Hyperaldosteronism - symptoms and treatment
In order not to miss hyperaldosteronism, it is first extremely important to identify the main risk factors that will help to suspect this disease. These include:
- arterial hypertension of the second degree, i.e. a stable increase in systolic (upper) blood pressure more than 160/179 mm Hg. Art., diastolic (lower) - more than 100/109 mm Hg. Art.;
- arterial hypertension, persistent and/or poorly controlled with medications (although this sign does not always indicate pathology);
- a combination of arterial hypertension with low levels of potassium in the blood (regardless of taking diuretics);
- arterial hypertension and incidentally detected (by ultrasound and/or CT) formation of the adrenal gland;
- burdened family history: the development of arterial hypertension and/or acute cardiovascular accidents before the age of 40, as well as relatives who have already been diagnosed with hyperaldosteronism [1][5].
The next stage of diagnosis is laboratory confirmation . To do this, the aldosterone-renin ratio (ARR) is examined. This study is the most reliable, informative and accessible. It should be carried out in the early morning hours: ideally no later than two hours after waking up. Before drawing blood, you need to sit quietly for 5-10 minutes.
IMPORTANT: Some drugs may affect plasma aldosterone concentrations and plasma renin activity, which in turn alters ARS. Therefore, two weeks before taking this test, it is important to stop drugs such as spironolactone, eplerenone, triamterene, thiazide diuretics, drugs from the group of ACE inhibitors, ARBs (angiotensin receptor blockers) and others. The doctor should inform the patient about this and temporarily prescribe a different treatment regimen for hypertension.
If APC is positive, a confirmatory saline test should be performed. It is carried out in a hospital setting, as it has a number of limitations and requires a study of the levels of aldosterone, potassium and cortisol initially and after a 4-hour infusion of two liters of saline. Normally, in response to a large amount of fluid administered, the production of aldosterone is suppressed, but with hyperaldosteronism it is not possible to suppress the hormone in this way.
Low levels of potassium in the blood are observed in only 40% of cases of the syndrome, so it cannot be a reliable diagnostic criterion. But the alkaline reaction of urine (due to increased excretion of potassium by the kidneys) is a fairly characteristic sign of pathology.
If familial forms of hyperaldosteronism are suspected, genetic typing (susceptibility testing) is carried out with consultation of a geneticist [3][6].
The third stage of diagnosis is topical diagnosis . It is aimed at finding the source of the disease. For this, various methods of visualizing internal organs are used.
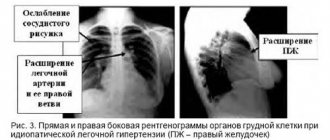
Ultrasound of the adrenal glands is a low-sensitivity diagnostic method. CT is preferable: it helps to identify both macro- and microadenomas of the adrenal glands, as well as thickening of the adrenal pedicles, hyperplasia and other changes [14].
To clarify the form of hyperaldosteronism (unilateral and bilateral lesions), selective blood sampling is carried out from the veins of the adrenal glands in specialized centers [9]. This study effectively reduces the risk of unnecessary adrenal gland removal based on CT findings alone [4].
Studies confirming the diagnosis of primary hyperaldosteronism
A positive ARS result mandates one of the recognized studies supporting PHA, but the ESH position authors suggest that in patients with idiopathic hypokalemia, plasma renin concentrations below the detection threshold or very low and resting aldosterone concentrations >200 pg/mL (20 ng/dL) PHA can be diagnosed without the need for a confirmatory study.
The ESH position lists the 4 most commonly used confirmatory tests: the oral and intravenous sodium loading test, the captopril test, and the fludrocortisone test, emphasizing that the choice of test should depend on the availability of the test and the experience of the particular institution. As with the ARS determination, medications that affect the renin-angiotensin-aldosterone system should be taken into account and contraindications to the test should be assessed - in the case of a sodium load test, these are: significantly elevated blood pressure, severe heart failure and renal failure.
The ESH position authors discussed the possibility of recommending a single test and cutoff values for diagnosing PHA. Due to the lack of sufficient data, no decision on specific values was made. Figure 2 suggests a diagnostic algorithm for PHA, developed on the basis of the discussed position and experience of the authors of this article. In practice, the most commonly used test today is the seated sodium load test. The most commonly used cutoff to diagnose PHA in this setting is an aldosterone concentration >60 pg/mL (170 nmol/L) with a cortisol concentration lower after a 0.9% NaCl infusion than initially determined. Threshold values for the supine sodium load test are also accepted: >100 pg/ml (definite diagnosis) and 50–100 pg/ml (probable diagnosis). The ESH position states that a sodium loading test performed in a sitting position may be the preferred method, and if it is contraindicated, a captopril test may be performed.
