Iron preparations are a group of medicines that contain salts of one of the trace elements - iron.
Macro- and microelements are a collective (generalized) name for mineral substances - food components that, together with vitamins, are involved in maintaining normal human life.
If minerals are needed by the body in relatively large quantities, they are called macroelements. Macroelements include magnesium, calcium, potassium, sodium, phosphorus, chlorine and sulfur.
The human body requires minerals called microelements in much smaller quantities. Microelements include zinc, iodine, selenium, iron, manganese, copper, etc.
Iron is an essential micronutrient that regulates the normal functioning of the human body.
Iron is part of the complex proteins hemoglobin and myoglobin, which are involved in the binding, transport (hemoglobin) or creation of a temporary reserve (myoglobin) of oxygen. In addition, iron is part of a number of enzyme proteins (in particular, cytochromes), which are involved in the processes of tissue respiration and energy production in cells, and also carry out the metabolism and transformation of drugs, steroid hormones, pigments in the liver and intestines.
Normally, the human body receives a sufficient amount of iron from food. However, under certain conditions, for example, malnutrition, pregnancy, during periods of active growth, pathological conditions (blood loss due to bleeding, diseases of the stomach and intestines, after removal of the stomach, etc.) there is a deficiency of this microelement. In this case, preparations containing iron are used.
Classification of drugs containing iron
Preparations containing iron are classified into:
- iron preparations for oral administration (oral): iron sulfate, iron fumarate, iron saccharate, iron hydroxypolymaltose complex;
- iron preparations for parenteral use (injection): iron hydroxypolymaltose complex, iron hydroxysucrose complex, iron carboxymaltose, iron dextran;
- combined preparations: iron hydroxypolymaltose complex + folic acid; iron sulfate + folic acid;
- iron ammonium citrate + folic acid + cyanocobalamin;
- iron sulfate + ascorbic acid;
- iron sulfate + D, L-serine;
- iron gluconate + manganese gluconate + copper gluconate;
Iron compounds are also included in multivitamin preparations containing mineral complexes.
Introduction
Iron deficiency anemia (IDA) is one of the most common diseases in the world [1, 2], and among women of fertile age it is in first place in terms of incidence, often being a complication of pregnancy and childbirth, as well as a number of gynecological diseases [3–7] .
Another group of patients that especially often suffer from IDA are young children [8, 9], and in them IDA accounts for about 90% of all anemia. Nevertheless, any person, regardless of gender and age group, has a fairly high risk of encountering this problem at one stage or another in their life. Among the world's population, the incidence of IDA is unevenly distributed. According to experts from the World Health Organization (WHO), in developing countries it is several times higher than in developed countries. The range of fluctuations in the prevalence of IDA in the population ranges from 5 to 40% or more [10]. IDA is a polyetiological disease; it can have many different causes and predisposing factors [11]. These include socio-economic conditions, availability of medical care, nutritional habits, level of sanitary culture, prevalence of parasitic infestations and many others.
Etiology and pathogenesis of IDA
The development of IDA is associated with iron deficiency in the body, which in turn may be a consequence of impaired intake, absorption or increased loss of this microelement. In different sex and age groups, the predominant causes of IDA are distributed as follows:
- in women of fertile age [3–7]: heavy menstrual bleeding and menorrhagia (including due to pathologies of the reproductive system, such as endometriosis and fibroids),
- pregnancy (multiple pregnancies accompanied by early gestosis are in a special risk area),
- childbirth (especially repeated births with short intervals between them, as well as complicated by bleeding),
- miscarriages, frequent abortions,
- lactation;
- blood loss due to pathology of the gastrointestinal tract - gastrointestinal tract (esophageal bleeding, peptic ulcer of the stomach and duodenum, ulcerative colitis, Crohn's disease, polyposis, rectal fissures, hemorrhoids, tumors, etc.),
- iron deficiency at birth,
Iron plays an important role in the functioning of all organs and systems. It is a key element of the hemoglobin molecule, acting as a component of the protoporphyrin prosthetic group, responsible for the binding and transport of oxygen. Iron also takes part in the process of oxidative phosphorylation in cell mitochondria, in collagen synthesis, porphyrin metabolism, is present in significant quantities in muscle myoglobin, and is also necessary for the full functioning of the immune system [1]. And yet, the participation of iron in the processes of tissue respiration determines the main biological significance of this microelement. It is natural that with the development of IDA, one of the most significant problems becomes precisely tissue hypoxia and the pathology that it provokes [23–25].
