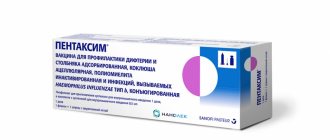
Menactra
5,200 rub.
Manufacturer USA, Sanofi Pasteur JSC
AgeChildren from 9 months, adults up to 55 years
Packaging 1 bottle / 1 dose / 0.5 ml No. 1
Made in the USA, Sanofi Pasteur JSC.
Menactra is the first menigococcal conjugate vaccine registered in Russia. The vaccine is produced by one of the world leaders in the development and production of vaccines. Thus, in 2014, the Russian Federation became one of the countries where a modern and effective vaccine became available to prevent a serious disease - meningococcal infection.
Menactra is a quadrivalent (type A, C, Y, W) conjugate meningococcal vaccine, which allows the prevention of all forms of meningococcal infection from the age of 9 months to 55 years. If there are medical indications, the vaccine can be used at an older age.
Previously, in Russia, only polysaccharide vaccines were available for the prevention of meningococcal infection, the use of which formed short-term immunity lasting no more than 5 years, so often these vaccines were used only in the presence of “outbreaks” of meningococcal infection. The conjugate meningococcal vaccine Menctra is recommended for routine prevention, as it causes long-term protection and the formation of immunological memory in vaccinated people.
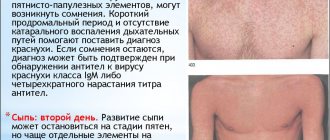
Meningococcal infection is a very pressing problem. This highly bacterial infection can have various clinical forms. The most dangerous of them are meningococcal sepsis and meningococcal meningitis. These forms of meningococcal infection are characterized by rapid development and progression, the absence of any specific manifestations in the initial stages of the disease that would make it possible to diagnose meningococcal infection and begin specific treatment. For this reason, every 6 children with meningococcal sepsis and meningococcal meningitis develop a fatal outcome. A severe form of meningococcal infection can take the life of a child in just 24 hours. 15% of survivors develop severe irreversible consequences in the form of limb amputations, hearing and vision loss, and other neurological disorders. Among the sick, 83% are children in the first 5 years of life, so the opportunity to vaccinate as early as possible is very important.
Currently, we can carry out routine prevention of this dangerous disease using the modern and effective Menactra vaccine.
Meningitis: causes and signs
The causative agents of infectious meningitis are most often microbes, less often viruses or fungi. The first signs of meningitis appear suddenly, 3-10 days after contact with a carrier of the infection. There are:
- Strong headache;
- fever;
- nausea and vomiting;
- lack of appetite;
- general weakness, disturbances of consciousness;
- pain in muscles, joints, neck (the child cannot bend his head forward);
- increased sensitivity to sounds and light (children scream);
- shortness of breath and palpitations;
- the skin is pale, the fingers and nasolabial triangle have a bluish tint.
In children, signs of meningitis are more difficult to suspect. Typically restlessness and constant crying, refusal to eat and sleep, bulging fontanelle, increased crying when parents try to pick up the child, bend their legs or move their head. Children burp like a fountain, press their arms and legs to their body, and take a pose with their back arched.
Who is recommended for vaccination?
Meningitis is especially dangerous for children under 2-3 years of age, whose immune system has not yet fully developed. In addition, the disease is dangerous for children with immunodeficiency, severe somatic diseases, and congenital malformations.
Vaccination against meningitis is not included in the compulsory vaccination schedule; it is provided free of charge according to indications if an outbreak of infection occurs or in risk groups. Parents can vaccinate for a fee in the following cases:
- children under two years of age attending children's groups (developmental classes, nursery or kindergarten);
- if you plan to travel to countries or regions with a high incidence of meningitis;
- frequently ill children of younger and preschool age.