Figure -2. Algorithm for diagnostic tactics in people with suspected primary hyperaldosteronism. The given limit values for individual diagnostic stages were proposed based on the position of ESH and the experience of the authors of this article. They should be considered as examples and take into account the methodology used and the experience of the institution
HELP AND FORECASTS
With complex treatment of hyperaldosteronism, complete recovery is possible (one of the few pathologies of the adrenal glands, which can be brought to almost complete remission). The treatment efforts themselves can be aimed at one of two things:
- elimination of factors that depress the adrenal glands;
- treatment of the disease that causes hyperaldosteronism, if it is a secondary form.
As a rule, they try not to use radical methods of treatment for this disease. Tactics are based on a conservative approach - supporting the body in terms of removing salts, replacement treatment to prevent dehydration, taking potassium-containing drugs, and a salt-free diet.
In cases where hyperaldosteronism is provoked by more serious disorders of the adrenal glands, pituitary gland, or GM, more complex treatment is required. Conservative tactics (drug program) and radical ones can already be used here.
Surgical treatment (resection) in combination with pharmacological support can give good results if the problem is detected early. Late diagnosis or development of tumor formations against the background of delayed treatment significantly complicates the prognosis.
Treatment of Conn's syndrome
Long-term treatment with mineralocorticoid receptor antagonists (spironolactone or eplerenone), or amiloride if they are intolerant; Often, combination with a thiazide diuretic can be the treatment approach of choice in patients:
- for whom surgical intervention is not possible;
- who do not want to carry it out;
- in whom arterial hypertension persists after surgery;
- the diagnosis of Conn's syndrome, in which it remains not fully confirmed despite the examination.
The use of mineralocorticoid receptor antagonists in individuals with Conn's syndrome provides a fairly clear reduction in blood pressure and allows regression of left ventricular hypertrophy. At the initial stages of treatment, doses of 50-100 mg/day or more of spironolactone or eplerenone may be required; subsequently, lower dosages (25-50 mg/day) are quite effective. The dose of these drugs can be reduced by combining them with thiazide diuretics. For long-term treatment of Conn's syndrome, a selective representative of mineralocorticoid receptor antagonists, eplerenone, with its inherent significantly lower frequency of side effects than spironolactone, can be considered as the drug of choice.
If other antihypertensive drugs are needed, the initial choice includes calcium channel blockers (eg, amlodipine), as they have some aldosterone receptor blocking properties in high doses. To control arterial hypertension, other classes of antihypertensive drugs can be used as components of treatment tactics.
In persons with adrenal carcinoma, drugs from the group of steroidogenesis antagonists can be used.
Causes
The causes of primary hyperaldosteronism are the following diseases:
- Adenoma or carcinoma of the adrenal cortex .
- Idiopathic hyperaldosteronism.
- Dexamethasone suppresses aldosteronism.
- Primary hyperplasia of the adrenal cortex .
The reasons for increased renin secretion and the development of secondary aldosteronism include:
- decrease in circulating blood due to fluid loss and blood loss, redistribution of fluid during edematous syndromes ( heart failure , ascites , nephrotic syndrome );
- loss of sodium when limiting it in the diet, diarrhea and taking diuretics, salt-wasting nephropathies ;
- pregnancy , in which the levels of renin and aldosterone often increase in the second and third trimesters;
- excessive potassium intake to stimulate aldosterone production;
- hypersecretion of renin in renin-secreting tumors and Bartter's syndrome .
Symptoms of secondary hyperaldosteronism
With hyperaldosteronism of the secondary form, one can usually detect a fairly high level of blood pressure, which gradually leads to tissue ischemia and damage to the vascular walls, as well as changes in the fundus (neuroretinopathy, hemorrhages), and deterioration in kidney function. The most characteristic symptom of secondary hyperaldosteronism is edema. Sometimes, (for example, pseudohyperaldosteronism in Barter syndrome), hyperaldosteronism of the secondary form occurs without the presence of arterial hypertension.
