Composition and release form
The drug is available from the pharmacy in the form of a finely granulated, odorless powder. It dissolves well in any liquid - propylene glycol, ethyl alcohol, methanol, water. It is also produced in the form of a solution with various concentrations of 2%, 5% and 15%, in the form of spray, foam and tablets for oral administration.
The active ingredient is minoxidil.
The solution has additional components: pure water, alcohol and propylene glycol.
You can purchase the medicine without a doctor's prescription.
Myth No. 1. Minoxidil is a hormonal drug
In fact, it is a chemically synthesized component that is a derivative of pyrimidine. It is not a hormone and does not exhibit androgenic or antiandrogenic activity. Does not affect hormone synthesis. Now there are a large number of minoxidil derivatives that have a similar chemical formula, related to cosmetics, the structure of which is similar to the structure of the drug molecule, for example, aminexil, copyrrole, diaminopyrimidine, stemoxidine and others. These substances are weakened analogues and do not cause such concerns in patients as the prototype itself.
pharmachologic effect
The drug has a complex therapeutic effect. It has a pronounced hypotonic and vasodilating property, also has a beneficial effect on hair follicles and stimulates their growth.
The drug helps dilate blood vessels, reduces the load on the heart muscle, provoking the development of tachycardia and increases cardiac output.
As a result of taking the medicine, activation of the follicles is also observed, which has a positive effect on the restoration of hair growth against the background of high exposure to male hormones. Stimulation of the bulbs occurs due to improved blood circulation in the scalp. The therapeutic effect is observed in both men and women. The best results from treatment are recorded in patients suffering from male pattern baldness for no more than 10 years, if the diameter of the receding hairline does not exceed 10 cm.
A more pronounced positive effect from taking the drug is observed in people suffering from a similar condition for 3-5 years.
Active hair growth begins after 3-4 months of regular use of the medicine. However, after stopping the drug, the bald patches on the head may return.
When administered orally, the drug is quickly absorbed into the bloodstream. The highest content of the active ingredient occurs after 60 minutes. It easily penetrates any placental barriers, so it easily passes into breast milk.
The drug is excreted through the kidneys in the urine.
Introduction
Minoxidil was first introduced as an oral drug to treat severe and refractory hypertension in the 1970s.
By chance, doctors noticed increased hair growth and generalized hypertrichosis in balding patients, which led to the development of the topical drug minoxidil to treat androgenetic alopecia (AGA), first in men and then in women. A 2% minoxidil solution was first introduced to the market in 1986, followed by 5% in 1993 [2]. Despite its worldwide acceptance for over 30 years, the mechanism underlying minoxidil's action on hair growth has yet to be fully elucidated. We aimed to review and update important clinical information on topical minoxidil, including pharmacology, mechanism of action, clinical efficacy, and side effects.
Pharmacology of topical minoxidil
Minoxidil is a piperidino-pyrimidine derivative with the following chemical structure: 2,6-diamino-4-piperidinopyrimidine-1-oxide (C9H15N5O) (Fig. 1) [2].
Minoxidil solution (MS) contains inactive ingredients including water, as well as ethanol and propylene glycol (PG), which are used as carriers to increase the solubility of minoxidil [3].
PG promotes effective drug delivery to hair follicles; however, its frequent induction of local irritation prompted scientists to create minoxidil foam (MF) without PG. Additional ingredients in the foam include cetyl alcohol, stearyl alcohol and butylated hydroxytoluene.
Compared to MS, MF provides better delivery of the active ingredient to the target site and easy drug penetration with less irritation; consequently, the US Food and Drug Administration (FDA) has granted approval for 5% MF for the treatment of AGA. MF is also easier to use because it dries faster and has less peripheral bleeding.
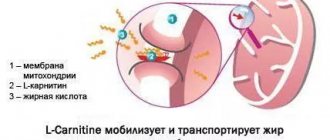
Minoxidil is a powerful vasodilator. It opens potassium channels located on the smooth muscle of the peripheral artery, causing hyperpolarization of the cell membrane [6]. Xu et al proposed that K+ channel activity is required for progression to the G1 stage of the cell cycle. Therefore, it may play a key role in early cell proliferation. This theory was further supported by animal studies in which minoxidil increased cellular DNA synthesis and increased cell proliferation. The positive effect of minoxidil on hair growth is due mainly to its metabolite, minoxidil sulfate, and the enzyme responsible for this conversion is sulfotransferase, which is localized in the hair follicles and varies in production between individuals [9].
There are two phenol sulfotransferases responsible for the sulfation of minoxidil in the human scalp. Patients with higher enzymatic activity responded better to topical minoxidil than patients with lower enzymatic activity. It should be noted that there is no correlation between serum or tissue concentrations of minoxidil and hair growth [10]. Salicylate and aspirin may inhibit sulfotransferase.
A recent study found that follicular enzyme activity decreased after 14 days of low-dose aspirin. Thus, prior or concomitant use of aspirin reduces the clinical response to topical minoxidil [11].
