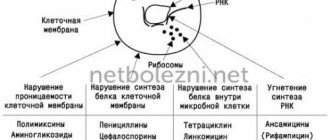
Spectrum of action
The activity of drugs from the cephalosporin group gradually changes from generation to generation:
- Medicines of 1-2 generations are most effective against infection with gram-positive flora (staphylo- and streptococci, corynebacteria).
- For the 3rd and 5th generations of cephalosporins, there is already an increased activity against gram-negative bacteria (Enterobacter, Haemophilus influenzae, gonococcus, meningococcus, Klebsiella, Moraxella, Proteus) and anaerobes (peptococcus, peptostreptococcus, clostridia, bacteroides) along with high efficiency against gram-positive flora. In addition, ceftazidime and cefixime are harmful to Pseudomonas aeruginosa.
- 4th generation cephalosporins are different: their effect is maximum for gram-negative bacteria, while cefepime also has an antipseudomonas effect.
Antibiotics from the cephalosporin series are classified as β-lactam antibiotics. Each of their representatives has 7-ACC (7-aminocephalosporanic acid) in its structure and is characterized by higher resistance to the special bacterial enzyme β-lactamase. By preventing the synthesis of components of the cell wall of bacterial cells, cephalosporins realize their bactericidal effect, i.e. completely destroy microbial cells.
III GENERATION CEPHALOSPORINS
MODERN ANTIMICROBIAL CHEMOTHERAPY
MODERN ANTIMICROBIAL CHEMOTHERAPY
L.S. Strachunsky, S.N. Kozlov. Guide for doctors
| Content | ANTIBIOTIC.ru |
Antibacterial drugs / Beta-lactam antibiotics / Cephalosporins
III generation cephalosporins have higher activity against gram-negative bacteria from the family Enterobacteriaceae
, including many nosocomial multidrug-resistant strains.
Some of the third generation cephalosporins (ceftazidime, cefoperazone) are active against P.aeruginosa
. Against staphylococci, their activity is slightly lower than that of first generation cephalosporins.
Like all other cephalosporins, third-generation drugs do not act on MRSA and enterococci, have low antianaerobic activity, and are destroyed by extended-spectrum β-lactamases.
Third-generation parenteral cephalosporins were initially used only for the treatment of severe infections in the hospital, but nowadays, due to the increase in antibiotic resistance, they are often used in outpatient settings.
For severe and mixed infections, parenteral cephalosporins of the third generation are used in combination with aminoglycosides of the second and third generations, metronidazole, vancomycin.
Oral cephalosporins of the third generation are used for moderate community-acquired infections caused by gram-negative flora, as well as as the second stage of step therapy after the prescription of parenteral drugs.
PARENTERAL CEPHALOSPORINS III GENERATION
CEFOtaxime
Claforan
The first, so-called “basic”, third generation cephalosporin, which has found wide use.
Activity spectrum
| Gram(+) cocci: | streptococci (including many penicillin-resistant pneumococci); staphylococci (but has a weaker effect than cefazolin). |
| Gram(-) cocci: | N.gonorrhoeae, N.meningitidis, M.catarrhalis , including β-lactamase (+) strains. |
| Gram(-) sticks: | E.coli, Proteus spp., H.influenzae, Klebsiella spp., Enterobacter spp., Citrobacter, Serration, Providence, etc., including strains resistant to gentamicin. |
| Anaerobes: | predominantly anaerobic cocci (peptostreptococci, etc.); no effect on B.fragilis . |
Pharmacokinetics
Penetrates well into various tissues and passes through the BBB. It does not displace bilirubin from its connection with plasma albumin, therefore it is preferable in newborns. Metabolized in the liver, and the metabolite (desacetylcefotaxime) has antimicrobial activity. Excreted by the kidneys. T1/2 - about 1 hour, metabolite - about 1.5 hours.
Indications
- Severe infections of the upper respiratory tract (acute and chronic sinusitis - if necessary, parenteral treatment).
- Severe NPD infections (community-acquired and nosocomial pneumonia).
- Infections of the gallbladder.
- Severe community-acquired and nosocomial UTI infections.
- Intra-abdominal and pelvic infections (in combination with antianaerobic drugs).
- Intestinal infections (shigellosis, salmonellosis).
- Severe infections of the skin, soft tissues, bones and joints.
- Bacterial meningitis.
- Sepsis.
- Gonorrhea.
Dosage
Adults
Parenteral - 3-8 g/day in 2-3 administrations; for meningitis - 12-16 g/day in 4 administrations; for acute gonorrhea - 0.5 g intramuscularly once.
Children
Parenteral - 50-100 mg/kg/day in 3 administrations; for meningitis 200 mg/kg/day in 4 doses. For meningitis in newborns, it is combined with ampicillin, which is active against listeria.
Release forms
Bottles of 0.25 g, 0.5 g, 1.0 g and 2.0 g of powder for the preparation of solution for injection.
CEFTRIAXONE
Rocephin, Lendatsin, Forcef, Ceftriabol
The spectrum of activity is similar to cefotaxime.
Main differences:
- among cephalosporins it has the longest T1/2 (5-7 hours), therefore it is administered 1 time per day, for meningitis - 1-2 times per day;
- high degree of binding to plasma proteins;
- dual route of elimination, therefore, in case of renal failure, no dosage adjustment is required (correction is carried out only in patients with both hepatic and renal insufficiency).
Indications
- Severe infections of the upper respiratory tract (acute and chronic sinusitis, acute otitis media - if necessary, parenteral treatment).
- Severe NPD infections (community-acquired and nosocomial pneumonia).
- Severe community-acquired and nosocomial UTI infections.
- Intra-abdominal and pelvic infections (in combination with antianaerobic drugs).
- Intestinal infections (shigellosis, salmonellosis).
- Severe infections of the skin, soft tissues, bones and joints.
- Bacterial meningitis.
- Bacterial endocarditis.
- Sepsis.
- Gonorrhea.
- Borreliosis (Lyme disease).
Warning
It should not be used for infections of the gallbladder, as it may precipitate in the form of bile salts (pseudocholelithiasis).
It is not recommended for use in newborns due to the possibility of displacing bilirubin from association with plasma albumin and the risk of developing kernicterus.
Dosage
Adults
Parenteral - 1.0-2.0 g/day in 1 administration; for meningitis - 2.0-4.0 g/day in 1-2 administrations; for acute gonorrhea - 0.25 g intramuscularly once. For intramuscular administration, dilute in a 1% solution of lidocaine.
Children
Parenteral - 20-75 mg/kg/day in 1-2 administrations; for meningitis - 100 mg/kg/day in 2 administrations (no more than 4.0 g/day). For acute otitis media - 50 mg/kg/day intramuscularly for 3 days (no more than 1.0 g per administration).
Release forms
Bottles of 0.25 g, 0.5 g, 1.0 g and 2.0 g of powder for the preparation of solution for injection.
CEFTAZIDIME
Fortum, Kefadim
Main differences from cefotaxime:
- highly active against P. aeruginosa
, often superior to piperacillin, aminoglycosides and ciprofloxacin; - less active against gram-positive cocci (staphylococci, pneumococci);
- has a longer T1/2 (2 hours).
Indications
- Pseudomonas infection, including meningitis.
- Nosocomial pneumonia.
- Severe community-acquired and nosocomial UTI infections.
- Intra-abdominal and pelvic infections (in combination with antianaerobic drugs).
- Neutropenic fever.
Dosage
Adults
Intravenously - 2.0-4.0 g/day in 2 injections, for meningitis - 6.0 g/day in 3 injections.
Children
Intravenously - 30-100 mg/kg/day in 2-3 injections, for meningitis - 200 mg/kg/day in 3 injections.
Release forms
Bottles of 0.25 g, 0.5 g, 1.0 g and 2.0 g of powder for the preparation of solution for injection.
CEFOPERAZONE
Cephobid
Main differences from cefotaxime:
- acts on P.aeruginosa
, but somewhat weaker than ceftazidime; - has a dual route of excretion: with bile (mainly) and with urine, therefore, in case of renal failure no dosage adjustment is required;
- penetrates the BBB worse;
- has a longer T1/2 (2 hours).
Indications
- Severe infections of the upper respiratory tract (acute and chronic sinusitis - if necessary, parenteral treatment).
- Severe NPD infections (community-acquired and nosocomial pneumonia).
- Severe community-acquired and nosocomial UTI infections.
- Intra-abdominal and pelvic infections (in combination with antianaerobic drugs).
- Severe infections of the skin, soft tissues, bones and joints.
- Sepsis.
- Neutropenic fever.
Warnings
May cause hypoprothrombinemia. You should not drink alcoholic beverages due to the risk of developing a disulfiram-like effect, which persists for several days after discontinuation of the drug.
Due to the fact that cefoperazone does not penetrate the BBB well enough, it should not be used for meningitis.
Dosage
Adults
Parenterally - 4-12 g/day in 2-3 administrations (for Pseudomonas aeruginosa infection, administered every 6-8 hours).