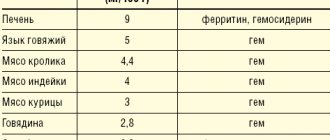
A natural source of iron is food, which provides about 10–20 mg of this microelement daily in a normal, complete diet. Of this amount, no more than 10% (about 2 mg) is absorbed, which provides the daily need for iron. At the same time, most healthy menstruating women lose up to 20-30 mg of iron every month. A similar or even more critical situation may arise with some chronic diseases. It can be difficult to replace what is lost with iron from food, even if its content in the diet is quite high. The problem is aggravated in the presence of impaired absorption and absorption of iron. A gradually developing imbalance between the intake and loss of iron depletes its reserves in the body, leading to an iron deficiency state, in particular to IDA with progressive hemic hypoxia and accompanying metabolic disorders.
Depending on the predominant mechanism of development of iron deficiency, anemia is divided into the following groups [25]:
- associated with blood loss;
- associated with impaired iron absorption;
- associated with an increased need for iron;
- nutritionally dependent.
In some cases, signs from several groups with varying degrees of severity may occur.
Clinical manifestations and diagnosis of IDA
The main clinical manifestations of IDA are characterized by hypoxic and sideropenic syndromes [1, 2, 27, 28].
Signs that make up hypoxic syndrome:
- pallor of the skin and visible mucous membranes;
- cardiopalmus;
- noise in ears;
- headache, dizziness;
- general weakness;
- loss of appetite;
- decreased performance, concentration, memory impairment.
Components of sideropenic syndrome:
- perversion of taste and smell;
- dry skin, pigmentation (“coffee with milk”);
- deformations and changes in the structure of nails (cross-striations, concavity, thinning, fragility);
- deterioration of hair condition (fragility, dullness, split ends, hair loss up to alopecia);
- manifestations of angular stomatitis (“jams”);
- burning sensation in the tongue.
In addition to the listed symptoms, the development of neurosis-like disorders, the manifestation of neurasthenia, disorders of peripheral circulation and microcirculation, decreased tolerance to physical activity, changes in metabolism in the myocardium are also possible, leading with long-term IDA to increasing symptoms of myocardial dystrophy, as well as shifts in the autonomic regulation of cardiac activity towards sympathicotonia .
Negative changes in the functioning of the gastrointestinal tract are noted. Patients with IDA often suffer from chronic gastritis and intestinal pathology. Syndromes of impaired absorption of iron in the intestine are often secondary in nature, further aggravating iron deficiency [17]. Regenerative disorders in the gastric mucosa are currently considered as the causes of decreased secretion and acid formation in IDA.
IDA negatively affects the immune system [29]. Thus, iron deficiency indirectly disrupts the functioning of the cellular component of anti-infective immunity: the proliferation of lymphocytes slows down and the microbicidal activity of granulocytes decreases. This may lead to a slight increase in the risk of developing infections and their unfavorable course.
The listed variety of clinical manifestations of IDA, which sharply reduce the quality of life of patients, once again emphasizes the particular importance of timely diagnosis and full correction of this condition. A separate problem is the low specificity of most clinical symptoms [28], which makes it difficult to confidently diagnose IDA solely on their basis.
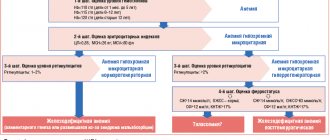
In this regard, laboratory data, incl. general blood analysis:
- assessment of hematocrit (diagnostic sign - its decrease);
- study of the number of red blood cells and reticulocytes in the blood (the number of red blood cells may be within normal limits or reduced, reticulocytosis is not typical, but can be observed with bleeding);
- determination of the average concentration and average content of hemoglobin in erythrocytes (these indicators are reduced, which reflects hypochromia of erythrocytes); determination of the shape and size of red blood cells (characteristic of anisocytosis with a tendency to microcytosis).
