therapy for many years. Aspirin in low doses (40-100 mg) irreversibly blocks the action of the enzyme cyclooxygenase-1 in platelets with a subsequent decrease in the formation of thromboxane A2, which is a powerful vasoconstrictor and proplatelet agent. Since ASA blocks COX-1 irreversibly, the antiplatelet effect persists throughout the life cycle of the platelet (7–10 days). The ability to irreversibly block platelet COX-1 distinguishes ASA from other non-steroidal anti-inflammatory drugs, the antiplatelet effect of which is short-term [1]. Considering the above, I would like to draw attention to the following clinical point.
What happens when a dentist prescribes an NSAID to a patient taking Aspirin? Data from epidemiological studies indicate that taking drugs from the NSAID group can cancel or significantly reduce the cardioprotective effect of aspirin.
Why? Aspirin and NSAIDs compete for the same substrate - COX 1. Therefore, with parallel administration, part of the platelets will be reversibly associated with NSAIDs, and the other part will be irreversibly associated with acetylsalicylic acid. After a few hours, platelets that were temporarily inactivated by NSAID drugs will restore their function and the patient will be left without cardioprotective action.
Which analgesics are contraindicated and which are the drug of choice in such patients can be learned in detail from my lectures.
In the RISK (Research on Instability in Coronary Artery Disease) study, when ASA was prescribed at a dose of 75 mg/day to patients with unstable angina, the risk of developing myocardial infarction decreased by 50% [2]. In another study in patients with acute MI, the effectiveness of ASA (160 mg/day) was comparable to the effectiveness of thrombolytic therapy [3].
Many patients receive dual antiplatelet therapy (Aspirin + Clopidogrel). In acute coronary syndrome, as well as after percutaneous coronary intervention (PCI) or stent placement, ASA is usually prescribed in combination with Clopidogrel. According to numerous studies, discontinuation of antiplatelet therapy in patients with an active stent is correlated with a 90-fold increase in the risk of stent thrombosis [4]. The literature has also documented thromboembolic complications with a fatal outcome due to unauthorized discontinuation of anticoagulant/antiplatelet drugs by the dentist before surgery. The risk of bleeding increases dramatically with dual antiplatelet therapy during surgery.