The course of hyperaldosteronism may be asymptomatic, but in quite rare cases.
Symptoms of Conn's syndrome
Conn's syndrome can be detected in any age group (the most typical age is 30-50 years), more often in women. Classic clinical and laboratory symptoms of primary aldosteronism include:
- arterial hypertension;
- hypokalemia;
- excessive excretion of potassium by the kidneys;
- hypernatremia;
- metabolic alkalosis.
Let's take a closer look at some of these manifestations.
Arterial hypertension
Arterial hypertension is present in almost all patients with Conn's syndrome.
Mechanisms of development of arterial hypertension
The pressor effects of an excess amount of aldosterone are predominantly associated with the development of sodium retention (this effect is realized through a complex of genomic mechanisms of the action of aldosterone on the sodium channels of tubular epithelial cells) and hypervolemia; a certain role is also assigned to the increase in total peripheral vascular resistance.
Arterial hypertension in persons with Conn's syndrome is usually characterized by high levels of blood pressure, often occurring as resistant, malignant hypertension. Significant left ventricular hypertrophy may be detected, often disproportionate to the severity and duration of arterial hypertension. In its development, an important role is assigned to the intensification of the processes of myocardial fibrosis due to the effect of an excess amount of aldosterone on myocardial fibroblasts. The profibrotic effects of excessive concentrations of aldosterone (realized through its non-genomic mechanisms of action on target cells) can also be quite clearly represented in the vascular wall (with an acceleration of the rate of progression of atherosclerotic lesions) and in the kidneys (with an increase in the processes of interstitial fibrosis and glomerulosclerosis).
Hypokalemia
Hypokalemia is a common but not universal manifestation of Conn's syndrome. The presence and severity of hypokalemia may depend on a number of factors. Thus, it is almost always present and quite clearly expressed in aldosterone-producing adrenal adenoma, but may be absent in bilateral adrenal hyperplasia. Hypokalemia may also be absent or insignificant in severity in the early stages of the formation of Conn's syndrome, as well as with significant restriction of sodium intake from food (for example, during the restriction of table salt when changing the lifestyle recommended for a patient with arterial hypertension).
Experts indicate that potassium levels may increase (and hypokalemia may be eliminated/mask) when:
- prolonged and painful venipuncture (mechanisms may include respiratory alkalosis during hyperventilation; release of potassium from muscle depots during repeated repeated clenching of the fist; venous stasis during prolonged compression with a tourniquet);
- hemolysis of any nature;
- the release of potassium from red blood cells in cases of delayed blood centrifugation and when the blood is kept in cold/ice.
Links[edit]
- ^ a b c d e f g h i j k l m n o p q r s t u v w
Schirpenbach C, Reincke M (March 2007).
"Primary aldosteronism: current knowledge and controversies in Conn's syndrome". Nature Clinical Practice Endocrinology and Metabolism
.
3
(3): 220–7. DOI: 10.1038/ncpendmet0430. PMID 17315030. S2CID 23220252. - ^ a b
"Primary hyperaldosteronism (Conn's syndrome or aldosterone-producing adrenal tumor)". Archived from the original on April 19, 2015. Retrieved April 8, 2015. - ^ a b c
Stowasser M., Taylor P.J., Pimenta E., Ahmed A.H., Gordon R.D.
(May 2010). "Laboratory study of primary aldosteronism". Clinical biochemist. Reviews
.
31
(2): 39–56. PMC 2874431. PMID 20498828. - ^ a b c d
"Primary hyperaldosteronism (Conn's syndrome or aldosterone-producing adrenal tumor)". Archived from the original on March 28, 2015. Retrieved April 8, 2015. - ^ a b c
Hubbard JG, Inabnet WB, Heerden CL (2009).
Principles and Practice of Endocrine Surgery
. London: Springer. item 367. ISBN. 9781846288814. Archived from the original on June 30, 2021. - ^ a b
"Primary hyperaldosteronism (Conn's syndrome or aldosterone-producing adrenal tumor)". Archived from the original on April 9, 2015. Retrieved April 8, 2015. - ^ a b
Conn JW, Louis LH (1955).
"Primary aldosteronism: a new clinical phenomenon". Proceedings of the Association of American Physicians
.