If IDA is suspected, it is also recommended to examine such indicators of iron metabolism as ferritin level (decreases), serum transferrin and serum iron-binding capacity (increases), serum iron and transferrin iron saturation coefficient (decreases).
For successful treatment, it is also important to determine the cause of the development of IDA, possible sources of blood loss, and concomitant diseases. For this purpose, X-ray examination of the chest organs, ultrasound examination of the abdominal organs, pelvis, and retroperitoneal space are used; electrocardiography, esophagogastroduodenoscopy, colonoscopy, as well as biochemical blood test, general urine test, etc. The results of basic studies help to choose the direction of further diagnostic search.
Treatment of IDA
The goal of treatment is to introduce iron into the body in an amount sufficient to normalize hemoglobin levels and replenish tissue iron stores [30–32]. At the same time, normal hemoglobin levels for women are 120–140 g/l, for men – 130–160 g/l, and a serum ferritin level above 40–60 μg/l indicates satisfactory accumulation of iron in the depot. This review examines the correction of IDA exclusively by pharmacological means. However, in some cases (severe IDA, concomitant cardiovascular pathology with the threat of decompensation due to iron deficiency, etc.), concomitant therapy with erythropoietin drugs, as well as blood transfusion therapy, can be prescribed on an individual basis.
When starting treatment for IDA, it is necessary to remember that anemia cannot be corrected with vitamin and mineral complexes containing iron. The vast majority of patients with IDA require special iron-containing medications [32]. Oral administration is a priority as a more convenient and safe way to use such drugs [33–35]. Parenteral iron preparations are used only if there are contraindications to oral administration, their poor tolerability or ineffectiveness. In this case, only the intravenous route of administration is recommended. Intramuscular injections of iron-containing drugs are ineffective and dangerous, because can cause infiltrates and even the development of myosarcomas.
Currently, preference is given to therapeutic regimens that involve taking 100–200 mg of elemental iron per day for adults (the optimal dose recommended by WHO is 120 mg/day). The use of more than 300 mg of iron per day is not advisable, because this usually does not increase the volume of absorption, while at the same time increasing the risk of developing undesirable side effects (stool disorders, signs of gastric irritation, etc.) [36]. When calculating the daily and course doses, the severity of the anemic syndrome, visceral lesions, and serum iron levels are taken into account.
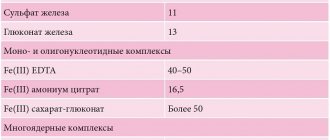
The modern pharmaceutical industry offers a fairly wide range of iron preparations, which differ in their quantitative (high- and low-dose) and qualitative compositions (single-component, combined). Oral iron preparations are also conventionally divided into ionic (salt) divalent (of iron salts, sulfate is most often used) and non-ionic (non-salt) ferric iron preparations based on polymaltose complex hydroxide (HPC) and protein succinylate [37, 38]. Randomized studies in recent years have proven equal effectiveness of both groups. For a more successful result, it is advisable to prescribe vitamins in parallel with iron supplements. For example, ascorbic acid allows for better absorption and assimilation of iron [39] and the full course of plastic processes in the body.
It should be remembered that the therapeutic effect of iron supplements, as a rule, develops quite slowly, so ferrotherapy is a long process. Depending on the severity of iron deficiency, treatment can last from 1 to 3 months or more. At the same time, data are accumulating on the successful use of low-dose drugs in intermittent courses (2 weeks per month), as well as on the benefits of treatment in an alternating mode - every other day for a month [40]. The listed regimens provide a much lower frequency of side effects without loss of effectiveness. To avoid side effects and to increase patient adherence to therapy, it makes sense to refrain from unnecessarily prescribing high-dose drugs and multiple doses throughout the day.
WHO experts recommend the use of drugs with sustained release of iron [41]. This allows you to reduce the frequency of taking the drug, while simultaneously improving its absorption and tolerability. The duration of their use is determined individually, taking into account laboratory parameters. The main course of treatment continues until the optimal hemoglobin level is achieved and iron metabolism in the blood serum is restored. Further, in order to replenish tissue depots, it may be necessary to extend the drug intake for up to 2 months. If iron deficiency is severe, the total duration of treatment with oral sustained-release iron medications is usually 3 to 6 months [37, 41, 42].