Pharmacokinetically, in the absence of scalp problems, approximately 1.4% of topical minoxidil is absorbed, while increased absorption is associated with drug concentration, frequency of drug use, and damage to the stratum corneum barrier function. Systemic absorption of topical minoxidil is less than 99% of the amount applied to the scalp. Minoxidil does not bind to plasma proteins and does not penetrate the blood-brain barrier. Approximately 95% of the systemically absorbed drug and its metabolites are excreted through the kidneys within 4 days.
Biological effects of minoxidil
Minoxidil has been used to treat hair loss for several decades. The drug acts on follicular cells, enhancing hair growth and reducing hair loss. Discontinuation of treatment results in progressive hair loss over 12 and 24 weeks [2].
Mori and Uno found that minoxidil shortened the telogen phase to 1-2 days in study rats, compared to about 20 days in control rats. An increase in the rate of DNA synthesis was observed during the anagen phase, suggesting that minoxidil stimulated secondary germ cells of telogen follicles and caused a rapid transition to the anagen phase [13].
Clinical trials in AGA patients treated with 2% or 5% minoxidil showed a noticeable increase in hair growth and reduction in hair loss, with particularly good results with the 5% formulation [14,15]. Hypertrichosis in minoxidil-untreated areas showed that the drug increased the anagen interval in humans. An experimental study showed that minoxidil prolongs the anagen phase in the dermal papilla (DP) by inducing β-catenin activity and stimulating follicular proliferation and differentiation [16].
Histologically, an increase in follicle size and percentage of follicles in the anagen stage was noted [17,18].
Laser Doppler velocimetry has shown that minoxidil may have an effect on blood vessels. However, electron microscopy has shown an increase in fenestration in the follicular capillary wall around the bulbs in the anagen phase with 4% minoxidil solution [19].
Vascular endothelial growth factor (VEGF) in DS cells regulated perifollicular vascularization. It increased sharply during the anagen phase and decreased during the catagen and telogen phases [20,21]. Minoxidil increased VEGF mRNA expression in a dose-dependent manner. VEGF mRNA expression increased sixfold with minoxidil. In addition, a recent study by Yum et al showed that topical minoxidil induces factor-1-alpha, which is activated by hypoxia and is dependent on VEGF induction [22].
Minoxidil also stimulated prostaglandin E2 production by activating prostaglandin endoperoxide synthase-1 [23], but inhibited prostacyclin production [24].
In addition, minoxidil increased the expression of prostaglandin E2 receptor, the most regulated target gene in the β-catenin pathway in DS cells. This circumstance may allow hair follicles to grow continuously and maintain the anagen phase [25].
The use of minoxidil for hair diseases
Topical minoxidil has been approved by the FDA for the treatment of AGA. In addition, it has been used as an off-label drug for the treatment of a number of hair diseases such as alopecia areata (AA), cicatricial alopecia, alopecia due to congenital hair shaft disorders, and to improve hair growth on body in other areas, including eyebrows and beard (Table 1).
Androgenetic alopecia
AGA is alopecia without scarring, in which the terminal hairs turn into vellus/vellus-like hairs. Typically, in men, hair loss and hair thinning occurs in the frontal and vertex areas [26], while in women, hair loss is characterized by a decrease in hair density on the crown without involvement of the frontal areas (also known as female pattern hair loss). female pattern hair loss, FPHL) [27].
Testosterone plays a role in the pathogenesis of AGA by being converted to doactive hydrotestosterone (DHT) by 5-alpha reductase [28,29].
A meta-analysis showed that topical minoxidil at any concentration produced better results than placebo. Regarding AGA, studies show that the mean differences between 2% and 5% minoxidil were 8.11 hairs/cm2 and 14.90 hairs/cm2, respectively, compared to the placebo group. While a comparison of the 2% minoxidil and placebo groups in female patients showed a mean difference of 12.41 hairs/cm2 [30].
A five-year follow-up study of 31 AGA patients treated with 2% and 5% minoxidil solutions showed peak hair growth after 1 year of use [31].
Numerous clinical trials have been conducted with topical minoxidil at multiple concentrations in different formulations to test effectiveness. In men with AGA, minoxidil 5% solution showed a significant increase in mean difference in hair density compared to minoxidil 2% solution and placebo [15,32,33].
An earlier response, characterized by a 45% increase in hair growth, was noted with MS 5% than in the MS 2% group [48]. MS with 2% and 5% showed promising results in the treatment of FPHL. Side effects such as dermatitis, headaches and hypertrichosis were more common at 5% MS. Hypertrichosis can be a problem in women, leading to poor treatment compliance, and hence 2% MS is considered preferable [34].
Interestingly, 1% MS was effective in Asian women with a significant increase in non-vellus hair compared with that in the placebo group [35]. Data from randomized controlled trials of topical minoxidil for AGA and FPHL are summarized in Tables 2 and 3, respectively.
The increased occurrence of contact dermatitis with MS has led to the development of non-PG foam for the treatment of hair loss. In patients with AGA, 5% MF was well tolerated and superior to placebo. Only 5% of patients experienced symptoms of scalp irritation after 52 weeks of treatment.
For male AGA, the recommended treatment is twice daily application of 1 ml of 5% MS and half a cap of 5% MF [38].