Children
Parenteral - 50-100 mg/kg/day in 2-3 administrations.
Release forms
Bottles of 1.0 g and 2.0 g of powder for the preparation of solution for injection with the addition of a solvent (water for injection).
CEFOPERAZONE/SULBACTAM
Sulperazon
It is a combination of cefoperazone with the β-lactamase inhibitor sulbactam in a 1:1 ratio and is the only inhibitor-protected cephalosporin.
Compared with cefoperazone, it is significantly more active against microorganisms that form β-lactamases - gram-negative bacteria of the Enterobacteriaceae
, Acinetobacter.
Unlike other cephalosporins, it works well against B.fragilis
and other non-spore-forming anaerobes, so for infections of the abdominal cavity and pelvis it can be used as monotherapy. In terms of activity against Pseudomonas aeruginosa, it corresponds to cefoperazone.
In other parameters (pharmacokinetics, adverse reactions), cefoperazone/sulbactam is practically no different from cefoperazone.
Indications
- Severe community-acquired and nosocomial (including Pseudomonas aeruginosa) infections:
- UDP (acute and chronic sinusitis - if necessary, parenteral treatment);
- NDP (community-acquired and nosocomial pneumonia, lung abscess);
- ZhVP (acute cholecystitis, cholangitis);
- MVP (acute pyelonephritis);
- intra-abdominal and pelvic;
- skin, soft tissues, bones and joints.
Dosage
Adults
Parenteral - 2.0-4.0 g/day in 2-3 administrations. In severe cases - up to 8 g/day.
Children
Parenteral - 40-80 mg/kg/day in 2-4 administrations. In severe cases - up to 160 mg/kg/day.
Release form
Bottles of 2.0 g of powder for the preparation of a solution for infusion.
ORAL CEPHALOSPORINS III GENERATION
CEFIXIM
Cefspan, Suprax
Activity spectrum
Compared with oral cephalosporins of the second generation, it is more active against gram-negative flora - H.influenzae, M.catarrhalis, N.gonorrhoeae
and the family
Enterobacteriaceae
. It acts against streptococci, including GABHS, but activity against pneumococci and staphylococci is lower than that of cefuroxime.
Pharmacokinetics
Bioavailability when taken orally is about 50%. It is excreted mainly in urine and partially in bile. T1/2 - 3-4 hours.
Indications
- Exacerbation of chronic bronchitis caused by H.influenzae
or
M.catarrhalis
. - UTI infections caused by multidrug-resistant flora.
- Shigellosis.
- Gonorrhea.
- The oral stage of step therapy after the use of parenteral cephalosporins of the III-IV generation.
Dosage
Adults
Orally - 0.4 g/day in 1-2 doses, regardless of food intake.
Children over 6 months
Orally - 8 mg/kg/day in 1-2 doses, regardless of food intake.
Release forms
Capsules of 0.1 g, 0.2 g and 0.4 g; powder for suspension 100 mg/5 ml.
CEFTIBUTEN
Tsedex
Among oral cephalosporins, it has the greatest resistance to β-lactamases, but is destroyed by ESBLs.
Activity spectrum
Compared with oral cephalosporins of the second generation, it is more active against gram-negative flora - H.influenzae, M.catarrhalis
, family
Enterobacteriaceae
. It has a weaker effect on pneumococci and staphylococci than cefuroxime.
Pharmacokinetics
Bioavailability is higher than that of cefixime (65%). Excreted mainly by the kidneys. T1/2 - 2.5-3 hours.
Indications
- Exacerbation of chronic bronchitis caused by H.influenzae
or
M.catarrhalis
. - UTI infections caused by multidrug-resistant flora.
- The oral stage of step therapy after the use of parenteral cephalosporins of the III-IV generation.
Dosage
Adults
Orally - 0.4 g/day in one dose, regardless of food intake.
Children
Orally - 9 mg/kg/day in 1-2 doses, regardless of food intake.
Release forms
Capsules 0.4 g; powder for suspension 180 mg/5 ml.
| Copyright © 2000-2007 ANTIBIOTIC.ru Posted: 05/15/2004 |
The address of this page: https://www.antibiotic.ru/books/mach/mac0106.shtml
Last modified date: 05/24/2004 18:56
Common representatives
Of the numerous list of cephalosporins, the most frequently used at the moment are representatives of the 3rd generation, namely ceftriaxone, ceftibuten, cefditoren. This is due to their wide spectrum of action and relatively low cost. In addition, the last two drugs are available in oral form, which is very convenient for patients to take.
It may seem that cephalosporins, available in tablet form, are rarely used drugs. This is not true: such drugs are applicable even for severe infections of various organs, when other antibiotics do not have the desired effect.
It is advisable to compare the most common representatives according to a number of important criteria:
Speed of effect (time of maximum concentration in blood):
| Ceftriaxone | Ceftibuten | Cefditoren |
|
|
|
Microbial spectrum of action:
| Ceftriaxone | Ceftibuten | Cefditoren |
| ||
|
|
|
Resistant strains of bacteria:
| Ceftriaxone | Ceftibuten | Cefditoren |
|
|
|
Side effects:
| Ceftriaxone | Ceftibuten | Cefditoren |
| Nervous system | ||
|
|
|
| Cardiovascular system, hematopoiesis | ||
|
|
|
| Gastrointestinal tract | ||
| ||
| Genitourinary system | ||
|
|
|
| Allergic reactions | ||
|
|
|
Use in pregnant and lactating patients:
| Ceftriaxone | Ceftibuten | Cefditoren |
|
|
|
See How to distinguish coronavirus from acute respiratory viral infections, flu, and colds. Pneumonia with coronavirus: symptoms, treatment Nobasit for covid Ingavirin for coronavirus Symptoms and treatment of coronavirus
Strategic approaches to the selection of cephalosporin antibiotics for respiratory tract infections
The problem of rational antibiotic therapy remains one of the most difficult in clinical practice. If previously a doctor, when choosing a drug, was guided by its effectiveness, tolerability and safety, today this is not enough. The factor of convenience of taking the drug and, which is especially unusual for our understanding, the issues of price and cost of treatment in conditions of serious restrictions on healthcare financing can often be decisive. Medical institutions spend 15–20% of their budget on the purchase of medicines, and 50–60% of these costs fall on antibacterial drugs, which forces them to reconsider existing ones and look for new approaches to their use.
The confusion that arises when using various antibacterial drugs, including cephalosporin antibiotics (CA), is associated with misunderstanding or simply ignorance of the basic principles of clinical chemotherapy. In this regard, we would like to dwell on some mistakes and “misconceptions” of practicing physicians that arise when prescribing antibacterial drugs, using the example of CA, and also determine their place in the treatment of respiratory tract infections.
Often we hear from practicing doctors about the unconditional advantages of the IV generation of drugs over the III, III generation over the II, etc. This is absolutely false. This point of view leads to the use of “reserve” and powerful drugs in the treatment of a banal infection, promotes the development of resistance, and therefore makes it impossible to use first-generation drugs and, finally, causes a significant and unjustified increase in the cost of treatment.
CAs occupy an important place in the treatment of upper and lower respiratory tract infections. The most critical step in antibacterial therapy for this category of patients is the choice of the initial drug. The effectiveness and safety of treatment, as well as its comfort, tolerability, cost, and epidemiological situation depend on the adequacy of the choice.
Analysis of available data on the use of target audiences in Russia for 1997–1998. allows doctors to identify preferences for a particular drug (group of drugs) and certain methods of its administration. As can be seen from Fig. 1, when prescribing cephalosporin antibiotics, the vast majority of doctors choose parenteral drugs.
This fact only confirms that in our country oral medications, and especially oral cephalosporins, are very little popular and are practically not used. This attitude towards tablet forms reflects a certain conservatism of practical doctors, due to the fact that even 15-20 years ago the then existing oral drugs could not be compared with parenteral drugs either in terms of the effectiveness of therapy or its tolerability. Only in recent decades since the creation of the first oral cephalosporin, cephalexin, and the advent of new oral bactericidal drugs, has this dosage form somewhat strengthened its position not only in outpatient, but also in inpatient practice. However, this did not radically affect the state of affairs.
It is obvious that this form of drug administration has undoubted advantages. This is manifested in the possibility of outpatient management of the patient, and in the convenience of taking the drug, and in reducing the risk of post-injection complications and length of hospital stay, and even in getting rid of the psychological discomfort associated with injections.
The presence of antibacterial drugs in two forms - for parenteral and oral use - makes it possible to use them for so-called step therapy. The essence of such treatment is to prescribe an intravenous or intramuscular drug and then, two to three days after achieving a clinical effect, transfer it to oral administration. The possibility of carrying out stepwise therapy with the same drug is a significant advantage of this drug over its analogues. Stepped therapy provides clinical and economic benefits to both the patient and the healthcare facility.