Monitoring the effectiveness of treatment for IDA
Monitoring the effectiveness of treatment with iron supplements is carried out by monitoring the following laboratory parameters [42]:
- hemogram;
- ferritin;
- iron binding capacity of serum;
- transferrin.
With adequate therapy, patients’ well-being improves already on the 5th–6th day of taking the drug. On the 7th–12th day, one should expect a reticulocyte crisis - an increase in reticulocytes by 2 or more times from the initial values. Hemoglobin concentration begins to increase after approximately 2.5–3 weeks of treatment. By the end of the 4th week it should increase by 10 g/l. Complete normalization of this indicator is usually observed no earlier than a month from the start of treatment. Hemogram values in mild anemia usually return to normal after 5–8 weeks of ferrotherapy. It is important to dynamically monitor hemoglobin levels during treatment and after completion of the course. This indicator should be monitored monthly throughout the year, which makes it possible to decide on the need for maintenance treatment.
If the target hemoglobin level and normal serum ferritin level cannot be achieved, conditions that interfere with iron absorption, as well as the possibility of adverse drug interactions (for example, with antacids), should be excluded.
Prevention of IDA
Primary prevention of IDA and latent iron deficiency is, first of all, a complete, balanced diet for a person at any age. Secondary prevention of IDA is facilitated by regular preventive examinations of the population for the purpose of timely diagnosis and treatment of diseases that can lead to iron deficiency in the body. In this case, screening studies to identify iron deficiency conditions occupy a central place [43]. You should remember about risk groups for the development of latent iron deficiency and IDA. These include people with irreparable causes of iron deficiency (chronic blood loss, malabsorption syndrome, celiac disease, program hemodialysis, inoperable tumors, etc.), vegans and vegetarians, regular blood donors, and pregnant women. They are recommended to take additional preventive iron supplements.
Conclusion
IDA is a very serious and quite common problem, and the medical community is currently actively searching for optimal ways to solve it. Some progress has already been made in the development of effective and safe drugs for the treatment and prevention of iron deficiency conditions. Competent pharmacological correction of IDA can improve the quality of life and increase performance in the vast majority of patients with a similar diagnosis. In this case, it is advisable to use modern drugs, the production technologies of which ensure the maximum possible bioavailability of iron, and also minimize the risk of developing the most common side effects from ferrotherapy.
Etiology and pathogenesis
· Free, unbound iron is toxic to living tissue and can locally destroy the intestinal mucosa.
· 10% of ingested iron (Fe 2+) is actively absorbed as ions in the small intestine. After absorption, iron accumulates as Fe3+ in the mucosal storage protein ferritin. From there, iron is transported to the liver, spleen and bone marrow for further storage in ferritin or for inclusion in the heme molecule.
· Iron is transported bound to the transport protein transferrin.
· When the blood's ability to bind iron is exceeded (serum iron > 90 µmol/L), free ions lead to tissue damage in most organs, including the liver and heart.
Progress, complications and prognosis
Gradient
The clinic is divided into four stages, which may overlap.
- Often transient symptoms such as lethargy, nausea, vomiting, diarrhea, abdominal pain. Leftover pills cause gray/black vomit and stool. Symptoms disappear after 6-8 hours. When consumed in large quantities, the local effect causes bloody diarrhea.
- Symptom of free interval pg transport and distribution of absorbed iron.
- The acute phase is observed in few patients. It manifests itself in the form of metabolic acidosis, shock, renal failure, and liver necrosis. Possible death within 1-3 days.
- Tension in the gastrointestinal tract due to damage. Complications: hypotension, shock, metabolic acidosis, liver and kidney failure, gastric fibrosis and pyloric obstruction.
Forecast
- The prognosis can be serious, but in most cases the poisoning is not life-threatening.
- Toxic doses for 2-3 years: 400 mg Fe2+.
- Lethal doses: 50 - 300 mg Fe2 + / kg.
- Patients who are alive 72 hours after administration make a full recovery.