The recommended treatment for FPHL is twice daily application of 1 ml of 2% MS and once daily application of half a cap of 5% MF. Daily topical application of 5% MF has been shown to be as effective as twice daily application of 2% MS in FPHL with significantly fewer adverse events [36,37].
Treatment should be continued for a long time, since stopping taking minoxidil leads to hair loss within 3-4 months. No data on teratogenicity or serious side effects were reported. As a safety precaution, the use of minoxidil should be avoided during pregnancy and lactation [39].
Response to minoxidil treatment in patients with AGA can be predicted by measuring sulfotransferase activity in plucked hair follicles. Studies have shown a sensitivity of 93% and specificity of 83% [40]. The enzymatic assay has also demonstrated high accuracy in 94% of non-responders [41].
A subsequent study using this assay showed that by 8 weeks of topical minoxidil application, sulfotransferase enzyme activity in hair stabilized. These data suggest that those who respond to minoxidil will not develop dosage resistance, and similarly, patients who do not respond to treatment will not develop into responders [42].
A study by McCoy et al showed that increasing the concentration of topical minoxidil to 15% in patients not predicted to respond had an increased clinical response compared with that associated with 5% minoxidil without cardiac side effects . Thus, patients who do not respond to treatment may experience a clinical response with increased minoxidil concentrations.
The use of minoxidil with a new formulation, minoxidil sulfate solution (MXS), has increased because it is an active metabolite of minoxidil. MXS has been shown to have higher efficacy than the conventional formula and may be a treatment option for patients with low minoxidil sulfotransferase activity [16,44].
However, due to its large molecular weight affecting its permeability, as well as higher degradation than MS, MXS may not be as effective [45].
To improve the response, MXS should be used at higher concentrations and the formulation should be kept in a small package to reduce solution instability [44].
A study conducted in 44 patients with AGA and FPHL unresponsive to 5% MS twice daily reported sensitivity to 10% MXS after treatment for 4 months. Side effects included mild irritation, erythema and folliculitis [46].
Therefore, we believe that MXS is a promising treatment for those who do not respond to minoxidil.
Attempts have been made to use oral minoxidil for patients with AGA or FPHL who were not satisfied with conventional treatment. The combination of low dose minoxidil 2.5 mg and spironolactone 25 mg in patients with FPHL showed positive results with decreased hair loss and increased hair density. The mean severity score decreased to 2.3 at 6 months and to 2.6 at 12 months. Mild side effects have been reported, including urticaria, postural hypotension, and facial hypertrichosis. There were no significant changes in blood pressure observed in the study.
Alopecia areata
Alopecia areata (AA) is an autoimmune disease of the hair follicles with clinical presentation ranging from patchy, scarless alopecia to complete scalp hair loss (alopecia totalis) and body hair loss (alopecia universalis) [48].
There are many treatments available, however, none have received FDA approval. Minoxidil is sometimes used off-label as monotherapy or in combination with other treatments [49,50]. Minoxidil was initially tested in patients with OA, but there were no satisfactory data on efficacy [51].
Monotherapy with topical minoxidil gave unsatisfactory results, since hair growth was stimulated only in cases of mild and not severe OA. Two randomized controlled trials showed that treatment with 3% MS increased hair growth to some extent compared with placebo. Hair growth was noted and the hair structure became denser in the treated area, but little or no effect was observed in patients with extensive OA. Only mild side effects of minoxidil have been reported, with no evidence of systemic effects [52,53].
Higher concentrations of topical minoxidil were preferred in the treatment of OA. For extensive OA lesions (more than 75% of the scalp), 5% MS resulted in 81% terminal hair growth compared with 38% in group 1 with %MS [54].
Additionally, Olsen et al demonstrated that the combination of prior systemic corticosteroid use (over 6 weeks) followed by 2% MS (three times daily) provided better results in permanent hair growth than without the combination [55].
Histological results with the use of minoxidil for OA demonstrated a decrease in perifollicular infiltration in respondents [56].
A significant reduction in perifollicular Langerhans cells and T cell infiltration was also observed [57].
However, other data showed no significant changes in perifollicular infiltration [58, 59].
Thus, the immunosuppressive effect of minoxidil is still unclear.
Overall, many studies have shown that topical minoxidil provided some benefit to patients with OA, as it slightly increased hair growth without altering disease progression but also without inducing remission [52].
As monotherapy, topical minoxidil did not demonstrate statistically significant improvement. Thus, it is recommended as an adjuvant therapy for OA [51].
Oral minoxidil 5 mg twice daily was studied in 65 treatment-resistant patients with OA. The drug was relatively well tolerated at a dosage of 2 g with limited intake of sodium-containing substances. Patients treated with oral minoxidil had better hair growth rates than patients with 5% MS. However, only 18% of patients had a positive clinical response at 34.8 weeks with a noticeable increase in terminal hairs.
Systemic symptoms of sodium and water retention developed in patients who did not adhere to the sodium restriction protocol. Other side effects include headache, palpitations, and facial hypertrichosis.
