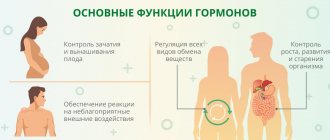
To do or not to do, to take or not to take medicine, to take a test or not to take a test. We are always faced with a choice and are often afraid of making a mistake. Iron can be said that you can’t go wrong with iron.
The adult human body contains about 3–4.5 g of iron. More than half of the iron is represented by heme in the composition of hemoglobin, a third is stored in the depot in the form of ferritin and hemosiderin. Moreover, iron deficiency is the most common deficiency condition worldwide, silently leading to anemia.
The main causes of iron deficiency:
Reduced intake of iron into the body due to impaired absorption due to diseases of the gastrointestinal tract, fasting, refusal, or reduced consumption of foods containing iron.
Increased iron loss during chronic, repeated blood loss (uterine, gastric, intestinal, etc.), as well as during acute massive bleeding.
Increased consumption of iron by the body in children during periods of growth, in women during pregnancy and subsequent breastfeeding of the child.
What does hemoglobin tell you?
The first point of the program is a clinical blood test. Be prepared for the unexpected.
If you smoke, detecting anemia may not be easy. The hemoglobin level and the number of red blood cells in smokers are always normal. Moreover, the more a person smokes, the higher he is. This occurs because the carbon monoxide found in cigarettes combines with hemoglobin to form a substance called carboxyhemoglobin. This form of hemoglobin does not have the ability to carry oxygen. To compensate for oxygen deprivation, hemoglobin levels increase. Therefore, smokers should not rely on the results of a conventional clinical analysis, but must conduct a comprehensive examination. In addition, smokers often have an increased number of red blood cells (erythrocytosis). It is very important to distinguish it in time from a malignant disease - erythremia, characterized by the same indicators of red blood.
If a clinical blood test shows low hemoglobin, you need to conduct a full study of iron metabolism. After all, the drugs will only help in case of true iron deficiency anemia. If you have chronic diseases, taking iron will only make them worse.
Comprehensive analysis is the second point of the survey program. It includes the study of serum iron, transferrin (transport iron) and ferritin, which reflects the true reserves of this important trace element in the body. In addition, ferritin is a tumor marker and an indicator of inflammatory diseases. Its reduced level indicates true iron deficiency anemia, and if it is normal or elevated, it means that the cause of anemia lies in another disease.
Complex tests can be done in all major biochemical laboratories and hematology centers.
What tests show iron deficiency?
- Be sure to look at a general blood test with a leukocyte formula, since iron deficiency ultimately leads to the development of anemia. Iron deficiency anemia is indicated by a decrease in hemoglobin levels, an indicator of MCV (erythrocyte hypochromia). Often, the examination begins when there are symptoms of anemia (pallor, weakness, dizziness, loss of strength, decreased performance and attention, brittle nails and hair, etc.).
- Decreased serum iron concentration
- Increasing the total iron-binding capacity of serum
- Decreased transferrin saturation coefficient with iron
- Decreased serum ferritin concentration
It is recommended to take tests in the morning, from 8 to 11 am, since the level of iron in the serum is higher in the morning. They should also be taken in the absence of inflammation, which helps lower transferrin levels and increase ferritin levels.
The iron content in the serum is checked 5-7 days after taking vitamin-mineral complexes and dietary supplements containing iron, or short-term use of iron supplements.
Five signs of a problem
How to determine if you are lacking iron.
1. Assess your condition. The most important sign of iron deficiency is muscle weakness, which is often attributed to chronic fatigue.
2. Pay attention to your skin, hair and nails. Increased fragility and layering of nails (sometimes they have a spoon-shaped concavity), hair loss, a pale yellowish tint to the skin, as well as a burning sensation of the tongue (as if it had been scalded) and a perversion of taste should also alert you.
3. Check your calendar. Has your menstrual cycle gone downhill or has your discharge become more abundant? Normally, during menstruation we lose an average of 30-50 ml of blood or 15-20 mg of iron. With heavy menstruation, losses increase.
4. Review your diet. Have you gone overboard by eliminating high-calorie meat products from your diet? Alas, a plant-based diet does not contribute to the accumulation of iron in the body. Its consumption also increases during physical activity, for example when playing sports.
5. Count your colds. And at the same time, remember what you used to treat them. Uncontrolled medication use often reduces iron stores.