The pediatrician may recommend vaccination individually if additional factors are present.
special instructions
The vaccine drug Menactra is recommended for use especially by people from risk groups who have an increased likelihood of developing meningococcal infection:
- people in direct contact with infected people;
- confirmed deficiency of properdin, complement components;
- tourists and those people who are going to visit areas unfavorable for meningococcal infection, located mainly in Africa;
- congenital or acquired absence of the spleen;
- accommodation in hostels, apartment-type hotels;
- people who are planning to serve in the army or are already recruits.
It is prohibited to combine the vaccine drug Menactra with any other drugs in the same syringe. Also, you cannot use subcutaneous, intravenous or intradermal methods of administering the vaccine. The studies conducted did not involve the use of the drug in people who have bleeding disorders and thrombocytopenia.
The vaccine is not used as a prophylactic against meningitis, which can be caused by other microorganisms or meningococci serogroup B. There have been cases of Guillain-Barré syndrome, which can progress after administration of the vaccine. Therefore, people who have previously suffered from this condition may be at risk of recurrence in the future. The decision to use the vaccine drug Menactra should be made by a doctor after assessing the possible risks of developing Siillain-Barré syndrome.
Patients who suffer from immune disorders and are being treated with immunosuppressive therapy may have a reduced immune response after using the vaccine.
Menactra has not been studied to affect driving.
Features of vaccination
Today, vaccinations are available against three types of meningitis – pneumococcal, Haemophilus influenzae or meningococcal. Each infection has its own characteristics of immunization.
Haemophilus influenzae infection. Some cases of Haemophilus influenzae infection lead to the development of meningitis, especially between the ages of birth and 6-7 years, when the immune system is still imperfect and may not fight the microbe as effectively. The effectiveness of vaccination against meningitis caused by Haemophilus influenzae reaches 95%. Repeated booster vaccinations increase immune protection.
Pneumococcal infection. Vaccination against meningitis caused by pneumococcus is especially important for children under two years of age. Microbes entering the body of children can provoke various manifestations - from colds to damage to the lungs (pneumonia) and the membranes of the brain. Vaccination reduces the risk of disease by 80%. If the baby is sick, the infection is easier. Source: A.A. Baranov, L.S. Namazova-Baranova, N.I. Briko, Yu.V. Lobzin, R.S. Kozlov, M.P. Kostinov, I.S. Koroleva, A.V. Rudakova, S.V. Sidorenko, V.K. Tatochenko, S.R. Kharit, M.V. Fedoseenko, E.A. Vishneva, L.R. Selimzyanova. Vaccinal prevention of pneumococcal infection in children // Pediatric pharmacology, 2021, v. 15, no. 3, p. 200-211.
Meningococcal infection. Most often, meningitis of this nature occurs in children in the first year of life. In addition, the infection is dangerous for weakened and frequently ill children. Having meningitis can lead to disability, so it is important to vaccinate your baby. When vaccinated, the risks are reduced by more than 90%. Source: A.A. Ergalieva, L.B. Seidulaeva, R.Zh. Baykhozhaeva, L.A. Umeshova. Meningoccal meningitis // National priorities of Russia, 2013, No. 2(9), special issue, pp. 154-156
Vaccination schedule
It is important to check with your pediatrician at what age your child should be vaccinated against a particular infection. Thus, vaccinations against meningococcus, depending on the specific drug, can be administered from 9 months, one and a half or 2 years.
The vaccine against hemophilus influenzae is administered simultaneously with DTP, and, accordingly, the number of times the vaccine against tetanus, diphtheria and whooping cough is given, the same number of times the anti-hemophilus component is administered. It is possible to carry out separate vaccinations according to an individual schedule.
Frequently ill children, especially those with recurrent bronchitis, are vaccinated against pneumococcal infection from two months of age. The vaccine can be administered up to 5 years of age; after 4 consecutive injections, lifelong protection is provided. There are drugs that are administered from 2 years of age, they provide protection against meningitis with other complications. It is important to remember how long the vaccine lasts. It protects for up to 10 years.
Immunological effectiveness
The immunological properties of the drug Menactra were studied by specialists during clinical studies in children and adults from different age groups:
- 9-18 months - 3 studies;
- from 2 to 10 years - 4 studies;
- from 11 to 55 years - 6 clinical studies.