68
: 215–31, discussion, 231–3. PMID 13299331. - Williams G. H. (2009). Textbook of Nephroendocrinology
. Amsterdam: Akadem. item 372. ISBN. 9780080920467. Archived from the original on June 30, 2021. - Cronenberg H. M. (2008). Williams Textbook of Endocrinology
(11th ed.). Philadelphia: Saunders/Elsevier. ISBN 978-1-4160-2911-3. - Brown MJ (September 2012). "Platt v. Pickering: What does the molecular understanding of primary hyperaldosteronism tell us about hypertension". JRSM Cardiovascular Diseases
.
1
(6): 1–8. DOI: 10.1258/cvd.2012.012020. PMC 3738367. PMID 24175075. - Choi M, Scholl UI, Yue P, Björklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Åkerström G, Wang W, Carling T, Lifton RP. (February 2011). "K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension". The science
.
331
(6018): 768–72. Bibcode: 2011Sci…331..768C. DOI: 10.1126/science.1198785. PMC 3371087. PMID 21311022. - ^ a b
Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, et al.
(April 2013). "Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension." Genetics of Nature
.
45
(4): 440–4, 444e1–2. DOI: 10.1038/ng.2550. PMID 23416519. S2CID 205346722. - ^ a b
Azizan E.A., Poulsen H., Tuluk P., Zhou J., Clausen M.V., Lieb A., et al. (September 2013).
"Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension." Genetics of Nature
.
45
(9): 1055–60. DOI: 10.1038/ng.2716. PMID 23913004. S2CID 205347424. - Åkerström T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, Stålberg P, Robinson B, Alexander Iwen K, Dralle H, Walz MK, Lehnert H, Sidhu S, Gomez-Sanchez C, Hellman P, Björklund P (January 2021). "Activating mutations in CTNNB1 in aldosterone-producing adenomas". Scientific reports
.
6
: 19546. Bibcode: 2016NatSR ... 619546A. DOI: 10.1038/srep19546. PMC 4728393. PMID 26815163. - Tiu SC, Choi CH, Shek CC, Ng YW, Chan FK, Ng CM, Kong AP (January 2005). "Use of the aldosterone-renin ratio as a diagnostic test for primary hyperaldosteronism and its test characteristics under different blood sampling conditions". Journal of Clinical Endocrinology and Metabolism
.
90
(1):72–8. DOI: 10.1210/jc.2004-1149. PMID 15483077. - United Bristol Healthcare NHS Trust, the premier teaching trust in South West England. Archived August 13, 2007 at Archive.today.
- ^ a b
Sponsor, John W.;
Carey, Robert M.; Fardella, Carlos; Gomez-Sanchez, Celso E.; Mantero, Franco; Stowasser, Michael; Young, William F.; Montori, Victor M. (2008). "Identification, diagnosis and treatment of patients with primary aldosteronism". Journal of Clinical Endocrinology and Metabolism
.
93
(9):3266–3281. DOI: 10.1210/jc.2008-0104. PMID 18552288. - Willenberg, H. S.; Colentini, C; Quinkler, M; Cupisti, K; Krausz, M; Schott, M; Sherbaum, Washington (January 2009). "Ratio of serum sodium to urinary sodium and (serum potassium)2 to urinary potassium (SUSPPUP) in patients with primary aldosteronism". European Journal of Clinical Research
.
39
(1): 43–50. DOI: 10.1111/j.1365-2362.2008.02060.x. PMID 19067735. S2CID 25616329. - Yin, G.S.; Zhang, S.L.; Ian, L; Lee, F; Qi, YQ; Chen, Z.K.; Cheng, H (13 April 2010). "[A new index of co-utilization of serum sodium and potassium and urinary sodium and potassium in screening for primary aldosteronism in patients with hypertension]." Zhonghua Yi Xue Za Zhi
.
90
(14):962–6. PMID 20646645. - Chen Cardenas, Stanley M.; Santhanam, Prasanna (4 September 2020). "11C-Methomidate PET in the diagnosis of adrenal neoplasms and primary aldosteronism: a review of the literature". Endocrine
.
70
(3):479–487. DOI: 10.1007/s12020-020-02474-3. ISSN 1559-0100. PMID 32886316. - O'Shea, Paula M.; O'Donoghue, Darragh; Bashari, Vail; Senanayake, Russell; Joyce, Mary B.; Powlson, Andrew S.; Brown, Darragh; O'Sullivan, Gerard J.; Chow, Heok; Mendikhovsky, Joseph; Quill, Denis (18 March 2021). "11 C-methomidate PET/CT is a useful adjunct for lateralization of primary aldosteronism in routine clinical practice". Clinical endocrinology
.