Based on the data presented, it is difficult to understand the logic of choosing a cephalosporin antibiotic of one generation or another and the principles that guide the doctor when prescribing the drug. Analysis of the use of cephalosporin antibiotics by generation (see Fig. 2 and 3) indicates the preferred use of drugs of the 1st and 3rd generations, with half of the 3rd generation drugs (61%) being cefotaxime, and the majority of the 1st generation drugs being cefazolin.
In clinical practice, the doctor begins to administer antibacterial therapy, in most cases without the results of microbiological verification of the infectious agent, and often without the prospect of obtaining this kind of data. Therefore, when choosing an antibacterial drug, one still has to rely on information obtained from the literature, microbiological monitoring data, as well as the characteristics of the clinical situation. All this makes it possible, with a greater or lesser degree of probability, to determine the etiological infectious agent, taking into account the clinical form of respiratory tract infection (pneumonia, chronic bronchitis, sinusitis, etc.), age (children, old people), concomitant diseases (diabetes mellitus, chronic alcohol intoxication , treatment with glucocorticoids and cytostatics). It is also necessary to keep in mind the peculiarities of the development of infection in an outpatient setting or in a hospital (treatment for another disease, stay in intensive care) in the corresponding epidemiological situation. It should be noted that when choosing a drug, it is important to distinguish a “hospital” or nosocomial infection that occurs two days after admission to the hospital, from an “outpatient” infection that is treated in a hospital. In the latter case, the tactics of antibacterial therapy should differ significantly.
Thus, the approximate etiology of bronchopulmonary infection serves as the basis for choosing among CA a specific drug (or generation of drugs) with appropriate antimicrobial activity.
In patients with outpatient infection of the upper and lower respiratory tract, the main pathogens of which are streptococci, H. influenzae, Moraxella catarrhalis, the drugs of choice may be I or II generation CA. In outpatient settings, preference should be given to oral cephalosporins (cefaclor, cefuroxime axetil, ceftibuten). At the same time, it is necessary to keep in mind the insufficient activity of CA in general against atypical bacteria (8–35% in the etiology of “domestic” pneumonia) and some anaerobic microorganisms, the likelihood of which increases in patients with chronic sinusitis and otitis.
During exacerbations of chronic bronchitis, drugs that are highly resistant to the action of b-lactamases produced by both gram-negative and gram-positive microorganisms (cefuroxime axetil) and have high activity against H. influenzae (ceftibuten) become of particular importance.
When identifying indications for hospitalization of patients with a “domestic” infection, implying a more severe course, Streptococcus pneumonia, Staphylococcus aureus, H. influenzae and Enterobacteriacea are more often detected. In this case, the prescription of parenteral cephalosporins of the second generation (cefuroxime, cefamandole) is more justified. However, it is precisely in such situations that mistakes are most often made: when a patient is hospitalized in a hospital with “domestic” pneumonia, benzylpenicillin, aminopenicillins and first-generation CAs are often prescribed (ineffective due to the high resistance of the pathogenic flora), or, for “reinsurance”, even in the presence of , III generation CA (cefotaxime, less often ceftriaxone). However, it is more reasonable - and this is determined by the spectrum of activity of the drug - to prescribe CA II. Among patients receiving hospital treatment for lower respiratory tract infections, mild cases predominate. Therefore, the ideology of prescribing second-generation CAs as “starter” drugs should dominate both from the position of adequate clinical effectiveness, economic feasibility, and from the position of maintaining a reserve in more severe situations.
The choice of CA as the initial antibiotic for community-acquired pneumonia in patients under 60 years of age without concomitant pathology should, apparently, not always be considered justified. This is due to the etiologically wide spectrum of pneumonia in this situation, which may include not only pneumococci and H. influenzae, but also the so-called atypical pathogens - Mucoplasma pneumoniae, Legionella, Chlamidia pneumoniae, which are not sensitive to cephalosporins of all generations. Meanwhile, rational empirical antibacterial therapy for bronchopulmonary infections, including pneumonia, should include the choice of a drug that is, if possible, active against all pathogens likely in a given situation. Unfortunately, today it is difficult to name a drug that fully satisfies these requirements, with the exception of the new generation of fluoroquinolones or “respiratory” fluoroquinolones. Some of them, for example, grepafloxacin, are currently being registered in our country. In cases of prescribing CA for this type of pneumonia, preference should be given to CA of the first and second generation. The use of third-generation CAs in such situations is irrational due to the high risk of developing resistance. The choice of a specific drug among I-II generation CAs should be based on the advantages of dosage forms, pharmacokinetic properties, cost, etc. For mild pneumonia, oral cephalosporins can be prescribed. In this case, it is necessary to keep in mind their different antimicrobial activity towards different microorganisms. For example, ceftibuten has the greatest activity against H. influenzae, and cefuroxime axetil has the greatest activity against S. aureus.
The general principles for choosing the initial CA remain in patients with pneumonia against the background of severe concomitant diseases (COPD, heart failure, diabetes mellitus, alcohol intoxication, etc.) and over the age of 60 years. H.influenzae, S.аureus, and some gram-negative microorganisms (E.coli, Clebsiella) acquire etiological significance in this clinical situation, and the frequency of beta-lactamase-producing bacteria increases. In this regard, the importance of drugs active against these pathogens is increasing. It is known that the antimicrobial effect of CA during the transition of activity from the first generation to subsequent ones is characterized by a decrease in antistaphylococcal activity and a predominance of activity against some gram-negative microorganisms. A valuable property is the resistance of second-generation CA to b-lactamases. In this regard, the doctor should focus in this situation on cephalosporins of the second or at least third generation.
A different approach that determines the choice of CA for the treatment of bronchopulmonary infection is observed in patients with “hospital” infection. Hospital-acquired pneumonia occupies a special place among all nosocomial infections due to the severity of the course and the difficulties of therapy. The main causative agents of hospital-acquired pneumonia are gram-negative microorganisms of the Enterobacteriacea family - Clebsiella, Protei, Enterobacter, Providencia, Serracia, as well as Staphylococcus aureus, both sensitive and resistant to methicillin. The likelihood of the etiological role of a particular infectious agent in hospital-acquired pneumonia is determined by the characteristics of the clinical situation (postoperative period, stay in intensive care, artificial ventilation, etc.). In patients in intensive care and burn departments, with immunodeficiencies and cystic fibrosis, the main microorganism of bacterial complications is Pseudomonas aeruginosa, detected in 70–95% of cases. Along with it, such patients are sown with Staphylococcus aureus or Haemophilus influenzae, resistant to CA II–III generations. The main place in the treatment of hospital-acquired pneumonia among CA is occupied by drugs of the III (ceftazidime, cefoperazone) and IV generations (cefpirome, cefepime). Taking into account the likelihood of the etiological role of Pseudomonas aeruginoza in appropriate situations (ventilation, the presence of a tracheostomy, previous glucocorticoid therapy), prescribed CAs should have antipseudomonas activity. Among the CAs available to the doctor, the third generation cephalosporins (ceftazidime, cefoperazone) and IV generation (cefpirome) have the greatest activity against Pseudomonas aeruginoza, which, however, do not have serious advantages over ceftazidime against Pseudomonas aeruginoza. The appearance of IV generation CA in the therapeutic arsenal expands the possibilities of antibacterial therapy for hospital-acquired pneumonia with a high probability of gram-negative flora, including Staphylococcus aureus, and can be considered as drugs for urgent situations.
| Cephalosporins, discovered more than 50 years ago, continue to occupy a strong position in the treatment of various bacterial diseases, despite the emergence of new antimicrobial agents. Cephalosporin antibiotics are divided into four generations, differing in their spectrum of action, antibacterial activity, stability in the presence of b-lactamases, and pharmacokinetic profile. All this, along with the variety of dosage forms and cost, determines their various indications. It is obvious that as new generations of cephalosporin antibiotics appear in clinical practice, an important problem arises in the differentiated prescription of the drug, taking into account the properties of both the antibiotic itself and the characteristics of the infectious and inflammatory process in a particular patient |
Thus, the rational choice of the initial CA for the treatment of upper and lower respiratory tract infections is determined primarily by the likelihood of the etiological role of a particular microorganism in a specific clinical situation. This approach requires the practitioner (namely, the adequate choice of drug depends on him) to be able to identify the characteristics of each case of pneumonia (epidemiological situation, background pathology, risk factors, etc.) and navigate the antimicrobial spectrum of the prescribed antibiotic. However, in clinical practice, when choosing CA, as well as other antibiotics, it is necessary to take into account other factors along with the approximate etiology of bronchopulmonary infection. Among the latter, the pharmacokinetics of the drug, the availability of various dosage forms, the risk of side effects, cost, etc. are important.
At present, the place of third-generation oral drugs in clinical practice has not been definitively determined, since their comparative clinical and bacteriological effectiveness differs little from that of second-generation drugs. Moreover, as mentioned above, the advantage of third-generation cephalosporins is their high activity against b-lactamase-producing bacteria, which most often cause serious hospital infections. But since in this case the patients are in the hospital, it is more reasonable to receive parenteral therapy. At the same time, due to reduced activity against gram-positive bacteria, which are often the cause of outpatient infections, the use of third-generation drugs has fewer advantages over second-generation drugs.