Telogen hair loss
Telogen effluvium is a common non-scarring alopecia characterized by excessive hair loss during the telogen phase. This condition can be caused by various factors, such as pregnancy, severe illness and surgery. Chronic telogen effluvium (CTE) hair loss is defined as hair loss that continues for 6 months [61].
Treatment for CTE is not always effective. Many medications have been tried, including minoxidil. There are also several clinical trials on topical minoxidil. It is believed that oral minoxidil may be a promising treatment option. A retrospective review was recently conducted among 36 patients with STE who received daily oral minoxidil in doses ranging from 0.25 mg to 2.5 mg. A noticeable reduction in hair loss was observed after 6 and 12 months. Some patients reported mild facial hypertrichosis, dizziness, and changes in blood pressure as side effects [62].
Scarring alopecia
For cicatricial alopecia, treatment should begin as early as possible. The goal of treatment is to preserve the remaining hair follicles and stop the progression of the disease [63].
Central centrifugal cicatricial alopecia is a common form of scarring alopecia among African American women. This usually results in hair loss at the top of the head and spreads to the periphery. In one small retrospective study, the combination of high-potency topical steroids and topical minoxidil did not show significant efficacy. However, a decrease in disease severity in some patients may indicate that medication may slow the progression of the disease [65].
Topical minoxidil is used to treat frontal fibrosing alopecia (FFA), a severe type of alopecia affecting the frontal and temporal areas. In a review, 50% of patients with FFA (n = 7) were treated with 2% MS twice daily and systemic steroids or finasteride. This therapy helped slow the progression of the disease [67].
Another type of scarring alopecia for which topical minoxidil has been reported to be effective is traction alopecia. A characteristic feature is the absence of scar changes and reversibility in the initial stages of the disease. This fact may be useful when using minoxidil. Two patients who did not respond to topical triamcinolone for 1 year experienced significant hair growth using only 2% minoxidil [68].
Thus, topical minoxidil may be beneficial and can be used as an adjuvant with other medications to treat cicatricial alopecia.
Chemotherapy-induced alopecia
Hair loss is one of the common side effects of chemotherapy [69].
A randomized controlled trial by Duvic et al showed that 1 ml of 2% MS applied to the entire scalp twice daily reduced the time to alopecia development in patients by approximately 50 days (Table 4). The drug was used during and up to 4 months after chemotherapy. However, in some cases, MS has failed to prevent hair loss, such as in patients with gynecological malignancies and solid tumors treated with doxorubicin-based chemotherapy [71,72].
In breast cancer, patients who used 1.5 ml of 5% MS twice daily did not experience significant hair regrowth 6 months after treatment [73]. Thus, the true effectiveness of topical minoxidil for chemotherapy-induced alopecia has not yet been proven.
Low doses of oral minoxidil (1 mg once daily) may be an effective treatment for permanent chemotherapy-induced alopecia (PCIA). A patient diagnosed with acute myeloid leukemia who had PCIA for 16 months showed increased hair growth after 6 weeks. Significant hair growth with newly emerged follicles in the anagen phase and reduced miniaturization was demonstrated histologically after 2 years of treatment [74].
Alopecia due to disorders of the hair shaft
Minoxidil has been used to treat monilethrix, a rare autosomal dominant hair disorder manifesting as brittle hair shafts with regular beady appearance [75]. Miliary follicular nodules with a pointed horny spine at the top, surrounded by an inflammatory rim, appear on the skin of the scalp. Hair in the affected area often splits at the base (a symptom of blackheads). Four patients with monilethrix who were treated with 1 ml of 2% MS twice daily for 1 year noted an increase in normal hair growth in the affected areas at 6 and 12 months, with no adverse reactions reported. The observed effect may be associated with prolongation of the anagen phase [76].
Topical minoxidil has also been reported to be effective in the treatment of loose anagen hair syndrome (LAS). A 2-year-old girl with LAS who was treated with 5% MS for 20 months showed significant improvement and the effect was maintained 28 months after stopping the medication. No side effects were noted [77].
Oral minoxidil has been used in many alopecias due to hair shaft disorders, but topical minoxidil may increase hair shaft fragility [78]. Low dose oral minoxidil (0.25 mg once daily) was used as treatment in two patients with monilethrix. Encouraging results were noted in one case where there was a decrease in hair loss, longer hair and an increase in hair density at 3 and 6 months. The improvement in hair condition was maintained with the same dosage after 18-24 months. No adverse events were observed [78]. In another case, oral minoxidil was used for alopecia due to hair shaft abnormalities in a 6-year-old girl with LAS who did not respond to 5% MS therapy. She received oral minoxidil 0.5 mg daily and showed improvement in hair color, density, and length within 3 months of treatment [79].
Strengthening hair on other areas of the face
Topical minoxidil has been used to stimulate eyebrow hair growth and beard growth (Table 4). The eyebrows play an important role as periocular landmarks on the face. Hypotrichosis of the eyebrows develops as a result of decreased eyebrow density and lack of growth [80,81].
A recent 16-week randomized controlled trial demonstrated that 2% MS twice daily was superior to placebo in the treatment of eyebrow hypotrichosis [82].