90
(5):670–679. DOI: 10.1111/cen.13942. ISSN 1365-2265. PMID 30721535. - Zhou, Yaqiong; Wang, Dan; Jiang, Licheng; Rahn, Fay; Chen, Xichao; Zhou, Peng; Wang, Peijian (December 31, 2021). "Diagnostic accuracy of adrenal imaging for the diagnosis subtype of primary aldosteronism: a systematic review and meta-analysis". BMJ Open
.
10
(12). DOI: 10.1136/bmjopen-2020-038489. ISSN 2044-6055. PMC 7780716. PMID 33384386. - Cotran RS, Kumar V, Fausto N, Nelso F, Robbins SL, Abbas AK (2005). Pathological basis of Robbins and Cotran's disease
. St. Louis, MO: Elsevier Saunders. item 1210. ISBN 978-0-7216-0187-8. - Trono D, Cereda JM, Favre L (August 1983). "[Pseudo-Conn's syndrome due to intoxication with non-alcoholic pastis]." Schweizerische Medizinische Wochenschrift
(in French).
113
(31–32): 1092–5. PMID 6623028. - "Inspra (eplerenone) [prescribing information]". Archived from the original on August 8, 2011. Retrieved July 17, 2011.
- Columbia adrenal center, hyperaldosteronism (Conn's syndrome). Archived May 26, 2011, at the Wayback Machine.
- "Spark-PA - Spark-PA is a clinical trial examining an investigational drug that may help people with primary aldosteronism (PA) lower their blood pressure". Retrieved March 5, 2021.
- "Welcome to the Primary Aldosteronism Foundation". Retrieved March 5, 2021.
Classification
Primary aldosteronism depending on the etiology is due to:
- aldosterone-producing adenoma;
- adrenal hyperplasia;
- bilateral adrenal hyperplasia;
- aldosterone-producing carcinoma .
Secondary aldosteronism (extra-adrenal) is caused by the presence of benign and malignant tumors:
- ovaries;
- testicles;
- thyroid gland;
- adrenal medulla;
- intestines.
As well as various somatic diseases (heart, kidneys, liver).
Conn's syndrome , the main cause of which is a hormonally active tumor of the adrenal aldosterome, refers to primary hyperaldosteronism. A benign tumor was described in 1955 by Conn, after whom this syndrome is named. Aldosteroma is a small benign tumor up to 1.5 cm in size that does not metastasize and is the cause of aldosteronism in 60-80% of cases. In the left adrenal gland, the tumor occurs 2 times more often.
Conn's syndrome is characterized by increased excretion of potassium and water in the urine, therefore polyuria with low urine density develops. Against the background of changes in electrolyte metabolism, patients develop neuromuscular disorders ( paresthesia , muscle weakness , sometimes cramps ), and nephropathy . Patients with adenoma have severe hypertension and severe hypokalemia .
Idiopathic hyperaldosteronism , which occurs in 30-40% of cases, is the second most common form of primary hyperaldosteronism. The term "idiopathic" indicates that the etiology of this form is unknown. The development of idiopathic aldosteronism is caused by bilateral hyperplasia of the adrenal cortex . In hyperplastic adrenal glands, a large amount of aldosterone is secreted with the ensuing consequences - arterial hypertension , hypokalemia and decreased plasma renin. The difference between this disease is the preservation of the sensitivity of the altered areas of the adrenal glands to the influence of angiotensin II. Adenocarcinoma of the adrenal gland is very rare.
Secondary aldosteronism is associated with increased renin secretion and other mechanisms. The causes of secondary hyperaldosteronism are: arterial hypertension that occurs against the background of renal artery stenosis, reninoma (renin-producing kidney tumor) and conditions that are accompanied by edema - ascites in liver cirrhosis , heart failure or nephrotic syndrome . Among kidney diseases, nephritis , cystinosis , and tubulopathies . In all of these diseases, the renin-angiotensin-aldosterone system is stimulated, which is accompanied by the release of aldosterone.