The primary immunological profile was studied in clinical trials in children aged 2 to 10 years. The immune response was studied before a single injection of the vaccine preparation and 28 days after that. As a result, experts found a significant increase in the average geometric antibody titers. Seroconversion was detected in all groups of participants. Antibody titers increased 4 times or more 28 days after vaccination.
Possible reactions and complications
The pediatrician will tell you in detail how each vaccination is tolerated, what side effects are possible, and what parents should do. The most common occurrence of fever is after vaccination. This is a completely acceptable reaction, since a foreign substance is introduced into the body, the production of antibodies is necessary. Usually the fever is low and goes away in 2-3 days.
Possible consequences and complications are rare, as the drugs are well tolerated. They include allergic reactions, severe redness at the injection site, severe weakness for two days.
Menactra
Trade name:
Menactra® [meningococcal polysaccharide vaccine (serogroups A, C, Y and W-135), conjugated with diphtheria toxoid]
Group name:
Vaccine for the prevention of meningococcal infections of serogroups A, C, W, Y, polysaccharide, conjugated
Dosage form:
Solution for intramuscular administration Vaccine Menactra® is a solution of purified capsular polysaccharides of Neisseria meningitidis groups A, C, Y and W-135, individually conjugated to a carrier protein (purified Corynebacterium diphtheriae toxoid).
Compound
One dose (0.5 ml) contains:
Active ingredients: Monovalent meningococcal conjugates (polysaccharide + carrier protein): Serogroup A polysaccharide - 4 µg Serogroup C polysaccharide - 4 µg Serogroup Y polysaccharide - 4 µg Serogroup W-135 polysaccharide - 4 µg Diphtheria toxoid ~ 48 µg
Excipients: Sodium chloride 4.35 mg, sodium hydrogen phosphate 0.348 mg, sodium dihydrogen phosphate monohydrate 0.352 mg, water for injection - up to 0.5 ml.
Description
Colorless transparent or slightly cloudy solution.
Pharmacotherapeutic group
MIBP vaccine.
ATX code
J07AH08
Pharmacological properties
The causative agent of meningococcal infection, including meningitis and septicemia, is the bacterium N. meningitidis; There are a number of serogroups of the pathogen. Administration of the Menactra® vaccine induces the production of specific antibodies that have bactericidal activity against capsular polysaccharides of the meningococcal pathogen serogroups included in the vaccine (A, C, Y and W-135).
Immunological efficacy Clinical efficacy studies have not been conducted because serum bactericidal antibodies (SBA) are considered to be an indicator of the effectiveness of meningococcal vaccines.
The immunological properties of the Menactra® vaccine were studied in 3 clinical studies in children aged 9-18 months, in 4 clinical studies in children aged 2-10 years and in 6 clinical studies in persons aged 11-55 years. Immunogenicity was assessed by the level of functional antibodies determined using the SBA assay.
The primary immunological profile of the vaccine was studied in clinical studies in participants aged 2-10 years. The immune response was assessed 28 days after administration of the Menactra® vaccine (compared to the initial level of antibodies before vaccination). A significant increase in geometric mean SBA titers was observed in study participants. In 86-100% of participants with initially undetectable SBA titer values (<1:8), seroconversion was noted for antigens of all serogroups of the pathogen, defined as a 4-fold (or more) increase in antibody titers 28 days after vaccination in comparison with the antibody titer before vaccination.
The ability of the Menactra® vaccine to induce the development of immunological memory after primary vaccination is confirmed by data from clinical studies in children and adults.