Almost all antibiotics and sulfa drugs can lead to so-called drug anemia. And frequent infections indicate reduced immunity - it may very well be that the same iron deficiency is to blame.
Did you answer “yes” to most questions? Then you will have to develop an action plan to eliminate iron deficiency.
How is iron deficiency treated?
It is necessary to identify and eliminate the cause of iron deficiency and adjust nutrition.
If these measures do not help, and iron loss is significant, your doctor may prescribe iron-based medications. Duration of treatment with iron preparations for the purpose of restoration and replenishment of iron reserves in the depot:
- for hidden iron deficiency 1-2 months,
- for mild anemia 3 months,
- with moderate anemia 4.5 months,
- for severe anemia 6 months.
Of course, first of all, iron supplements are used in the form of tablets, drops, capsules, syrups. If your doctor has prescribed you iron supplements, you need to be prepared for long-term and comfortable treatment. This will help by following simple rules.
The dosage of the drug is also determined by the presence of anemia (this is the full therapeutic dose) and the type of iron ion that is included in the drug.
TOP - iron source products
Everyone knows that the source of iron is red meat. However, there are other products that contain this element. It is found in food in heme and non-heme forms.
Heme Iron Products
Heme iron can be obtained from animal products that contain hemoglobin, such as fish, meat and poultry. In this form, it is well absorbed by the body (up to 40% of the total volume).
Foods to include in your diet to obtain heme iron:
- Pork and beef.
- Chicken.
- Veal.
- Fish: perch, salmon, haddock, halibut, tuna, grouper.
- Oysters and mussels.
An excellent source of heme iron is the liver.
Non-heme iron foods
Non-heme iron is found in plant foods. It can be obtained from vegetables, grains, and also from foods enriched with special additives.
It is known that a person receives about 90% of total iron from the non-heme form and only 10-15% from the heme form. However, the bioavailability of non-heme iron is much lower, that is, it is less absorbed by the body [1].
Foods containing the maximum amount of non-heme iron:
- Rice, wheat, oats.
- Dark green leafy vegetables: kale and spinach.
- Apricots and raisins.
- Soy and lentils.
What iron supplements are there?
Iron in preparations can be represented by a di- or trivalent ion, designated Fe2+ or Fe3+, which is part of salts or complex complexes (for example, polymaltose complex hydroxide).
When using iron salts, you may encounter problems with its absorption. Unfortunately, not all iron from the drug is absorbed in the gastrointestinal tract. Moreover, this process is poorly controlled, since it depends on the state of the gastrointestinal tract, food taken, and other medications. Therefore, both a lack of incoming iron and an overdose are possible. In addition, not all forms of medication are convenient to use; if some tablets are chewed, the enamel of the teeth is often stained and a metallic taste remains in the mouth for a long time. Trivalent iron preparations usually do not have these side effects.
Bioavailability of iron and the influence of calcium ions on its effectiveness
The iron content in the human body averages 4.2 g. About 75% of its total amount is included in the hemoglobin of red blood cells, which carry oxygen from the lungs to the tissues, 20% of iron is reserve (bone marrow, liver, macrophages), 4% is part of myoglobin, about 1% is contained in respiratory enzymes that catalyze respiration processes in cells and tissues, as well as in other enzymatic structures. Iron carries out its biological function by being part of biologically active compounds, mainly enzymes. Iron-containing enzymes perform the following main functions:
- electron transport (cytochromes);
- transport and storage of oxygen (hemoglobin, myoglobin);
- participation in the formation of active centers;
- redox functions (oxidases, hydroxylases, superoxide dismutases, etc.);
- transport and deposition of iron in blood plasma (transferrin, ferritin).
Iron has several special properties that distinguish it from other biologically active ions and substances.