Tumors of the endocrine glands secrete mineralocorticoids, which is unusual in normal conditions. Secondary changes in steroid biosynthesis are also possible. Glucocorticoids, estrogens , catecholamines , androgens and toxic metabolic products contained in tumors block the synthesis of steroids through a negative feedback mechanism, while the production of adrenocorticotropic hormone is reduced, and the excess biosynthesis of mineralocorticoids is compensatorily activated. Secondary hyperaldosteronism is characterized by decreased potassium levels, high levels of renin and aldosterone, and alkalosis while maintaining normal blood pressure.
Bartter's syndrome is a rare genetic disorder that affects the renal transport of sodium, potassium, and chloride. In the clinic, this is manifested by hypochloremic alkalosis and various electrolyte disturbances. Bartter's syndrome is characterized by elevated renin levels and hyperaldosteronism. Despite this, the pressure remains normal, which is associated with a decrease in plasma volume and the formation of vasodepressor prostaglandins , which counteract the increase in pressure.
Bartter's syndrome is divided into two variants: neonatal and classic. In neonatal cases, an increased level of chloride is detected in the amniotic fluid. Children are born prematurely with low weight, they have severe polyuria and dehydration (dehydration), life-threatening, convulsions , diarrhea and hypercalciuria, in which nephrocalcinosis (kidney calcinosis) develops very early. Nephrocalcinosis progresses and kidney function declines over several years.
In the classic version, the altered gene encodes the renal chlorine channel, which transports it through the membrane into the blood. When this transport is disrupted, loss of sodium chloride develops - hypovolemia - activation of the renin-angiotensin-aldosterone system - loss of potassium - development of hypokalemic alkalosis.
Nephrocalcinosis is absent in the classic version of the syndrome. The child has polyuria, episodes of fever and diarrhea, muscle twitching and muscle weakness. Chronic hypokalemia is accompanied by the formation of kidney cysts, and significant polyuria provokes hydronephrosis . Treatment consists of administering potassium chloride intravenously to replenish potassium and taking spironolactone . The basis of treatment is taking Indomethacin a 2-5 mg/kg per day. Treatment should be lifelong. Patients often develop symptoms of indomethacin toxicity to the kidneys, gastrointestinal tract, and liver.
Pathophysiology[edit]
Aldosterone acts on most or all cells of the body, but clinically the most important actions occur in the kidney, on cells of the late distal convoluted tubule and medullary collecting duct. In principal cells, aldosterone increases the activity of the basolateral membrane sodium-potassium ATPase and apical epithelial sodium channels, ENaC, as well as potassium channels, ROMK. These actions increase sodium reabsorption and potassium secretion. Because more sodium is reabsorbed than potassium excreted, it also makes the lumen more electrically negative, causing chloride to follow sodium. Water then follows sodium and chloride by osmosis. In Conn's syndrome, these actions cause an increase in extracellular sodium and fluid volume and a decrease in extracellular potassium. Aldosterone also acts on intercalated cells by stimulating apical proton ATPase, causing proton secretion that acidifies the urine and alkalinizes the extracellular fluid. [ citation needed
]
Thus, hyperaldosteronism causes hypernatremia, hypokalemia, and metabolic alkalosis. [ citation needed
]
More subtle notes on aldosterone include the fact that it stimulates sodium-potassium ATPase in muscle cells, increasing intracellular potassium, and also increases sodium reabsorption throughout the intestine and nephron, possibly due to widespread stimulation of sodium-potassium ATPase. Finally, the epithelial cells of the sweat gland ducts and the distal surface of the colon respond in the same way as the main cells of the nephron. These responses are important for adaptation to climate change and as a cause of constipation due to elevated aldosterone levels [ citation needed
] .
Sodium retention results in increased plasma volume and increased blood pressure. Increased blood pressure will increase the glomerular filtration rate and cause a decrease in the release of renin from the granule cells of the juxtaglomerular apparatus in the kidney, reducing sodium reabsorption and returning renal sodium excretion to near normal levels, allowing sodium to "escape" the effects of mineralocorticoids (also known as the aldosterone escape mechanism in primary hyperaldosteronism, also contributing to an increase in ANP levels). If there is primary hyperaldosteronism, the decrease in renin (and subsequent decrease in angiotensin II) will not lead to a decrease in aldosterone levels (a very useful clinical tool in the diagnosis of primary hyperaldosteronism). [ citation needed
]