The thickness and diameter of the eyebrows also increased significantly. Statistical differences in both subjective and objective assessments were noted from weeks 8 to 16. Similar results were also noted with 1% MS used twice daily, with significant changes in total photographic score, hair count, hair diameter, and patient satisfaction. Additionally, MS 3% has been shown to be more effective when applied topically than bimatoprost 0.03% in enhancing eyebrow growth. However, contact dermatitis was more common in the 3% MS group [84].
Beard hair enhances the appearance of the face and symbolizes masculinity. Compared to placebo, minoxidil was superior in enhancing beard growth with only minor side effects. Statistically significant differences were found in overall photographic scores, hair count, and patient self-esteem when using 0.5 ml of 3% minoxidil twice daily than when using placebo [85].
Consequently, minoxidil was considered a safe and effective treatment for eyebrows and beards.
Side effects of minoxidil
Topical minoxidil is considered safe. However, some patients experienced side effects after use. The most common side effect is contact dermatitis with typical symptoms of itching and burning. The prevalence of side effects is lower at 2% MS than at 5% MS. Allergic contact dermatitis can also occur due to PG or minoxidil itself. A patch test should be performed to identify the inciting agents. Although an allergic reaction to minoxidil can occur, it is rare. If patients are allergic to PG, the “carriers” of the active substance in the drug should be replaced with butylene glycol, glycerin or polysorbate. Additionally, PG-free MF should be prescribed instead, and if reactions persist, an allergy to minoxidil should be suspected. In this case, the use of all minoxidil preparations should be discontinued.
Hypertrichosis is dependent on the concentration of minoxidil, with the highest incidence of unwanted hair growth observed with 5% MS [87].
This side effect is more common in women than men, and although there is no clear explanation, it is believed that some female patients may have a higher number of follicles that are sensitive to minoxidil. Spontaneous resolution of hypertrichosis occurs first on the face and hands after 1–3 months, and then on the legs after 4–5 months after stopping minoxidil use. It has been suggested that excessive topical application may cause systemic absorption, resulting in excessive hair growth in areas not treated [88,89].
Twice daily use of topical minoxidil does not produce systemic side effects such as hypotension, abnormal heart rate, and weight gain [2]. Therefore, minoxidil is considered safe and effective for various hair conditions.
Oral minoxidil is primarily metabolized through the liver by conjugation with glucuronic acid. Metabolized minoxidil is excreted through the kidneys 3–4 hours after administration, but the vasodilation effect may persist for up to 72 hours [1].
Serious side effects, including sodium and fluid retention, and cardiovascular complications (eg, coronary artery disease, pericardial effusion, and pulmonary hypertension) have been reported with systemic administration of minoxidil [12].
Sodium and fluid retention causes weight gain and, in severe cases, congestive heart failure. This is due to the redistribution of blood flow in the kidneys, as well as the activity of plasma renin [1,2].
Coronary heart disease has been reported and may be caused by higher oxygen demand, increased heart rate, and increased cardiac output [4]. Pericardial effusion caused by minoxidil occurs in approximately 5% of patients, the mechanism is unknown [12].
Pulmonary hypertension has also been described as a consequence of increased pulmonary artery pressure and increased cardiac output after taking minoxidil [12].
Other adverse effects include hypertrichosis, intermittent throbbing headache, pruritus, skin rashes such as bullous eruptions, and polymenorrhea [12,89,90].
In our opinion, the benefits of oral medications are not sufficient to justify the risk of potential side effects.
Conclusion
Minoxidil is a common drug prescribed for the treatment of problems associated with hair loss, which provides good clinical results in patients with alopecia. To date, the FDA has only approved minoxidil for AGAs. However, minoxidil is used off-label to treat some other hair conditions, as well as to increase hair growth in other areas. Although topical minoxidil is considered an effective and safe treatment option for a variety of hair conditions, more evidence-based data on minoxidil is needed for the treatment of some alopecia conditions.