The human body does not have any special mechanisms for removing iron. Iron is mainly excreted through the skin and intestines (I. Guinote et al., 2006). In addition, it is also lost in hair, nails, urine and sweat. The total amount of iron excreted in a healthy person (male) is about 1 mg per day. The same amount is normally absorbed from food consumed (Linder, 1991). The difference is during the menstrual period, when intake should be about 4 mg of iron per day. Thus, the concentration of the element in the blood serum depends on its absorption in the gastrointestinal tract, on its accumulation in the spleen, bone marrow and skeletal muscles (myoglobin), as well as on the synthesis and breakdown of hemoglobin and its release from the body. Iron can be present in food in two forms - heme and non-heme, which are characterized by different absorption mechanisms. Heme iron (a porphyrin ring with an iron atom in the center, bound to 4 nitrogen atoms) is released from protein chains in the gastrointestinal tract and is absorbed by intestinal enterocytes in the form of metalloporphyrin. There, nonspecific endosomal penetration of heme into the cell occurs, followed by its destruction. Next, with the help of the protein transport system IREG1, iron ions are oxidized to ferric iron, bind to transferrin and leave the enterocyte, entering the bloodstream (Linder et al., 2006). In the blood plasma, iron moves in conjunction with the same protein, which performs both the depot and transport functions. The presence of free iron ions in the blood is not typical and is a pathology. Absorption of heme iron occurs in the range of 15–50% (average 20–30%).
Non-heme ferrous iron in the stomach is bound by the protein gastroferrin and transported to the intestine. Once in the duodenum and proximal part of the jejunum, iron enters the enterocyte using the nonspecific ion transporter DMT1 (Divalent metal transport). This proton-dependent transporter is also involved in the transport of many other divalent cations, such as Mn, Cu and Zn (M. Arredondo et al., 2006). In addition, it has been shown that this carrier can also transport some monovalent ions, such as Cu+1, which is formed by the action of ascorbate on Cu+2 (M. Linder et al., 2006). Thus, it can be assumed that depending on the concentration of these ions in the diet or multivitamin tablet, they may compete for the DMT1 transporter. At the same time, there is evidence that there is specificity of the DMT1 transporter in relation to various divalent ions, associated with their location along the gastrointestinal tract, which was shown by transcription of various iron-independent mRNAs of the DMT1 transporter, and there is no competition for the transporter.
The literature describes several mechanisms of iron transport within the enterocyte, based mainly on experiments carried out on tissue culture Caco2 (M. Linder et al., 2006). According to the first theory, divalent iron, which enters the enterocyte using the DMT1 transporter, is delivered through vesicles with transferrin (some assign it the role of an intracellular receptor) or in a free state to the basolateral membrane of the enterocyte, where another transporter is present - IREGI/ferroportin/MTP1 (Donovan et al. al., 2000). This transporter oxidizes ferrous iron to ferric iron and transports it into the blood, where it combines with plasma transferrin. According to the second theory, iron is transported inside the enterocyte, apparently in vesicles along with apotransferrin, which enters the enterocyte from the bloodstream by endocytosis (endo-/exocytosis) (Ma et al., 2002). During this transport, divalent iron is oxidized to ferric iron and enters the blood through the basilar membrane of the enterocyte through exocytosis. The already mentioned IREG system may participate in this process. According to the literature, it is the mechanism of iron transport through the basolateral membrane of the enterocyte into the blood that is limiting in the process of iron adsorption (Roy and Enns, 2000). Absorption of inorganic ferrous iron usually occurs in the range of 6–15%.
Non-heme ferric iron can be reduced by ferrous reductase to ferrous iron and absorbed by DMT1. The recovery of ferric iron is highly dependent on the acidity of gastric juice. Unreduced iron can be absorbed through the specific integrin-mobiferin IMP system. The absorption of ferric iron is the least complete and rarely exceeds 4%.
The amount of iron entering the effector cell, where it is transported with the blood, is directly proportional to the number of membrane receptors. The cell releases iron from transferrin. Plasma apotransferrin is then returned to circulation. An increase in the need of cells for iron during their rapid growth or hemoglobin synthesis leads to the induction of the biosynthesis of transferrin receptors and, on the contrary, with an increase in iron reserves in the cell, the number of receptors on its surface decreases. Iron released from transferrin inside the cell binds to ferritin, which delivers the trace element to the mitochondria, where it is incorporated into heme. In addition to heme synthesis, ferrous iron is used in mitochondria to synthesize iron-sulfur centers. There is a constant redistribution of iron in the human body. In quantitative terms, the metabolic cycle is of greatest importance: plasma - red bone marrow - red blood cells - plasma. Typically, 70% of plasma iron goes to the bone marrow. Due to the breakdown of hemoglobin, about 21–24 mg of iron is released per day, which is many times higher than the intake of iron from the digestive tract (1–2 mg/day).