The goal of antibacterial therapy is not only to achieve a clinical effect, but also to completely eradicate the pathogen, i.e., bacteriological effectiveness. This is mainly determined by adequate dosing of the drug to achieve the required concentration at the site of infection. A high degree of drug accumulation in tissues is a necessary requirement for a medicinal substance.
First-generation CAs penetrate tissues less well, which reduces the degree of bacterial eradication.
Data on the bioavailability of oral CA should be kept in mind when differentially prescribing them to patients with concomitant intestinal pathology associated with malabsorption, as well as when taking antisecretory drugs and antacids simultaneously, taking into account the effect of food on the absorption of CA.
Knowledge of the ways of eliminating CA, along with an assessment of the functional state of the liver and kidneys (age, concomitant pathology) can also determine the choice of a more adequate drug for a given situation. When choosing CA for the treatment of severe hospital-acquired pneumonia, for example, in newborns and the elderly or in patients with kidney pathology, in the presence of renal failure, preference should be given to cefoperazone, taking into account its predominantly biliary excretion.
When making a differentiated choice of CA, it is necessary to take into account the risk of side effects. The most typical reactions are hypersensitivity reactions (fever, skin rash), hematological syndromes (cytopenia, eosinophilia), disorders of the gastrointestinal tract (nausea, vomiting, diarrhea), liver (increased transaminase activity), kidneys (increased creatinine levels), central nervous system ( headache), phlebitis with intravenous administration. Therefore, anamnestic and clinical laboratory data on the presence of any pathology in patients should influence the choice of the appropriate drug.
Phlebitis often occurs when cephalothin, cefotaxime, or cefepime are administered. Cefuroxime, cefoperazone, ceftibuten can cause anemia (usually hemolytic), and when cephalothin, cefamandole, cefotaxime, ceftazidime are prescribed, antibodies fixed on erythrocytes are sometimes detected. An increase in the activity of liver enzymes is possible during treatment with cefoperazone, ceftriaxone, ceftazidime, cefuroxime. Creatinine levels may increase during treatment with cephalexin and cefpodoxime. Oral cephalosporins most often cause gastrointestinal disorders (nausea, vomiting, diarrhea). When treated with parenteral CA, an increase in prothrombin time was noted, with the exception of ceftazidime, which does not affect the synthesis of prothrombin complex factors and blood clotting parameters. Hypersensitivity reactions (skin rash, fever, eosinophilia) are possible with the use of almost all third-generation CAs.
Thus, the differentiated choice of CA for the treatment of upper and lower respiratory tract infections should be based on taking into account and adequately assessing many factors, including both the characteristics of the clinical situation and the antimicrobial activity and pharmacokinetic characteristics of the prescribed drug.
Classification of cephalosporins
The series of cephalosporins includes drugs of five generations. Their division into groups occurred gradually, parallel to the discovery of new substances and their properties. Within each generation, oral (taken by mouth) and parenteral (entered into the body through injection) forms are distinguished.
1st generation:
| Representatives | Tradename | Method of application, price |
| Cefazolin (parenteral) | Cefazolin | Powder for the preparation of injection solution: 0.5 g. (diluted in 2 ml of sterile water) – 2.0 g. (diluted in 4 ml of sterile water) per day in 3-4 doses, administered intravenously. 20-910 rub. |
| Cefazolin-AKOS | Powder for the preparation of injection solution: 0.5 g each. x 2 times a day intravenously (diluted in 5 ml of sterile water) or intramuscularly (diluted in 2 ml of sterile water). 30-50 rub. | |
| Cephalothin (parenteral) | Cephalothin | Powder for making an injection solution: 0.5-1.0 g. every 6 hours intravenously or intramuscularly. 800-1000 rub. |
| Cephalexin (parenteral, oral) | Cephalexin | Capsules: 0.25-0.5 g. every 6 hours, washed down with water, 30-60 minutes before meals. 80-120 rub. |
| Granules for preparing a suspension inside a bottle: add 80 ml of distilled water, shake, drink the resulting mixture according to a measuring spoon (the bottle contains 0.25 g of substance). 1.0-2.0 gr. per day, while 1 ml of the finished mixture contains 25 mg of cephalexin. 80-100 rub. | ||
| Ecocephron | Capsules: 0.25-0.5 g. every 6 hours, washed down with water, 30-60 minutes before meals. 80-100 rub. | |
| Cefadroxil (oral) | Duracef | Capsules, tablets, granules for suspension are excluded from the register of used drugs in the Russian Federation. |
| Biodroxyl |
2nd generation:
| Representatives | Tradename | Method of application, price |
| Cefuroxime (parenteral, oral) | Zinatsef | Powder for making an injection solution: 0.75-1.5 g. intravenously x 3 times a day. 130-250 rub. |
| Zinnat | Capsules: 0.25-0.5 x 2 times a day after meals. 220-400 rub. | |
| Granules for preparing a suspension orally in a bottle: 0.125-0.25 g. per day during meals. 250-330 rub. | ||
| Axoseph | Tablets: 0.25-0.5 g. x 2 times a day. 400-600 rub. | |
| Powder for making an injection solution: 0.75-1.5 g. intravenously x 3 times a day. Maximum 6.0 g. per day. 120-250 rub. | ||
| Cefamandole (parenteral) | Tzefat | Powder for making an injection solution: 0.5-1.0 g. every 6 hours intramuscularly (dissolved in 3 ml of sterile water) or intravenously (dissolved in 10 ml of isotonic sodium chloride). 120-360 rub. |
| Cefaclor (oral) | Ceclor | Capsules, tablets, granules for suspension are excluded from the register of used drugs in the Russian Federation. |
| Cefaclor Stada | ||
| Alphacet |
3rd generation:
| Representatives | Tradename | Method of application, price |
| Cefotaxime (parenteral) | Claforan | Powder for the preparation of injection solution: 0.5-2.0 g. (depending on the infection) x 1 time per day intramuscularly (first dissolving 1.0 g in 4 ml, 2.0 g in 10 ml of sterile water) or intravenously (first dissolving in 40-100 ml of sterile water) slowly. 130-150 rub. |
| Cephosin | Powder for the preparation of injection solution: 1.0 g. every 8-12 hours intramuscularly (dissolving 1.0 g in 4 ml of sterile water), slowly intravenously (first dissolving 1.0 g in 4 ml, 2.0 g in 10 ml of sterile water) or drip ( 50-100 ml of isotonic sodium chloride solution per 1.0-2.0 g of substance). 50-70 rub. | |
| Ceftazidime (parenteral) | Fortum | Powder for the preparation of injection solution: 1.0-6.0 g. x 1 time per day in 2-3 intravenous or intramuscular infusions. 450-520 rub. |
| Ceftidine | Powder for the preparation of injection solution: 1.0-6.0 g. x 1 time per day (usually 1.0 g every 8 hours) intravenously or intramuscularly. 150-200 rub. | |
| Ceftriaxone (parenteral) | Ceftriaxone | Powder for the preparation of injection solution: 1.0-2.0 g. x 1 time per day intramuscularly/intravenously. 30-900 rub. |
| Azaran | Powder for the preparation of injection solution: 1.0 g. dissolve in 3.5 ml of 1% lidocaine hydrochloride solution, apply intramuscularly once a day. 2300-2700 rub. | |
| Cefoperazone (parenteral) | Cephobid | Powder for the preparation of injection solution: 2.0-4.0 g. per day intramuscularly, dividing the total daily dose into 2 doses. 250-300 rub. |
| Tsefpar | Powder for the preparation of injection solution: 2.0-4.0 g. per day intravenously or intramuscularly, divide the dose into equal parts every 12 hours. 30-100 rub. | |
| Cefixime (capsules, suspension) | Suprax | Capsules: 0.4 g. once a day. 700-780 rub. |
| Pantsef | Tablets: 0.4 g. x 1 time per day or 0.2 g. x 2 times a day. 380-590 rub. | |
| Granules for preparing a suspension inside a bottle: shake the bottle well, add 66 ml of boiled water at room temperature, shake again, take 0.4 g. x 1 time per day or 0.2 g. x 2 times a day (using a measuring cap). 390-700 rub. | ||
| Suprax Solutab | Effervescent tablets: 0.4 g. x 1 time per day or 0.2 g. x 2 times a day, dissolved in a glass of water. 800-1000 rub. | |
| Ceftibuten (capsules) | Tsedex | Capsules: 0.4 g. x 1 time per day. 800-1100 rub. |
| Cefditoren (in tablets) | Spectraceph | Tablets: 0.2/0.4 g. twice a day. 1300-1400 rub. |
4th generation:
| Representatives | Tradename | Method of application, price |
| Cefepime (parenteral) | Maxipim | Powder for the preparation of injection solution: 0.5-2.0 g. every 12 hours, administered slowly intravenously (diluted in 5/10 ml of sterile water) or intramuscularly (diluted in 1.3/2. ml of sterile water). 350-400 rub. |
| Cefepime | Powder for making an injection solution: 0.5-1.0 g. every 12 hours, intravenous or intramuscular administration (dilution volumes are similar). 120-150 rub. | |
| Cefpir (parenteral) | Cefanorm | Powder for the preparation of injection solution: 1.0-2.0 g. intravenously every 12 hours. 1300-1500 rub. |
| Izodepom | Powder for making an injection solution: 0.25/0.5/1.0/2.0 g, dividing the administration in half every 12 hours. Administer intravenously slowly/drip or intramuscularly (dilute the dose in 25/50/100/200 ml of sterile water or isotonic sodium chloride solution, respectively). 600-900 rub. |
5th generation:
| Representatives | Tradename | Method of application, price |
| Ceftobiprole (parenteral) | Zeftera | Lyophilisate for the manufacture of injection solution - excluded from the register of used drugs in the Russian Federation. |
| Ceftaroline (parenteral) | Zinforo | Powder for the preparation of injection solution: 0.6 g each. every 12 hours as an intravenous infusion for 60 minutes (after adding 20 ml of sterile water to the powder, shake the resulting mixture and transfer it to a bottle, where add 50/100/250 ml of isotonic sodium chloride solution). 25000-27000 rub. |
The combination of cefoperazone + sulbactam in parenteral preparations was created specifically to protect against the destructive effects of bacterial β-lactamase enzymes:
- Sulperazone (powder for the preparation of an injection solution: 1.0-2.0 g of cefoperazone + 1.0-2.0 g of sulbactam in a 1:1 ratio, dividing the dose into 2 doses, administered intravenously, intramuscularly). 480-550 rub.