Results of 3 clinical studies conducted in children aged 9-18 months with a single or double dose of Menactra® vaccine alone or concomitantly with other pediatric vaccines (pneumococcal conjugate vaccine (PCV) or measles, mumps, rubella and varicella vaccine (MMRV) )) confirmed that the majority of participants in the two-dose Menactra vaccine groups, when administered alone or concomitantly with other pediatric vaccines, had an increase in SBA titres of ≥1:8 to all vaccine serogroups. ≥91% and ≥86% of participants in the separate two-dose Menactra vaccine group experienced an increase in SBA titer of ≥1:8 to serogroups A, C, Y and serogroup W-135, respectively. After a second dose of Menactra® vaccine was administered concomitantly with PCV or with MRCV (or MRCV + vaccine for the prevention of infections caused by Haemophilus influenzae type b), the majority of study participants experienced an increase in SBA titer ≥1:8 (in >90% of study participants to serogroups A, C and Y, in >81% of participants to serogroup W-135). The values of geometric mean titers of SBA after vaccination to all four serogroups of meningococci included in the vaccine were high.
Results from studies conducted in participants aged 11 to 18 years confirmed a significant immune response to a single dose of Menactra® vaccine. The values of geometric mean SBA titers on the 28th day after vaccination were significantly higher than the initial ones. In addition, in 98-100% of adolescents in whom the antibody titer was not initially determined (<1:8), by the 28th day there was a 4-fold (or more) increase in the SBA titer to all serogroups of the pathogen included in the vaccine . The results obtained indicate the high immunogenicity of the vaccine in adolescents.
When analyzing by serogroup, it was revealed that 93-100% of adult participants in clinical trials with initially undetectable antibody titers (<1:8) by the 28th day had a 4-fold (or more) increase in the SBA titer to all serogroups of the pathogen included included in the vaccine. In each of the 3 studies, the immunological response induced by Menactra® vaccine was similar when assessed by sex, age and race.
Immune response kinetics: There are no data on the kinetics of the initial response to Menactra® vaccine, however, as with other polysaccharide and conjugate vaccines, immune protection usually develops 7-10 days after vaccination.
Duration of protection: The ability of the Menactra® vaccine to induce the formation of immune memory after primary vaccination has been proven in clinical studies. One study demonstrated that the persistence of bactericidal antibodies in the blood 3 years after a single administration of the Menactra® vaccine was higher compared to a group of vaccinated individuals who received a single immunization with an unconjugated 4-valent polysaccharide meningococcal vaccine against serogroups A, C, Y and W -135. In the group vaccinated with the Menactra® vaccine, a higher concentration of SBA was observed, as well as a higher proportion of individuals who had specific high-avidity antibodies, which indicates the formation of immune memory.
Immunogenicity in adolescents and adults after booster vaccination In a clinical study conducted in the United States, 834 people were given a single booster dose of Menactra® vaccine 4 to 6 years after vaccination. Before booster vaccination, the proportion of participants with an SBA titer <1:8 was 64.5%, 44.2%, 38.7%, and 68.5% for serogroups A, C, Y, and W-135, respectively. In the subgroup of study participants whose response was assessed on day 6 after revaccination (n=112), 86.6%, 91.1%, 94.6%, 92.0% of patients achieved a ≥4-fold increase in SBA titer for serogroups A, C, Y and W-135, respectively. The proportions of study participants (n=781) who achieved a ≥4-fold increase in SBA titer on day 28 after revaccination were 95.0%, 95.3%, 97.1% and 96% for serogroups A, C, Y and W-135, respectively. The proportion of study participants achieving an SBA titer ≥1:8 at day 28 was >99% for each of the 4 serogroups.
Indications for use
Prevention of invasive meningococcal infection caused by N. meningitidis serogroups A, C, Y and W-135 in persons aged 9 months to 55 years.
Contraindications
- Known hypersensitivity with systemic manifestations to any component of the vaccine, including diphtheria toxoid, or to previous administration of other vaccines containing the same components. — Acute infectious and non-infectious diseases, exacerbation of chronic diseases (in these cases, vaccination is carried out after recovery or in remission).