Figure 1. Chemical structure of minoxidil
Table 1. Hair diseases for which topical minoxidil is used
| Indications for use of minoxidil ( FDA ) |
|
| Application of Off - label (not according to instructions) |
|
Table 2. Randomized clinical trials for the treatment of androgenetic alopecia in men
| Author, year | Age, years | Intervention group | Comparison | Length of weeks | Results, mean difference in hair density/cm2 (SD) between baseline and end of study | Side effects |
| Olsen et al., 2002 | 36.5 (average) | 2% minoxidil solution 2 times/day, N=141 5% minoxidil solution 2 times/day, N=139 | Placebo N=78 | 48 | 2% minoxidil solution: 12.7 (20.7) 5% minoxidil solution: 18.6 (25.4) Placebo: 3.9 (21.7) | 2% minoxidil solution: itching 1%, headache 0.6% 5% minoxidil solution: itching 4%, headache 3% |
| Olsen et al., 2007 | 39.2 (average) | 5% minoxidil solution 2 times/day, N=180 | Placebo N=172 | 16 | 5% minoxidil solution: 20.9 (22.5) Placebo: 4.7 (19.7) | Erythema 3.9%, dryness/flaking 2.8%, folliculitis 1.1% |
| Berger et al., 2003 | 40 (average) | 5% minoxidil solution 2 times/day + shampoo with placebo, N=50 | Placebo shampoo, N=50 | 26 | 5% minoxidil solution: 12.3 Placebo: -0.58 | Local intolerance 7% |
Table 3. Randomized clinical trials for the treatment of female pattern hair loss
| Author, year | Age, years | Intervention group | Comparison | Length of weeks | Results, mean difference in hair density/cm2 (SD) between baseline and end of study | Side effects |
| Lucky et al., 2004 | 37 (medium) | 2% minoxidil solution 2 times/day, N=108 5% minoxidil solution 2 times/day, N=101 | Placebo N=51 | 48 | 2% minoxidil solution: 20.7 (17.6) 5% minoxidil solution: 26.0 (19.5) Placebo: 9.4 (14.6) | 2% minoxidil solution: itching 0.6% headache 2% 5% minoxidil solution: itching 5%, headache 4%, hypertrichosis 3% |
| Tsuboi et al., 2007 | More than 20 | 1% minoxidil solution 2 times/day, N=120 | Placebo N=120 | 24 | 1% minoxidil solution: 8.15 Placebo: 2.03 | Not reported |
| Blume-Pevtavi et al., 2003 | 49.9 (average) | 5% minoxidil foam 1 time/day N=50 | 2% minoxidil solution 2 times/day, N=50 | 24 | 5% minoxidil foam: 31.9 (19.9) 2% minoxidil solution: 28.4 (19.9) | 5% minoxidil foam: facial hypertrichosis 33%, hair loss 12.5%, itching 8.9%, dermatitis 3.6%, headache 3.6%, nausea and shortness of breath 1.8%, palpitations and tachycardia 1.8 %, papules and pustules 1.8%, maculopapular rash 1.8% 2% minoxidil solution: facial hypertrichosis 51%, hair loss 17.5%, headache 7%, palpitations and tachycardia 3.5%, ear edema 1.8%, papules and pustules 1.8%, dermatitis 1.8% |
Table 4. Randomized clinical trials in the treatment of non-AGA alopecia
| Author, year | Age, years | State | Intervention group | Comparison | Length of weeks | Results, mean difference in hair density/cm2 (SD) between baseline and end of study | Side effects |
| Duvic M, 1996 | 44 (medium) | Alopecia after chemotherapy | 2% minoxidil solution 2 times/day, N=10 | Placebo N=9 | There was no significant increase in density (148.5 day average) | Undefined | itching 60%. hypertrichosis 38.1%, folliculitis 25% |
| Lee et al, 2014 | 30.6 (average) | Hypertrichosis of the eyebrows | 2% minoxidil solution 2 times/day, N=39 | Placebo N=39 | 16 | 2% minoxidil solution: 5.1 (5.2) Placebo: 1.0 (4.7) | Mild to moderate side effects 12.8% |
| Worapunpong et al, 2017 | 30.7 (average) | Hypertrichosis of the eyebrows | 1% minoxidil solution 2 times/day, N=40 | Placebo N=40 | 16 | 1% minoxidil solution: 10.5 (15.0) Placebo: 8.1 (10.6) | Mild itching and burning 22.5% |
| Ingprasert et al., 2016 | From 20 to 60 | To improve beard growth | 3% minoxidil solution 2 times/day, N=23 | Placebo N=23 | 16 | 3% minoxidil solution: 5.0 (0.7) Placebo: 0.4 (0.3) | Not reported |
Contraindications
It is prohibited to take the drug in tablets in the following cases:
- Individual intolerance to medicinal components.
- Mitral stenosis.
- Re-development of pulmonary hypertension.
- Pheochromocytoma.
- Time of bearing a child and breastfeeding.
- Children under 12 years old.
- Contraindications to the use of the solution are:
- Skin damage.
- Dermatosis on the scalp.
Why is minoxidil so popular?
Minoxidil has already become a traditional remedy that is prescribed all over the world for the treatment of alopecia, especially androgenetic alopecia (AGA). It is prescribed despite its numerous contraindications and side effects, which minoxidil has like any drug. The fact is that for a long time there was no alternative - minoxidil was practically the only drug that gave an effect. Both doctors and patients got used to it. Today, no less effective and at the same time harmless means have appeared, as all advanced trichologists say. But force of habit, coupled with the minoxidil business, sometimes prevents ordinary consumers from learning the truth. At the same time, the very fact that minoxidil is prescribed to “everyone”, regardless of gender and causes of hair loss, should already be alarming. After all, there is no universal therapy, for example, for cardiovascular or bacterial diseases - treatment is always selected individually and, as a rule, is complex. Contraindications for the use of minoxidil:
- Chronic diseases of the circulatory system;
- Acute skin diseases;
- Infectious diseases;
- Tumors (the drug does not cause the growth of tumors, but increases the risk of their metastasis);
- Open wounds (cuts, abrasions), ulcers, burns on the scalp;
- Pregnancy and lactation;
- Age under 18 and over 65 years.