- Sulperacef (powder for the preparation of an injection solution: 0.5-1.0 g cefoperazone + 0.5-1.0 g sulbactam in a 1:1 ratio, administered intramuscularly or intravenously every 12 hours). 2400-3000 rub.
All drugs from the list of cephalosporins are absolutely prohibited for use together with alcoholic beverages of any strength. Otherwise, the antabuse effect develops - an acute, deadly toxic effect on the body in the form of respiratory disorders, cardiac activity and bronchospasm.
The place of third-generation oral cephalosporins in the treatment of acute pyelonephritis in women and children
The role of third-generation oral cephalosporins in the treatment of upper urinary tract infections in women (pregnant and non-pregnant) and children is discussed. Data on the sensitivity of modern pathogens of uncomplicated outpatient pyelonephritis in these groups of patients are presented. Antibiotic treatment regimens for acute uncomplicated pyelonephritis, proposed by the clinical guidelines of the European Association of Urology 2014, are considered. The place of third-generation oral cephalosporins in pyelonephritis of varying severity is determined.
Urinary tract infections are one of the most common infectious diseases in both outpatient and hospital practice [1]. Acute pyelonephritis, or the so-called acute upper urinary tract infection, is a serious infectious and inflammatory disease. As is known, the main risk of this condition is the high probability of developing severe sepsis, the mortality rate of which reaches 30–50%. The key components of the success of treatment of patients with acute pyelonephritis are:
- timely and accurate diagnosis, determination of the severity of the process;
- early identification and, if possible, elimination of factors that complicate the course of the infectious-inflammatory process in the kidney and/or reduce the effectiveness of antibiotic therapy;
- actually rational antibiotic therapy.
Let us briefly describe the principles of diagnosing pyelonephritis, since it is correct and timely diagnosis that largely determines the effectiveness of therapy. In accordance with modern clinical recommendations of the European Association of Urology [2], in addition to assessing the clinical manifestations of the disease, which are well known, mandatory diagnostic tests for suspected acute pyelonephritis are:
- general blood analysis;
- general analysis and culture of midstream urine;
- Ultrasound examination of the kidneys to exclude a number of complicating factors (dilatation of the upper urinary tract, stones, abscesses, etc.).
It is difficult to overestimate the role of bacteriological examination (culture) of urine in acute pyelonephritis, since this method allows not only to confirm the presence of a urinary infection, but also to timely adjust antibiotic therapy if the empirically prescribed drug is ineffective. In accordance with the clinical guidelines of the European Association of Urology, urine culture should also be repeated in all patients on days 5–10 of pyelonephritis therapy. If sepsis is suspected, the necessary studies are to determine the level of serum procalcitonin and bacteriological examination of the blood.
Let us consider in more detail the principles of antibiotic therapy for uncomplicated pyelonephritis in women of childbearing age (non-pregnant and pregnant) and upper urinary tract infections in children. Note that these categories of patients make up the vast majority of patients suffering from upper urinary tract infections. It is in these patients that the use of third-generation oral cephalosporins seems most appropriate.
Such widespread use of oral cephalosporins in patients with uncomplicated pyelonephritis is due to the following properties of the drugs in question:
- high natural sensitivity of the main uropathogens - enterobacteria (primarily Escherichia coli);
- the ability of drugs to reach high concentrations in urine and kidney parenchyma;
- favorable pharmacokinetics, which allows maintaining high efficiency when taken once a day (which in turn ensures good compliance with therapy);
- low toxicity and good tolerability, allowing their use during pregnancy, lactation and childhood.
It is obvious that the effectiveness of empirical antibiotic therapy for pyelonephritis directly depends on the prevailing uropathogens and their resistance profile in a particular region. That is why it is so important to regularly update data on the sensitivity of pathogens of urinary tract infections and review the recommended drugs each time. In accordance with modern clinical recommendations of the European Association of Urology, empirical use of an antibacterial drug is not advisable if more than 10–20% of microorganism strains in the population are resistant to it.
Considering the above, the results of the DARMIS study are of particular interest, during which the characteristics of almost a thousand pathogens of both uncomplicated and complicated urinary tract infections in adult patients from 20 regions of Russia were studied [3]. In outpatient (non-hospital) uncomplicated urinary tract infections in women, the main uropathogen (Escherichia coli) demonstrated the highest sensitivity to fosfomycin (98.6%), nitrofurantoin (98.6%), as well as two oral cephalosporins - ceftibuten (97.9%). ) and cefixime (95.9%). Among all members of the family Enterobacteriaceae
the highest sensitivity was noted to ceftibuten (95.9%), fosfomycin (95.7%), cefixime (93.5%) and furazidine (90.5%). We emphasize that neither fosfomycin, nor nitrofurantoin, nor furazidin can be used to treat pyelonephritis, since they are not able to reach high concentrations in the renal tissue. It is interesting that earlier, in the study of S.V. Yakovleva 2006 [4], the resistance of outpatient pathogens of urinary tract infections to third-generation cephalosporins did not exceed 10%, which means that over the past five years, resistance to this group of antibiotics among outpatient uropathogens has not increased.
In a study by S. Mårild et al. 2009 [5] in children with acute uncomplicated pyelonephritis, all 368 (100%) isolated strains of Escherichia coli
were sensitive to the oral cephalosporin ceftibuten.
Thus, at present, the levels of resistance of pathogens of acute uncomplicated pyelonephritis to third-generation cephalosporins in both adults and children remain low (does not exceed 10%), which allows us to consider them as one of the optimal options for empirical therapy.
For non-pregnant women of reproductive age, the clinical guidelines of the European Association of Urology indicate the following antibiotic treatment regimens for acute uncomplicated pyelonephritis of mild to moderate severity:
- ceftibuten per os
400 mg once a day for 10 days; - levofloxacin per os 500 mg once a day for 7–10 days;
- levofloxacin per os
750 mg once a day for 5 days; - ciprofloxacin per os
500–750 mg twice a day for 7–10 days; - amoxicillin clavulanate per os
500/125 mg three times a day for 14 days (only for gram-positive uropathogen).
The choice of one or another regimen should be determined by the low level of resistance of local uropathogens. This principle, as we have seen above, is fully consistent with oral cephalosporins, in particular ceftibuten. Treatment of these patients in the vast majority of cases is carried out on an outpatient basis.
In severe cases (with high fever, severe systemic manifestations) of acute uncomplicated pyelonephritis in non-pregnant women, treatment begins with parenteral drugs in a hospital setting. In this case, third-generation cephalosporins in parenteral form can be considered as the drugs of choice. Oral cephalosporins in this category of patients can be used in step-down therapy, that is, while continuing the course of antibiotic therapy on an outpatient basis after the patient’s condition improves and they are discharged from the hospital.
In pregnant women, as is known, the range of antibiotics approved for use is significantly narrowed, and cephalosporins seem to be the optimal drugs for many bacterial infections, including pyelonephritis. Although no urinary infection during pregnancy can be considered uncomplicated, European Association of Urology guidelines allow outpatient treatment with an appropriate antibiotic (eg, the third-generation oral cephalosporin ceftibuten) if symptoms are mild and closely monitored. The regimen for taking ceftibuten in pregnant women suffering from acute uncomplicated pyelonephritis is similar to that in non-pregnant women. In addition to third-generation cephalosporins, all drugs recommended by the European Association of Urology for pregnant women with pyelonephritis imply parenteral (and, accordingly, inpatient) use.