Use during pregnancy and breastfeeding
Animal studies have not revealed a negative effect of the Menactra® vaccine on pregnancy and embryofetal development, the birth process and postnatal development. Because there are no studies of vaccination in pregnant women, and post-marketing experience with its use in pregnant women is limited, administration of the vaccine to pregnant women is recommended only when absolutely necessary, such as during an outbreak of meningococcal disease, before traveling to an endemic area, and only after assessing the balance of benefits and risks of vaccination.
It is currently unknown whether the active ingredients contained in the vaccine are able to pass into breast milk. However, it has previously been shown that antibodies to polysaccharides are detected in young breastfed mice.
Studies in mice have not demonstrated any adverse effects on the postnatal development of offspring receiving antibodies induced by Menactra® vaccine in their mother's milk. At the same time, the effects in children of the first year of life whose mothers were immunized with the Menactra® vaccine during breastfeeding have not been studied. Before deciding to immunize a nursing woman, the risks and benefits of this immunization must be assessed.
Directions for use and doses
Vaccination is carried out with one dose of 0.5 ml.
The vaccine should be administered intramuscularly, taking into account the age of the person being vaccinated: for children aged 9 to 12 months - in the anterolateral region of the thigh; for children 12 months and older and adults - into the deltoid muscle of the shoulder.
Do not administer the vaccine intravascularly. Before insertion, you must ensure that the needle does not enter a blood vessel.
In children aged 9 to 23 months, the course of vaccination with the Menactra® vaccine consists of 2 injections of one dose of vaccine (0.5 ml) with an interval of at least 3 months.
In persons aged 2 to 55 years, vaccination is carried out with a single dose of 0.5 ml.
If the risk of meningococcal disease remains, a single booster dose may be given in accordance with national recommendations if at least 4 years have passed since the previous dose.
Side effect
The nature and frequency of side effects identified in studies varied depending on the age of those vaccinated.
In clinical studies in children aged 9 to 18 months, the most common symptoms reported within 7 days of vaccination were injection site tenderness and soreness. During clinical studies in children aged 2 to 10 years, the most commonly observed pain and redness at the injection site, irritability, diarrhea, drowsiness, and anorexia; In adolescents aged 11 to 18 years and adults aged 18 to 55 years, the most common symptoms reported were pain at the injection site, headache, and fatigue.
The incidence of the following side effects is classified according to the recommendations of the World Health Organization (WHO) and includes the following categories:
- Very common: ≥ 10%
- Common: ≥ 1% and < 10%
- Uncommon: ≥ 0.1% and < 1%
- Rare: ≥ 0.01% and < 0.1%
- Very rare: <0.01%
- Frequency unknown: cannot be determined from available data.
Children aged 9 to 18 months Most of the recorded local and general reactions observed within 7 days after vaccination were mild and lasted less than 3 days. In addition, the following side effects have been noted:
Metabolic and nutritional disorders Very common: loss of appetite
Nervous system disorders Very common: drowsiness
From the gastrointestinal tract Very common or common: vomiting
General and injection site conditions Very common: soreness, injection site erythema, injection site swelling, irritability, abnormal crying, fever
Children aged 2 to 10 years Most of the reported local and general reactions observed within 7 days after vaccination were mild. In addition, the following violations were noted:
Metabolic and nutritional disorders Very common or common: decreased appetite
Nervous system disorders Very common or common: drowsiness
From the gastrointestinal tract Very common: diarrhea Common: vomiting
From the skin and subcutaneous tissues Common: rash, urticaria
Musculoskeletal and connective tissue disorders Common: arthralgia
General and injection site disorders Very common: soreness and tenderness at the injection site Very common or common: irritability, redness at the injection site, swelling at the injection site, fever
Persons aged 11-55 years Most of the reported local and general reactions observed within 7 days after vaccination were mild. In addition, the following violations were noted:
Metabolic and nutritional disorders Very common or common: decreased appetite
Nervous system disorders Very common: headache
From the gastrointestinal tract Very common or common: diarrhea Common: vomiting
Skin and subcutaneous tissue disorders Common: rash
Musculoskeletal and connective tissue disorders Very common: arthralgia
General disorders and disorders at the injection site Very common: pain, induration, redness and swelling at the injection site, fatigue, general malaise Common: chills, fever
Revaccination In adolescents and adults, serious adverse events occurred in 1.3% of cases after revaccination with the Menactra® vaccine.