There is a pronounced inverse relationship between the body's supply of iron and its absorption in the digestive tract. Iron absorption occurs primarily in the duodenum and proximal jejunum and is absent in the ileum.
The absorption of iron depends on the following reasons: age, the body's supply of iron, the state of the gastrointestinal tract, the amount and chemical forms of incoming iron and other food components. For optimal absorption of iron, normal gastric juice secretion is necessary. Taking hydrochloric acid promotes the absorption of iron in achlorhydria. The table shows the main substances found in human foods that can enhance or reduce the absorption of iron contained in that food or multivitamin tablet. Ascorbic acid, which reduces iron and forms chelate complexes with it, increases the availability of this element in the same way as other organic acids. It is one of the most powerful stimulators of iron absorption. Another food component that increases iron absorption is “animal protein factor,” which contains myoglobin and hemoglobin. Simple carbohydrates improve iron absorption: lactose, fructose, sorbitol, as well as amino acids such as histidine, lysine, cysteine, which form easily absorbed chelates with iron.
The most powerful inhibitors that block iron absorption are phytates and polyphenols. Phytates are a storage form of phosphates and minerals found in cereal grains, vegetables, seeds and nuts. They actively inhibit iron absorption, acting in direct proportion to the dose. Iron absorption is reduced by drinks such as tea containing tannin, as well as other polyphenolic compounds that bind this element tightly. Phenolic compounds exist in almost all plants and are part of the defense system against insects and animals. Therefore, tea is used to prevent increased iron absorption in patients with thalassemia. Various diseases have a great influence on the absorption of iron. It increases with iron deficiency, with anemia (hemolytic, aplastic, pernicious), hypovitaminosis B6 and hemochromatosis, which is explained by increased erythropoiesis, depletion of iron reserves and hypoxia.
Of the listed substances that can reduce iron absorption, calcium ion attracts special attention. Calcium is highly biologically active, is found in significant quantities in staple foods, and is usually present in one multivitamin tablet with iron.
| Table. Activators and inhibitors of iron absorption contained in the human diet |
In this regard, the question of the possible effect of calcium on the bioavailability of iron has been studied for a long time, both in animal experiments and in human studies.
It should be noted that the cellular mechanisms of absorption, i.e., the flow of iron and calcium ions from the intestinal lumen into the bloodstream through intestinal enterocytes, are different. Numerous studies have shown that various cellular transporters are involved in this process (J. Hoenderop et al., 2005). In addition, there is evidence that calcium reduces the intake of both heme (L. Hallberg, 1991) and non-heme iron. Taken together, this indicates that calcium may influence the bioavailability of iron by exerting an inhibitory effect either on its transport in the gastrointestinal tract or on binding to receptors located on the apical membrane of enterocytes.
Experiments on an isolated intestinal loop in vivo in rats showed a decrease in the absorption of iron from a FeCl2 solution injected directly into the loop with the addition of calcium. Moreover, the effect depended on the absolute concentration of calcium in the duodenum, and not on the molar Ca/Fe ratio (Barton et al., 1983). A study of the effect of various calcium-containing salts on cellular iron transport showed that the greatest inhibitory effect is caused by CaCO3, while the effects of CaSO4 and Na2CO3 are present, but to a lesser extent (Prather, 1992). This calcium salt, added in an amount of 500 mg, can reduce the absorption of non-heme iron contained in foods by 32% when consuming foods that do not contain additional inhibitory substances, and by 42% when consuming foods in combination with eggs, coffee, etc. (Cook et al., 1991). CaCO3 also reduces the absorption of iron when used together in one tablet. In this case, 300 mg of calcium salt, when taken together with 37 mg of iron, present as FeSO4, reduces iron absorption by 15% (Seligman et al., 1983; Cook et al., 1991).
Volunteers were tested on the absorption of iron when combined with calcium-containing foods. Iron absorption was determined by the radioisotope method using Fe55 and Fe59. Healthy women (21 subjects) consumed additional milk and cheese (~930 mg calcium per day) for 10 days. This resulted in a 30–50% reduction in iron absorption (Hallberg, 1995). Based on the data obtained, the authors suggest that inhibition of iron absorption occurs at the intestinal lumen-enterocyte stage.