Side effects
During the course of treatment with Minoxidil, side effects may occur associated with a disorder of any organ or system. However, the central nervous system and sensory organs are most often affected.
The most common adverse reactions associated with the use of the drug are:
- Headache, dizziness.
- Decreased visual acuity.
- Tachycardia, symptoms of angina pectoris.
- Heart rhythm disturbance.
- Change in laboratory data - there is a decrease in the level of leukocytes and platelets in the blood.
- Signs of an allergic reaction.
- Increased pigmentation of the skin.
- Eczema, dermatitis, folliculitis.
Myth 4. Stops working after 2-3 years
Minoxidil continues to work for a long time. Studies have been conducted that have demonstrated that even after 10 years of treatment, the condition is better than before starting to use the drug. However, hair growth in the first 2-3 years of use is indeed more significant. But this is not due to the fact that the drug begins to work worse, but to the fact that the negative effects of hormones continue, but the component has no effect on them, that is, the aggressiveness of alopecia increases over time, but the effectiveness of the drug does not.
Is there a future for minoxidil?
Despite the emergence and development of new treatment methods, minoxidil remains the most popular drug due to its relatively low cost and high effectiveness compared to many cosmetic substances.
At the moment, there is a return to the administration of this substance orally, in tablet form. A group of researchers led by one of the world's leading trichologists, Rodney Sinclair, has accumulated a large number of successful cases of treating androgenetic alopecia using tablets. A dose has been developed that does not give systemic side effects, but gives good hair growth in androgenetic alopecia.
Lotions and sprays are being improved. A search is underway for optimal conductors that can improve the penetration of the drug to the level of the hair follicle, thereby increasing its clinical effectiveness.
Minoxidil is the only topical medication approved by the FDA for the treatment of androgenetic alopecia. But remember that it was and remains a remedy that should be used as directed, as well as under the supervision of a medical specialist.
Instructions for use of the medicine
According to the instructions, the drug in tablet form is intended for oral administration. Adults and children are prescribed 5 mg up to 2 times a day. If necessary, the therapeutic dose can be increased.
Moreover, the average dosage of the drug is 10-40 mg, the largest amount of the drug per day should not exceed 100 mg.
For patients under 12 years of age, tablets are prescribed once a day at a therapeutic dose of 0.2 mg per kg of body weight. Depending on clinical indications, the dosage is increased to 1 mg/kg, maximum 50 mg/kg body weight.
Minoxidil solution is used externally. It is applied to the head directly to the scalp, which has been previously washed and dried. Dosage regimen: twice a day, 1 mg. The drug is rubbed in gently with massage movements, starting from the center of the applied area.
An effective and safe alternative
Minoxidil, in addition to its disadvantages, does not help everyone. Therefore, even if you decide to use minoxidil, you should not choose it as monotherapy. Treatment for hair loss should always be comprehensive, and if it contains minoxidil, use it no more than once every 7-10 days. Therapy should be multidirectional. It may include taking vitamin complexes, cosmetic procedures, and the use of external products based on vitamins, amino acids, plant extracts, and peptides. Which set of tools will help you? Only a competent trichologist will answer this question. He, if necessary, can prescribe consultations with other specialists if, for example, the loss is associated with poor circulation due to cervical osteochondrosis or hormonal imbalance. It should also be understood that minoxidil can only affect one cause of hair loss - deterioration of local blood circulation. However, volume loss may be associated with other factors:
- nutritional deficiencies;
- the action of the hormone dihydrotestosterone (DHT), which causes dystrophy of hair follicles in AGA;
- low rates or lack of new hair growth.
Modern means of combating hair loss affect all these causes at once, helping to improve local blood flow, nourish the roots with necessary substances, block DHT, and stimulate new hair growth with the help of safe growth factors. These products include serums based on peptides, vitamins and plant extracts. Moreover, they have no side effects, contraindications, withdrawal syndrome and are not addictive. Serums with peptides are a worthy and safe alternative to products containing minoxidil and its analogues, so today there is no longer any need to put up with the shortcomings of minoxidil.
View the entire HairFOOD line
Interaction with other drugs
An increase in the hypotensive effect of a drug occurs when it interacts with diuretics, adrenergic blockers and various antihypertensive drugs.
The activity of the main substance is reduced when Minosoxidil is taken in parallel with non-steroidal anti-inflammatory drugs, female hormones, contraceptives and sympathomimetics.
You should not combine any form of the drug with Guanethidine, as a severe orthostatic form of low blood pressure may develop.
How to recognize minoxidil?
Today, minoxidil products are produced by various manufacturers under different names. Therefore, be careful and read the composition on the label - look for the active ingredient “minoxidil”. Such products are allowed to be sold only in pharmacies - you will not find them in cosmetic stores. However, there is a popular analogue of minoxidil - aminexil (a light version of minoxidil). It is allowed to be included in cosmetics and therefore can be purchased anywhere. But in its shortcomings it is similar to minoxidil, so you need to carefully study the composition when purchasing even anti-hair loss cosmetics. Aminexil is referred to as "aminexil".