The most common local and systemic reactions reported within 7 days after vaccination were injection site pain (60.2%) and myalgia (42.8%). The average frequency of local and general reactions in adolescents and adults after a single primary immunization and after revaccination with the Menactra® vaccine did not differ. Most reported reactions were mild or moderate and resolved within 3 days.
During the post-registration period, information was additionally received on the following adverse events after administration of the drug (currently, the incidence of these events and their cause-and-effect relationship with the use of the Menactra® vaccine cannot be determined):
From the blood and lymphatic system Lymphadenopathy
From the immune system Hypersensitivity reactions, such as anaphylactic shock, anaphylactoid reactions, wheezing, difficulty breathing, swelling of the upper respiratory tract, urticaria, redness of the skin, itching, decreased blood pressure.
From the nervous system: Guillain-Barré syndrome (GBS), paresthesia, loss of consciousness (caused by dysregulation of the autonomic nervous system), dizziness, convulsions, facial paralysis, acute disseminated encephalomyelitis, transverse myelitis.
Musculoskeletal and connective tissue disorders Myalgia
General Disorders and Injection Site Disorders A widespread injection site reaction, manifested by severe swelling of the extremity, may be accompanied by redness, a feeling of heat at the injection site, increased sensitivity, or pain at the injection site.
Post-marketing safety study The risk of GBS after administration of Menactra® vaccine was assessed in a retrospective cohort study (USA). In a medical study of 1,431,906 patients who received Menactra® vaccine, 72 cases of GBS were reported. In all cases, Menactra® vaccine was administered to patients no earlier than 42 days before the onset of symptoms. An additional 129 cases of GBS were not confirmed or were excluded from the analysis due to missing or insufficient medical information. Based on the results of the analysis, the additional risk of GBS was estimated to range from 0 to 5 additional cases of GBS per 1,000,000 vaccinated within 6 weeks after vaccination.
Overdose
No cases of overdose have been reported.
Interaction with other drugs
The Menactra® vaccine was used in persons aged 18-55 years simultaneously with a polysaccharide vaccine for the prevention of typhoid fever and in persons aged 11-17 years simultaneously with an adsorbed vaccine against diphtheria (with reduced antigen content) and tetanus, intended for use in older children age, teenagers and adults.
In clinical studies in children aged 4 to 6 years inclusive, the Menactra® vaccine was used together with the vaccine for the prevention of diphtheria, tetanus, whooping cough (acellular). In clinical studies in children younger than 2 years of age, Menactra® was given with one or more of the following vaccines at the time of the second dose at 12 months of age: pneumococcal 7-valent conjugate vaccine (PCV7), measles, mumps, rubella vaccine ( with or without a varicella component), varicella vaccine, hepatitis A vaccine, or Haemophilus influenzae type b vaccine.
When Menactra® and Diphtheria, Tetanus, Pertussis (Acellular) Vaccine are co-administered in children 4 to 6 years of age, it is preferable to administer both vaccines at the same time, or Menactra® should be administered before Diphtheria, Tetanus, Pertussis (DTP) Vaccine ( acellular). A decreased immune response to Menactra® vaccine has been demonstrated when administered one month after administration of diphtheria, tetanus, and pertussis (acellular) vaccine. There are no data available to evaluate the immune response to Menactra after administration of diphtheria, tetanus, pertussis (acellular) vaccine in children under 4 years of age or to Menactra after administration of other vaccines containing diphtheria toxoid to persons under 11 years of age.
Pneumococcal antibody titers against some PCV7 serotypes were reduced after coadministration of Menactra® and PCV7.
BCG vaccine should not be used simultaneously with Menactra® vaccine.