Human studies also examined the effects of artificial mineral supplements: ferrous sulfate, calcium citrate and calcium phosphate, etc. The work was carried out on 61 healthy subjects. The dual radioisotope method was also used to assess absorption. When calcium citrate (600 mg) was consumed, iron absorption was reduced by 49% and phosphate by 62% (Cook et al., 1991). Interestingly, in this study, the effect of calcium supplementation was observed only in the presence of food intake. It is likely that competition between cations occurred when the intestine was full. It is theoretically possible that high concentrations of calcium may alter the rheological properties of the bolus in the lumen of the upper small intestine (Conrad et al., 1993). The differences in the effects of calcium on heme and non-heme iron intake have also been studied in humans. Thus, in a study on 27 volunteers using a complete colonic lavage to measure iron absorption, calcium supplementation (450 mg) showed a 20% reduction in the absorption of heme iron alone. In this study, calcium supplementation did not affect the absorption of non-heme iron (ZK Roughead, 2005). Another study conducted on 44 men and 81 women observed a decrease in the absorption of heme iron from the diet with calcium supplementation in doses ranging from 40 to 300 mg. The maximum reduction was observed at a dose of 300 mg and amounted to 74%. A further increase in calcium content to 600 mg did not lead to an increase in iron ion inhibition (L. Hallberg et al., 1991). The conflicting results obtained in different studies are likely due to the difficulty of replicating the accuracy of methodological approaches carried out in humans.
All of the above studies showed, to one degree or another, a decrease in iron absorption in the gastrointestinal tract by 20–60% when combined with calcium-containing products during a single meal or tablet preparations. It is characteristic that the doses of calcium used did not exceed the daily norm for an adult (in all the described cases, the total intake of calcium per day was less than 1000 mg). However, the direct mechanism of the antagonistic effect of calcium on iron absorption remains unclear.
A series of studies conducted on volunteers with long-term joint meals containing a certain amount of iron and calcium did not provide a clear answer about the effect of calcium ion on the bioavailability of iron, and most importantly, on the hemoglobin level in these subjects. An effect was often detected (19% inhibition) but was not statistically significant (Reddy et al., 1997). Long-term studies in humans appear to be complicated by dietary controls and dietary controls (SR Lynch, 2000).
An analysis of the literature allows us to conclude that experimental studies on animals and work carried out on subjects have confirmed that calcium ions can reduce the level of iron absorption. The extent to which the effect was detected depended on the methodological approaches used, which differed from one study to another, making it difficult to interpret the results. However, the possibility of such interactions may be most relevant and should certainly be taken into account for people suffering from iron deficiency conditions (anemia) or who are at risk for this condition (children, pregnant women, etc.). To treat and prevent such conditions, it is necessary to increase iron intake, both through an appropriate diet and through mineral supplements. But it should be remembered that the effectiveness of these measures may be significantly reduced by the consumption of dietary calcium or calcium-containing vitamin complexes. It is not advisable to limit calcium intake, since in many cases (pregnancy, age 12–18 years) there is an increased need for both elements. A way out of the situation can be the separate use of calcium and iron. Experimental data have shown that an interval between calcium and iron intake of even 4 hours eliminates the inhibitory effect (A. Gleeprup et al., 1993). In addition, while taking an iron supplement, you should refrain from consuming any foods containing calcium, i.e. you need to exclude the entire range of dairy products, as well as green parts of plants.
In this case, it is convenient to use vitamin-mineral complexes, which provide for the separate use of iron and calcium in advance. And this is not the only combination of vital micronutrients that exhibit antagonistic properties. Thus, proper separation of the components of vitamin-mineral complexes according to the time of administration is a necessary condition for the effectiveness of their use.
Literature
- Arredondo M., Martinez R., Nunez MT et al. Inhibition of iron and copper uptake by iron, copper and zinc. Biol. Res. 2006; 39: 95–102.
- Barton JC, Conrad ME, Parmley RT Calcium inhibition of inorganic iron absorption in rats. Gastroenterology. 1983; 84:90–101.
- Conrad ME, Umbreit JN A concise review: iron absorption- the mucin - mobilferrin - integrin parthway. A competitive partway for metal absorption. Am J Hematology. 1993; 42:67–73.
- Cook JD Adaption in iron metabolism. Am J Clin Nutrition. 1990; 42:67–73.
- Cook JD, Dassenko SA, Whittaker P. Calcium supplementation: effect on iron absorption. Am J Clin Nutrition. 1991a; 53: 106–111.