In children, the duration and treatment algorithm for pyelonephritis directly depend on the severity of the urinary infection. Thus, for a simple urinary infection (not a very high temperature, the child drinks well, sufficient compliance with therapy), the European Association of Urology offers the following antibiotic treatment regimens (treatment duration is 5–7 days):
- third generation cephalosporin per os
: ceftibuten 9 mg/kg once a day, cefixime 8 mg/kg twice a day; - amoxicillin per os
50–100 mg/kg/day in two to three doses; - amoxicillin clavulanate per os
37.5–75 mg/kg/day in three divided doses.
The clinical effectiveness of treating pyelonephritis in children with oral cephalosporins is high. Thus, in accordance with the data of S. Mårild et al. [5], when treated with ceftibuten in a standard dosage for 10 days, it is 93%.
For severe urinary infection in children (fever 39 °C or higher, vomiting, severe dehydration and poor compliance), the European Association of Urology recommends the following regimens of primary empirical antibiotic therapy (treatment course of 10–14 days):
- ceftriaxone IV 50–100 mg/kg/day in one administration until fever disappears, then a third-generation cephalosporin per os
(for example, ceftibuten 9 mg/kg/day in one dose); - amoxicillin clavulanate IV 60–100 mg/kg/day in three doses until fever disappears, then per os
37.5–75 mg/kg/day in three doses.
From the above schemes it is clear that in children with severe urinary infections, stepwise therapy also seems optimal: the transition from parenteral to oral therapy with a drug of the same pharmacological group.
Despite a long history of use, third-generation cephalosporins remain highly effective against pathogens of community-acquired uncomplicated pyelonephritis in women (pregnant and non-pregnant) and children. Oral cephalosporins, in particular ceftibuten, are used not only in the treatment of outpatient pyelonephritis, but also in the stepwise therapy of severe upper urinary tract infections; they have high clinical efficacy and good tolerability.
Use in childhood
Antibiotics of the cephalosporin group are for the most part not contraindicated for use in pediatric patients. Average daily dosages for children are:
| Cephalosporin | Dose |
| Cefazolin |
|
| Cephalothin | 0.02-0.04 g/kg per day, dividing the administration every 6 hours. |
| Cephalexin |
|
| Cefuroxime |
|
| Cefamandole |
|
| Cefotaxime |
|
| Ceftazidime |
|
| Ceftriaxone |
|
| Cefoperazone | 0.05-0.2 g/kg per day (administer 2 times). |
| Cefixime |
|
| Ceftibuten |
|
| Cefditoren |
|
| Cefepime |
|
| Cefpir |
|
| Ceftaroline | There is no complete information about the safety and effectiveness of the drug in children under 18 years of age. |
Cephalosporins of all generations do not lose their relevance at the present stage of development of medicine. Due to the wide spectrum of action of these drugs, it is possible to cure a wide range of infectious diseases. Unfortunately, microorganisms are constantly changing their structure, trying to become immune to the harmful effects of antibiotics. To avoid this, do not take antibacterial drugs without a doctor's prescription.
Author:
Selezneva Valentina Anatolyevna physician-therapist
CLINICAL PHARMACOLOGY OF IV GENERATION CEPHALOSPORINS
Cephalosporin antibiotics currently occupy a leading place in the treatment of infections in hospitals of various locations, being the means of choice for many infections. At the same time, a limitation of the use of cephalosporins is the development of strains resistant to them. The article is devoted to the new highly effective cephalosporin antibiotics that have appeared in recent years, the chemical feature of the molecule is the presence of both negative and positive charges. This bipolar structure is characteristic of fourth-generation cephalosporins, among which the most important and studied are cefepime and cefpirome.
Today cephalosporin antibiotics occupy a prominent place in the inpatient treatment of infections at different sites, which are the drugs of choice in many infections. At the same time the limited use of cephalosporins is the development of their resistant strains. The paper deals with novel highly effective cephalosporin antibiotics which have recently come into use. The chemical feature of their molecule is the simultaneous presence of negative and positive charges. This bipolar structure is typical of fourth generation cephalosporins among which cefepime and cefpirome are most important and well studied.
S.V. Yakovlev - Moscow Medical Academy named after. THEM. Sechenov SV Yakovlev – IM Sechenov Moscow Medical Academy
N
Despite great strides in the field of antimicrobial chemotherapy achieved in recent years, the incidence of severe hospital infections remains high, and in many cases they cause death in patients.
Particularly difficult to treat are infections in intensive care units and in patients with neutropenia. Difficulties in treating hospital infections are associated with the characteristics of the infectious agents, which are often resistant to many antibacterial drugs. Such “problem” microorganisms include primarily Staphylococcus aureus and coagulase-negative staphylococci (mainly Staphylococcus epidermidis), including strains resistant to methicillin, multidrug-resistant enterococci (Enterococcus faecalis and E. faecium), as well as penicillin-resistant pneumococci (Streptococcus pneumoniae) . Among the gram-negative microorganisms, one should highlight Pseudomonas aeruginosa and other pseudomonas (Pseudomonas spp.), a group of enterobacteria - Enterobacter cloacae, Klebsiella pneumoniae, Serratia spp., producing extended-spectrum b-lactamases and resistant to third-generation cephalosporins, Stenotrophomonas maltophilia, which causes hydrolysis of most b-lactam antibiotics, including carbapenems. These microorganisms are common causative agents of hospital infections of various localizations (pneumonia, peritonitis, wound infection, sepsis). The search for new antibacterial agents with an extended spectrum of action for the treatment of patients with severe infections is an urgent task of modern chemotherapy. Table 1. Comparative in vitro activity of cephalosporin antibiotics (schematically) [2, modified]
| Generations of cephalosporins | Activity regarding | Stability to b -lactamases | ||
| Gram positive bacteria | Gram negative bacteria | Gram positive bacteria | Gram negative bacteria | |
| First | +++ | +/- | + | — |
| Second | ++ | + | + | +/- |
| Third | + | +++ | +/- | + |
| Fourth | ++ | +++ | + | ++ |
Cephalosporin antibiotics currently occupy a leading place in the treatment of infections in hospitals of various locations. A wide spectrum of antibacterial activity, good pharmacokinetic characteristics, low toxicity, and good compatibility with other antibacterial agents make cephalosporins the drugs of choice for many infections. At the same time, a limitation of the use of cephalosporins is the development of strains of microorganisms resistant to them as a result of the production of b-lactamases by bacteria. This problem has become especially relevant in recent years due to the widespread use of cephalosporins, sometimes unjustified and often uncontrolled. Rice. Chemical structure of cefepime and cefpirome
The group of cephalosporin antibiotics includes more than 50 drugs, which are usually divided into generations. The comparative antimicrobial activity of cephalosporins is presented in table. 1. In recent years, new highly effective cephalosporin antibiotics have appeared, the chemical feature of the molecule is the presence of both negative and positive charges. This bipolar structure is characteristic of fourth generation cephalosporins (Fig. 1), among which the most important and studied are cefepime and cefpirome. The cephem core of antibiotics carries a negative charge. The quaternary nitrogen of the cyclopentopyridine group carries a positive charge and gives the molecule a zwitterionic structure. Table 2. Spectrum of antimicrobial activity of IV generation cephalosporins
| Usually sensitive (MIC < 4 mg/l) Escherichia coli Salmonella spp. Shigella spp. Proteus spp. Providencia spp. Klebsiella spp. Citrobacter spp. Enterobacter aerogenes Serratia spp. Neisseria spp. Moraxella catarrhalis Haemophilus influenzae Streptococcus pneumoniae Streptococcus spp. Staphylococcus spp. MS Peptostreptococcus spp. Clostridium perfringens Propionbacterium spp. Lactobacillus spp. | Moderately sensitive (MIC 4-32 mg/l) |
| Pseudomonas aeruginosa Pseudomonas spp. Acinetobacter spp. Enterobacter cloacae Enterococcus faecalis Yersinia spp. | |
| Resistant (MIC > 32 mg/l) Pseudomonas cepacia Stenotrophomonas maltophilia Staphylococcus spp. MR Enterococcus faecium Clostridium difficile Bacteroides spp. Listeria |
Features of the chemical structure of IV generation cephalosporins give them a number of properties [3, 4]. The bipolar structure of cefpirome and cefepime ensures rapid penetration of the molecule through the outer membrane of gram-negative bacteria; the positive charge serves as a conductor for the molecule to find a favorable position in the porin channel of the bacterial cell. The aminothiazoline-methoxy-imino group, attached at position 7 of the cephem core, has a more pronounced effect on gram-negative microbes and imparts resistance to b-lactamases. Penetrating into the microbial cell, IV generation cephalosporins reach high concentrations in the periplasmic space and bind to penicillin-binding proteins (mainly type 3), for which they have high affinity. These properties of fourth-generation cephalosporins (rapid penetration through the outer membrane of bacteria, low affinity for b-lactamases and effective binding to penicillin-binding proteins) ensure their activity against gram-negative bacteria, including strains resistant to third-generation cephalosporins.