Patients' feelings
Most note that they feel a slight warmth at the time of application. In some cases, itching and burning appear, which indicates an individual allergic reaction. After applying the product, you can notice an increase in the speed of blood circulation and its increased flow to the hair follicles. Follicles that are in the resting phase awaken and intensive hair growth begins. Under the influence of the drug, the hormone dihydrotestosterone is blocked. It plays a key role in the process of male pattern baldness.
It is worth noting that strong hair growth is usually not observed over the entire surface of the body, even with long-term use of the pills. Moreover, this effect is not observed with local use of the drug. But almost every person who has tried the drug Minoxidil can note that their hair began to fall out less. This is an important result, especially for a person with a tendency to go bald.
Price, reviews, analogues
The instructions for use of Minoxidil give a general idea of the drug, on the basis of which we can draw a conclusion about its effectiveness. But you need to trust advertising promises with some caution. Moreover, it is best to undergo a preliminary diagnosis in order to know exactly what you are going to treat. At the same time, you can ask a specialist for advice. What do those who have already tried the effects of Minoxidil tablets say? There are plenty of reviews online. Can they be trusted? It’s difficult to say, because this is everyone’s personal choice. There are practically no negative responses. Some write that the drug helped well and the effect was noticeable after a couple of weeks. Others say that even after a few months the improvement in hair condition was not as noticeable as they would like, but it was there.
The price of the drug is quite affordable. In different cities of Russia it can be ordered for 990 rubles. Delivery is available to Ukraine, Kazakhstan and other republics. To date, there are no analogues. There are medications with a similar mechanism of action. These are “Regaine”, “Dualgen 15%”, “Spectral DNS”. Sulsena anti-dandruff paste has a similar therapeutic effect. Compositions with a similar effect include pharmacy and homemade masks with pepper and mustard. All of them have a vasodilating effect locally. This ensures a rush of blood to small vessels and capillaries and improves nutrition of the scalp. But you shouldn't make the decision on your own. It is better to consult a doctor.
How much product should I use at one time?
A single dosage of minoxidil is no more than 1 ml of the product. The lotion package contains a special measuring pipette. With its help, it is possible to obtain the required dose of the composition. As a result, there are no problems with dosing.
When using the spray, it is enough to follow the instructions for minoxidil, which say that seven clicks on the sprayer will give the required portion.
A single dose of foam is measured with a cap - half is enough for one time.
It is not advisable to apply more than 2 ml of minoxidil per day.
Care products
If you use shampoo with Minoxidil simultaneously with one of the products from this line presented above, the effect is enhanced several times. This drug serves as a kind of conductor for other nutrients. They improve the absorption of vitamins and microelements, which has a positive effect on hair growth. Research confirms that it is possible to quite effectively combine the use of antiandrogen drugs and Minoxidil.
Indications for use
Minoxidil tablets are prescribed for arterial hypertension, which usually occurs in severe forms, in combination with other drugs. But today it has migrated from cardiologists to trichologists. Thanks to the drug, the supply of nutrition and oxygen to the bulbs increases. This has a positive effect on hair growth, thickness and condition.
Anyone can buy Minoxidil tablets. This imposes additional responsibility on the buyer. After all, he makes the decision himself, which means he must study all the information about side effects and contraindications.
Where can I buy
The drug "Minoxidil" is not sold in pharmacies. This is a little alarming, because most often they try to sell us drugs over the Internet that have not been certified. At best, these are useless supplements; at worst, you can cause irreparable harm to your health. Therefore, it is best to first consult a doctor, and only then decide which medicine to take and in what dosage.
How to choose the appropriate concentration?
Preparations are available in 2%, 5%, 10%, and 15%. Each of them differs only in the concentration of minoxidil, but the composition and application are the same. Trichologists recommend starting use with 5%. The specified concentration almost always gives noticeable results and does not exhibit side effects.
Less concentrated preparations are suitable for women. They are much more sensitive to minoxidil and even a 2% solution allows you to achieve stable hair growth.
Higher concentration products are usually not recommended for beginners. 10% and 15% minoxidil may be needed if the effect of the drug with a lower concentration is insufficient. You can purchase minoxidil on our website at the best prices.
How long should you use minoxidil?
The first results become noticeable after three months of regular use. You should expect complete hair restoration no earlier than six months. Of course, the question of how long to use minoxidil after the desired result is also of concern. If you stop applying the composition, then after a short time the hair will begin to fall out again and the problem will return. Thus, if you want to maintain the results obtained, you should continue to use the product. There is no problem here, because... Within six months, a habit will have already developed, like brushing your teeth.
Advantages of Finasteride in the treatment of hair loss
- Reviews about Finasteride are positive. The drug leads to a quick stop of hair loss. This has been proven by millions of men who have used the drug to treat alopecia for several years.
- The product is suitable for monotherapy and for use in combination with other drugs. Finasteride is most active in the initial stages of hair loss, and produces less pronounced results as alopecia progresses. A good effect is achieved if you use the product after hair transplantation.
- Finasteride, reviews of which indicate its maximum effectiveness, leads to a reduction in testosterone activity in the hair.