- Donovan A., Brownile A., Zhou Y. et al. Positional cloning of zebrafish ferraportin identifies a conserved vertebrate iron exporter. Nature. 2000; 403:776–781.
- Gleerup A., Rossander-Hulten L., Hallberg L. Duration of the inhibitory effect of calcium on non-haem iron absorption in man. Eur I Clin Nutr. 1993; 47:875–879.
- Guinote, Fleming R., Silva R. et al. Using skin to assassinate iron accumulation in human metabolic disorders. Ion Beam Analysis. 2006; 249:697–701.
- Hallenberg L., Brune M., Erlandsson M. et al. Calcium: effect of different amounts on nonheme- and heme-iron absorption in humans. Am J Clin Nutrition. 1991; 53: 112–119.
- Hoenderop J. Gj., Nilius B., Bindels RJM Calcium absorption across epithelia. Physiol. Rev. 2005; 85:373–422.
- Linder MC Nutrition and metabolism of the trace element. Nutritional Biochemistry and Metabolism. 1991: 151–198.
- Linder MC, Moriya M., Whon A. et al. Vesicular transport of Fe and interaction with other metal ions in polarized Caco2 Cell monolayers. Biol. Res. 2006; 39: 143–156.
- Lynch SR The effect of calcium on iron absorption. Nutr. Res. Rev. 2000; 13: 141–158.
- Ma Y., Specian RD, Yen KY et al. The transcytosis of divalent metal transporter 1 and apo-transferrin during iron uptake in intestinal epithelium. Am J Physiol. 2002; 283:965–97.
- Prather Ta and Miller DD Calcium carbonate depresses iron bioavailability in rats more than calcium sulfate or sodium carbonate. J Nutrition. 1992; 122:327–332.
- Reddy MB and Cook JD Effect of calcium on nonheme-ironabsorption from a complete diet. Am J Clin Nutrition. 1997; 65: 1820–1825.
- Roughead ZK, Zito CA, Hunt JR Inhibitory effects of dietary calcium on the initial uptake and subsequent retention of heme and nonheme iron in humans: comparisons using an intestinal lavage method. Am J Clin Nutr. 2005; 82(3): 589–597.
- Roy CN and Enns CA Iron homeostasis: New tales from the crypt. Blood. 2000; 96: 4020–4027.
- Seligman PA, Caskey JH, Frazier JL et al. Measurements of iron absorption from prenatal multivitamin-mineral supplements. Obstetrics and Gynecology. 1983; 61:356–362.
N. A. Medvedeva , Doctor of Biological Sciences, Professor of Moscow State University, Moscow
How to increase the effectiveness of treatment
To reduce the likelihood of side effects, iron salts are taken before meals.
The absorption of iron is higher when taken simultaneously with ascorbic and succinic acids, fructose, and cysteine. This property is used in some combination drugs.
Iron absorption is reduced by certain substances from food or medications. Thus, it is reduced by tannin (strongly brewed tea), calcium salts (in milk or medications), magnesium and manganese (in mineral complexes or antacid preparations, such as phosphalugel), tetracycline antibiotics, phosphoric and phytic acids (cereal seeds, legumes). This effect is smoothed out when using preparations based on ferric iron.
Iron absorption is increased in severe iron deficiency. Therefore, you should be more careful about the prescribed dosage. Doctors often rely on a therapeutic dose of 200 mg of iron ion/day. If you understand that an iron supplement is not suitable for you due to the development of adverse reactions, difficulties in taking it, or lack of improvement in your well-being, tell your doctor about this. Under no circumstances should you change the dosage of the drug yourself. Exceeding the dose can lead to overdose and poisoning, reducing the dose can lead to useless use and lack of effect.
What helps iron absorption?
When all problems with iron absorption are resolved or prevented, you need to think about the transport of iron in the blood. For good transport of iron, copper is necessary.
It is this that ensures the high-quality production of cerulloplasmin, an element that transports iron. Therefore, iron deficiency is often a consequence of a lack of copper in the body and, as a result, a lack of important cerulloplasmin.
Don't forget about the importance of zinc and cobalt for the absorption of iron, which can be found in liver, cocoa and seafood. And, of course, when fighting anemia you cannot do without ascorbic acid (vitamin C). Vitamin C helps iron to be absorbed well, which is the reason for its frequent inclusion in iron-containing preparations.