Table 3. Antimicrobial activity in vitro of IV generation cephalosporins [4-9]
| Microorganisms | Drugs | MIC, mg/l | % sensitive strains | ||
| Range | IPC 50 | IPC 90 | |||
| Gram-negative | |||||
| Escherichia coli | Cefepime Cefpirom | 0,015-2 0,06-32 | 0,03 0,06 | 0,12 0,25 | 100 99 |
| Proteus mirabilis | Cefepime Cefpirom | 0,06-0,12 0,06-32 | 0,06 0,06 | 0,12 1 | 100 96 |
| Proteus vulgaris | Cefepime Cefpirom | 0,06-16 0,06-32 | <0,5 0,06 | 0,5 2 | 100 93 |
| Klebsiella pneumoniae | Cefepime Cefpirom | 0,008-2 0,06-32 | 0,03 0,06 | 1 4 | 100 92 |
| Enterobacter cloacae | Cefepime Cefpirom | 0,015-8 0,06-32 | 0,06 0,12 | 8 16 | 100 90 |
| Enterobacter aerogenes | Cefepime Cefpirom | 0,03-0,06 0,06-32 | 0,03 0,12 | 0,03 4 | 100 94 |
| Serratia marcescens | Cefepime Cefpirom | 0,06-8 0,06-32 | 0,5 0,12 | 8 8 | 100 99 |
| Citrobacter freundii | Cefepime Cefpirom | 0,015-0,12 0,06-16 | 0,03 0,12 | 0,12 2 | 100 97 |
| Haemophilus influenzae | Cefepime Cefpirom | 0,007-2 0,06-0,25 | <0,06 0,06 | 0,06 0,13 | 100 100 |
| Acinetobacter spp. | Cefepime Cefpirom | 1-8 0,06-32 | 8 4 | 8 i32 | 100 67 |
| Pseudomonas aeruginosa | Cefepime Cefpirom | <0,5-64 0,06->32 | 2 4 | 8-16 i32 | 87 69 |
| Pseudomonas spp. | Cefepime Cefpirom | 0,06-32 0,06->32 | 4 8 | 32 i32 | 77 63 |
| S. maltophilia | Cefepime Cefpirom | 1->64 0,06->32 | 32 i32 | >64 >32 | 17 17 |
| Gram-positive | |||||
| Staphylococcus aureus MS | Cefepime Cefpirom | 0,125-16 0,06-32 | 2 0,5 | 4 1 | 98 98 |
| Staphylococcus aureus MR | Cefepime Cefpirom | 8->64 8->64 | >64 >64 | >64 >64 | 9 8 |
| Staphylococcus CN MS | Cefepime Cefpirom | 0,03-16 0,06-32 | 0,5 0,5 | 8 4 | 74 96 |
| Streptococcus pneumoniae | Cefepime Cefpirom | 0,007-0,25 0,005-0,25 | 0,03 0,05 | 0,06 0,06 | 100 100 |
| Enterococcus faecalis | Cefepime Cefpirom | 0,06-32 | 64 8 | >64 i32 | 11 55 |
| Enterococcus faecium | Cefepime Cefpirom | 0<06->32 | >64 32 | >64 i32 | 9 21 |
| MS – methicillin sensitive MR – methicillin resistant CN – coagulase negative | |||||
Antimicrobial activity
IV generation cephalosporins have a wide, well-balanced antimicrobial spectrum, which combines the activity of I-II generation cephalosporins against gram-positive bacteria with the high activity of III generation cephalosporins against gram-negative bacteria. The antimicrobial spectrum of cefepime and cefpirome covers gram-negative bacteria (family Enterobacteriaceae, Neisseriaceae, Haemophilus influenzae, Moraxella catarrhalis, Pseudomonas spp., Acinetobacter spp.), gram-positive bacteria (methicillin-sensitive staphylococci, streptococci, pneumococci) and some anaerobic microorganisms (Table 2 ). The antimicrobial activity of cefepime and cefpirome against the most important clinical strains of microorganisms is presented in table. 3. The activity of IV generation cephalosporins against gram-negative bacteria is not inferior to or higher than that of the most active III generation cephalosporins - cefotaxime and ceftriaxone and is comparable to the activity of fluoroquinolones and carbapenems. IV generation cephalosporins, to a greater extent than III generation cephalosporins, are resistant to hydrolysis by b-lactamases produced by gram-negative bacteria, including the extended spectrum. An important property of cefepime and cefpirome is that they often remain active even against strains resistant to third-generation cephalosporins [10, 11]. According to the severity of the effect on gram-negative enterobacteriaceae (Enterobacteriaceae), the most active drugs are arranged in the following order: meropenem > ciprofloxacin > cephalosporins IV = imipenem > cephalosporins III = amikacin. IV generation cephalosporins have moderate activity against P. aeruginosa; in terms of activity against this microorganism, they are slightly inferior to ceftazidime, meropenem and ciprofloxacin, but superior to gentamicin, piperacillin/tazobactam and equal in activity to imipenem and amikacin. In general, cefepime is slightly superior to cefpirome against gram-negative enterobacteriaceae and P. aeruginosa.
Table 4. Pharmacokinetic parameters of cefepime and cefpirome [4, 10, 12, 13]
| Options | Cefepime 1 g IV | Cefpirom 1 g IV |
| Cmax, mg/l | 67-82 | 53-97 |
| Vdss, l | 18 | 21 |
| F with i.m. administration, % | ~ 100 | >90 |
| AUC, mg•h/l | 137-149 | 119-156 |
| CLt, ml/min | 122-155 | 109-177 |
| T 1/2, h | 1,3-2,3 | 1,5-2,5 |
| Protein binding, % | 16-19 | 5-13 |
| Cmax - maximum concentrations in the blood Vdss - steady-state volume of distribution F - absolute bioavailability AUC - area under the pharmacokinetic curve in the interval 0-24 hours CLt - total clearance T? - half-life | ||
The activity of IV generation cephalosporins against staphylococci is comparable to the activity of I and II generation cephalosporins and superior to III generation cephalosporins. Cefepime and cefpirome are highly active against pneumococci, including strains with reduced sensitivity to penicillin. Cefpir has moderate and inconsistent activity against Enterococcus faecalis. The clinical significance of this phenomenon is not clear: perhaps, when using cefpirome, there may be a lower risk of developing enterococcal superinfection, which is typical for all other cephalosporin drugs. IV generation cephalosporins, like other cephalosporins, are not active against methicillin-resistant staphylococci, Enterococcus faecium and Listeria. Cefpirome is moderately superior to cefepime against Gram-positive bacteria.
Cefepime and cefpirome have some activity against some anaerobes, but they do not act on the most common pathogens of anaerobic infections of the abdominal cavity and wounds and therefore in these cases, as a rule, require combination with metronidazole or clindamycin.
Table 5. Penetration of cefepime and cefpirome into body fluids and tissues [4, 10, 14-15]
| Tissues and fluids | Penetration coefficient (tissue/blood concentration ratio) | |
| Cefepime | Cefpir | |
| Inflammatory fluid | 0,8 | 0,9 |
| Peritoneal fluid | 0,66 | 0,98 |
| Sputum | 0,1 | 0,05-0,2 |
| Bronchial tissue | 0,6 | 0,46 |
| Prostate fluid | 0,43 | 0,31-0,46 |
| Female genital organs | 0,6 | 0,58 |
| Cerebrospinal fluid (for meningitis) | 0,2 | 0,19-0,25 |
| Breast milk | <0,01 | 0,04 |
Pharmacokinetics
Cefpirome and cefepime are poorly absorbed from the gastrointestinal tract and therefore are used only parenterally - intravenously or intramuscularly. When administered intramuscularly, both drugs are characterized by high bioavailability. When administered intravenously, high concentrations are quickly reached in the blood, which then decrease biexponentially with a half-life of about 0.3 hours. The half-life of cefepime and cefpirome does not depend on the dose and duration of use and is about 2 hours. Antibiotics are detected in the blood in therapeutic concentrations within 12 hours, which is the rationale for their use 2 times a day. 12 hours after intravenous administration at a dose of 1 and 2 g, serum concentrations of cefpirome are 1 and 2.5 mg/l, which is higher than the MIC values for most sensitive gram-negative and gram-positive bacteria, with the exception of P. aeruginosa, Acinetobacter spp. and Enterococcus spp. Cefepime and cefpirome have similar pharmacokinetic parameters (Table 4). Both drugs are slightly metabolized in the body and are excreted unchanged in the urine. Renal clearance accounts for 80-70% of the total clearance. Table 6. Dosing of cefepime and cefpirome
| Diseases | Single dose, g | Interval, h |
| Infections in patients with neutropenia | 2 | 8-12 |
| Infections in the intensive care unit | 2 | 12 |
| Hospital pneumonia | 2 | 12 |
| Intra-abdominal infection | 2 | 12 |
| Sepsis | 2 | 12 |
| Infection caused by | 2 | 12 |
| P. aeruginosa | ||
| Community-acquired pneumonia | 1 | 12 |
| Skin and soft tissue infections | 1 | 12 |
| Urinary tract infections | 0,5-1 | 12 |
Cefepime and cefpirome bind to plasma proteins to a small extent and penetrate well into body fluids and tissues, and drug concentrations in lung tissue, skin and soft tissues, and ascitic fluid exceed the MIC of the main causative agents of these infections (Table 5). In elderly patients, there is some change in the pharmacokinetic parameters of cefepime and cefpirome, characterized by an increase in half-life by 1.7-2 times and a decrease in total clearance. However, these changes in pharmacokinetics do not require routine adjustment of the drug dosage regimen in the elderly. Liver diseases do not affect the pharmacokinetics of cefepime and cefpirome, while impaired renal function requires adjustment of the dosage regimen taking into account the degree of renal failure. In patients with uremia, the recommended doses of cefepime and cefpirome are 0.5 g at 24-hour intervals, plus an additional 0.25 g after hemodialysis. In children, no pronounced changes in the kinetics of these drugs are observed.