Vaccines must always be administered to different parts of the body, using separate syringes for each of them.
special instructions
Vaccination is especially indicated for the following groups at high risk of meningococcal disease:
- persons who had direct contact with patients infected with meningococci of serogroups A, C, Y or W-135 (in the family or in closed institutions); - persons with deficiency of properdin and complement components; - persons with functional or anatomical asplenia; - tourists and persons traveling to areas hyperendemic for meningococcal infection, such as sub-Saharan African countries; — employees of research, industrial and clinical laboratories who are regularly exposed to N. meningitidis in solutions capable of aerosol formation; - students of various universities, and especially those living in dormitories or apartment-type hotels; - conscripts and new recruits.
It is prohibited to administer Menactra® vaccine subcutaneously or intradermally as there is no data on the safety and effectiveness of the vaccine when administered subcutaneously or intradermally.
Do not mix Menactra® vaccine in the same syringe with other vaccines or medications.
The vaccine has not been studied in people with thrombocytopenia or bleeding disorders. As with other vaccines administered intramuscularly, the benefit versus risk of using the vaccine should be assessed in individuals at increased risk of bleeding from intramuscular injection.
The risk of developing Guillain-Barré syndrome (GBS) after administration of the Menactra® vaccine was assessed in a post-marketing retrospective cohort study. Cases of the development of GBS have been described, characterized by a temporal connection with the administration of the Menactra® vaccine. Individuals who have previously been diagnosed with GBS may be at increased risk of developing this condition after receiving the Menactra® vaccine. The decision to use Menactra® vaccine in this situation should be made after assessing the potential benefits and risks.
The vaccine is not intended for the prevention of meningitis caused by other microorganisms or for the prevention of invasive meningococcal infection caused by meningococcal serogroup B.
Although a humoral immune response to diphtheria toxoid may be observed after administration of Menactra® vaccine, this vaccine cannot be considered an immunizing agent against diphtheria. It is not recommended to change the vaccination schedule with standard diphtheria vaccines in connection with the introduction of the Menactra® vaccine.
In persons with an impaired immune status, as well as against the background of immunosuppressive therapy, a reduced immune response to the administration of the Menactra® vaccine may be observed.
As with any vaccination, protective immunity may not be developed in 100% of those vaccinated.
Before administering a vaccine, the health care provider or prescriber should inform the patient, parent, guardian, or other responsible adult of the potential benefits and risks associated with receiving the vaccine.
Before administering the vaccine, the necessary precautions should be taken to prevent severe adverse reactions, namely: review the patient's vaccination history, find out the presence of contraindications to immunization, and assess the patient's current health status.
The vaccine is administered under the supervision of a medical professional, and the necessary means of anti-shock therapy (for example, solutions of epinephrine hydrochloride (1:1000) and glucocorticosteroids for injection) must be available in the office where vaccination is carried out.
Cases of fainting have been reported following administration of the Menactra® vaccine. Provisions should be made to prevent injuries associated with fainting and to provide medical care in the event of fainting.
Impact on the ability to drive vehicles and machinery
No studies have been conducted to study the effect of the vaccine on the ability to drive a car or use other machinery.
Release form
Solution for intramuscular administration 0.5 ml/dose. 1 dose (0.5 ml) of the drug in bottles of transparent borosilicate glass (type I) with a capacity of 3 ml, which are sealed with a stopper made from a mixture of chlorobutyl (latex-free) and synthetic polyisoprene, and rolled up with an aluminum cap equipped with a tear-off plastic cap "flip-off" type. 1 or 5 bottles along with instructions for medical use in a cardboard pack.
Best before date
2 years. Do not use after the expiration date stated on the package. The expiration date is the last day of the month indicated on the package.
Storage conditions
At temperatures from 2 to 8 °C. Do not freeze. Keep out of the reach of children. The drug that has been frozen cannot be used.
Transportation conditions
At temperatures from 2 to 8 °C. Do not freeze.
Vacation conditions
Dispensed by prescription.
Manufacturer
Sanofi Pasteur Inc., USA