Finding ascorbic acid in its natural form is quite simple: lemons, oranges, apples, grapefruits, kiwis, wild strawberries, strawberries, bell peppers, tomatoes and cauliflower are rich in it.
It should also be noted that any nutrition consultant who has completed a dietetics course or nutrition school will advise combining iron with folic acid. It ranks second in a number of causes of iron deficiency anemia. There is a lot of folic acid in parsley, young nettles and dried apricots.
Interestingly, special carrier proteins mucin-b3-integrin and DMT1 are involved in iron transport. During anemia, they are synthesized much faster - this is a protective function of the body, ensuring better absorption of iron from foods and vitamin complexes.
Proteins transport Fe only once, so new protein molecules are also needed to transport new Fe molecules. They take approximately 4-6 hours to produce, so taking iron too often will not increase its absorption by the body. There will simply be more unabsorbed iron, and this will increase the risk of developing side effects when taking it.
How is the effectiveness of treatment assessed?
During the first days of treatment with iron supplements, physical sensations are assessed.
When treating anemia, on the 5-8th day of treatment, a reticulocyte crisis is determined (an increase in the number of reticulocytes compared to the initial value, usually by 2-3% or 20-30‰).
After 4 weeks of treatment, the increase in hemoglobin is assessed. An increase in hemoglobin concentration by 10 g/l by the end of the first month of treatment with iron preparations indicates that the dose has been correctly selected and the effectiveness of therapy. After hemoglobin is restored, therapy is continued to saturate the iron depot, while the dose of the drug is reduced by 2 times.
For hidden iron deficiency, half doses of the drugs are used for 4-8 weeks. Depot saturation is determined using a comprehensive biochemical study (ferritin, transferrin, TLC).
At Lab4U you can take tests to identify iron deficiency and monitor the effectiveness of therapy with a discount of up to 50%.
What interferes with calcium absorption?
Calcium deficiency is just as insidious as iron deficiency. There are a lot of nuances in its assimilation. For example, doctors have long established that normal absorption of calcium is promoted exclusively by physical activity. In other words, if the muscles do not work, then you should not expect that calcium will be well absorbed from food or vitamins.
So osteoporosis, a disease characterized by brittle bones, can occur against the background of calcium deficiency and lack of physical activity even at 30 years of age. It is unlikely that even greater arguments are needed for playing sports.
Do not forget that the absorption of calcium is hindered by nicotine, alcohol, caffeine and carbonated drinks, which remove calcium through the kidneys even before it is absorbed into the blood.
Please note that foods that interfere with calcium absorption include anything that contains large amounts of sodium and phosphorus. The same can be said for oxalic acid. But, fortunately, it is easily neutralized by heat treatment of products.
Why is calcium poorly absorbed?
Calcium has many functions. The health of teeth, nails, and the entire skeletal and vascular system depends on it. It also helps with weight loss, as it promotes the production of enzymes that break down subcutaneous fat. Any nutrition consultant will tell you this with confidence.
But in order for calcium to be well absorbed, many different conditions will have to be observed, otherwise it will be quickly “washed out” from the body. For example, with a sedentary lifestyle and during stress, calcium loss increases significantly.
This microelement loves movement. It has long been proven that as muscle mass increases, bone mass also increases, therefore, calcium is better absorbed and accumulates faster. So, if you spend time passively, it will be very difficult to replenish calcium reserves.
It must be remembered that this microelement does not tolerate combinations with alcohol, nicotine, coffee and sweet soda. Just think: just one cup of coffee blocks the absorption of calcium from food and vitamin complexes for 3 hours.
This means that if you wash down a plate of cottage cheese with an invigorating drink, you will not get anything out of it. In addition, coffee will “wash” iron, zinc, potassium, B vitamins and niacin from the body. Not a very pleasant prospect, is it?
As for alcohol, nicotine and harmful sodas, they promote the excretion of calcium in the urine before it enters the blood. That is, the specified microelement will simply pass through your body in transit.
Another calcium antagonist is salt. By adding salt to dairy and fermented milk products, we significantly reduce the absorption of calcium from them. You should not combine this mineral with starchy and refined foods, oxalic acid, bran and fiber. Sodium and phosphorus, as well as products containing them, are also not the best company for calcium. They speed up its “washing out” from the body. Therefore, make sure that there is a break of at least 2-3 hours between taking them.