Clinical Application
IV generation cephalosporins have been used in clinical practice since the early 90s, and during this period a large number of comparative and non-comparative studies of these drugs have been conducted for various infections, mainly hospital-acquired. An analysis of the results of clinical trials of cefepime and cefpirome is presented in fundamental reviews by L. Barradell & H. Bryson [10], H. Giamarellou [16] and S. Norrby [17]. Cefepime and cefpirome showed high effectiveness, exceeding 80% in various hospital infections - pneumonia, skin and soft tissues, intra-abdominal, pelvic, urinary tract, sepsis. According to summary data, the clinical effectiveness of cefpirome and cefepime in severe community-acquired pneumonia was >70% [4, 10]. A high clinical effect of these drugs was observed in particularly severe infections in hospitals - in intensive care units, in hematology-oncology departments, and in febrile patients with neutropenia. In comparative studies, cefepime and cefpirome showed similar clinical efficacy to third-generation cephalosporins - cefotaxime, ceftriaxone and ceftazidime, while the positive bacteriological effect when using fourth-generation cephalosporins was in some cases higher than the comparison drugs. Most studies have noted that cefpirome and cefepime at a daily dose of 4 g are not inferior to ceftazidime at a daily dose of 6 g in the treatment of severe nosocomial infections. Two studies examined the comparative effectiveness of IV generation cephalosporins and imipenem: for infections of the skin and soft tissues, the clinical effect with the use of cefpirome and imipenem was achieved in 95 and 98% of patients [4], for complicated intra-abdominal infections, the clinical and bacteriological effectiveness of cefepime with metronidazole (88 and 89%) was higher than with imipenem (76 and 76%) [18]. In a multicenter study conducted in Russia in 6 clinical institutions in 111 patients with various hospital infections [19], the high clinical and bacteriological effectiveness of cefpirome was noted, which amounted to 91 and 88% for lower respiratory tract infections, and 95 and 70% for urinary tract infections. %, for surgical infections of the skin and soft tissues - 96 and 80%. Cefpirome and cefepime are well tolerated even when treating severely ill patients. Analysis of the results of controlled studies showed that the frequency of adverse events when using these drugs does not exceed that when using other cephalosporin antibiotics [4, 20]. The dosage of IV generation cephalosporins is presented in Table. 6.
Conclusion
The most important properties of IV generation cephalosporins, which determine the scope of their application, include: * Wide spectrum of antimicrobial activity (wider compared to III generation cephalosporins); * Stability to various b-lactamases, including extended spectrum; * Activity against many strains of gram-negative bacteria resistant to third generation cephalosporins; * Good penetration of drugs into tissues, maintaining bactericidal concentrations there for 12 hours; * Convenient dosing (every 12 hours); * Good tolerability and lack of toxicity; * Proven effectiveness in comparative clinical studies. These properties of cefepime and cefpirome explain their high clinical effectiveness as monotherapy in the treatment of various hospital infections, including particularly severe infections. Cefpirome and cefepime can be prescribed as first-line drugs for the empirical treatment of severe hospital infections of various localizations: * Severe hospital-acquired pneumonia, including ventilator-associated pneumonia; * Peritonitis (in combination with metronidazole); * Gynecological infection; * Sepsis caused by gram-negative microorganisms; * Infections in patients in the intensive care unit; * Infections in febrile patients with neutropenia; * Infections in cancer patients. In addition, cefpirome and cefepime are indicated for the isolation of hospital strains of microorganisms resistant to third generation cephalosporins. In these clinical situations, IV generation cephalosporins are effective as monotherapy. For mixed aerobic-anaerobic infections, it is advisable to combine cefepime and cefpir with antianaerobic agents (metronidazole or clindamycin). For infections caused by P. aeruginosa, cefepime and cefpir should be combined with aminoglycosides or fluoroquinolones. Thus, doctors now have at their disposal new highly effective IV generation cephalosporin antibiotics - cefepime and cefpirome, which can be used as monotherapy in the treatment of severe hospital infections, including those caused by multi-resistant microorganisms.
Literature:
1. Neu HC. Infection problems of the 90's — do we have an answer? Scand. J. Inf. Dis. 1993;91:7-13. 2. Piriti P. Introduction: cephalosporin generations. J Chemotherapy 1996;8(Suppl. 2):3-6. 3. Laws A, Page M. The chemistry and structure-activity relationships of C3-quaternary ammonium cephem antibiotics. J Chemotherapy 1996;8(Suppl. 2):7-22. 4. Yakovlev V.P., Yakovlev S.V. Cefpirome: a fourth-generation cephalosporin antibiotic. Moscow: JSC Farmarus, 1997;112. 5. Hancock RE, Bellido F. Antibacterial in vitro activity of fourth generation cephalosporins. J Chemotherapy 1996;8(Suppl. 2):31-6. 6. Kessler RE, Fung-Tomc J. Susceptibility of bacterial isolates to beta-lactam antibiotics from US clinical trials over a 5-year period. Am. J. Med. 1996;100(Suppl. 6A):13S-19S. 7. Schafer V, Shah PM, Doerr HW, et al. Invitro activity of cefpirome against isolates from patients with urinary tract, lower respiratory tract and wound infections. J. Antimicrob. Chemother. 1992;29(Suppl. A):7-12. 8. Thornsberry C, Brown SD, Yee YC, et al. Invitro activity of cefepime and other antimicrobials: survey of European isolates. J. Antimicrob. Chemother. 1993;32(Suppl. B):31-53. 9. Verbist L. Epidemiology and sensitivity of 8625 ICU and hematology/oncology bacterial isolates in Europe. Scand. J. Infect. Dis. 1993;Suppl. 91:1424. 10. Barradell LB, Bryson HM. Cefepime. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1994;47:471-505. 11. Blahova J, Krcmery V, Kralikova K. In vitro activity of cefepime against multiresistant nosocomial gram-negative bacteria. 8th European Congress of Clinical Microbiology and Infectious Diseases, Lausanne, May 25-28;1997;165. 12. Rybak M. The pharmacokinetic profile of a new generation of parenteral cephalosporin. Am. J. Med. 1996;100(Suppl. 6A):39S-44S. 13. Van der Auwera P, Santella PJ. Pharmacokinetics of cefepime: a review. J. Antimicrob. Chemother. 1993;32(Suppl. B):103-116. 14. Baldwin DR. The penetration of the fourth generation parental cephalosporins. J Chemotherapy 1996;8(Suppl. 2):71-82. 15. Craig WA. The pharmacokinetics of cefpirome: rationale for a twelve-hour dosing regimen. Scand. J. Infect. Dis. 1993;Suppl. 91: 33-40. 16. Giamarellou H. Clinical experience with the fourth generation cephalosporins. J Chemotherapy 1996;8(Suppl. 2):91-104. 17. Norrby SR. Cefpirome: efficacy in the treatment of urinary and respiratory tract infections and safety profile. Scand. J. Infect. Dis. 1993;Suppl. 91:41-50. 18. Barie PS, Vogel SB, Dellinger EP, et al. A randomized, double-blind clinical trial comparing cefepime plus metronidazole with imipenem-cilastatin in the treatment of complicated intra-abdominal infections. Arch. Surg. 1997;132:1294-1302. 19. Yakovlev S.V., Dvoretsky L.I., Shakhova T.V., Eremina L.V. Cefpirome is a IV generation cephalosporin antibiotic for the treatment of severe hospital infections. Antibiotics and Chemotherapy 1996;41(12):34-39. 20. Neu HC. Safety of cefepime: a new extended-spectrum parenteral cephalosporin. Am. J. Med. 1996;100(Suppl. 6A):68S-75S.