What are bacteriophages?
It is well known that bacteriophages are able to adapt to new conditions thanks to mutations, but among the signs of “living” they have only the ability to reproduce and transmit hereditary information to descendants. It is these properties that have allowed humans to use them as an alternative to antibiotics to fight infections and destroy pathogenic bacteria.
Bacteriophages are viruses, tiny natural structures similar to molecular crystals. But, unlike most viruses known to mankind, they do not infect higher organisms (for example, humans), but only lower ones - single-celled ones; it is not for nothing that “bacteriophage” is literally translated as “eater of bacteria”. Bacteriophages are so simple that they cannot even reproduce on their own - for this, like other viruses, they need a “foreign” living cell.
Phage cocktails
A new round of interest in phage therapy has occurred in recent years. The fact is that antibiotics have also not become a panacea for the treatment of bacterial infections: these days, the development of new drugs has not kept pace with the increase in the number of bacteria with acquired resistance to existing antibiotics. Already today, in hospitals in England, about 40% of staphylococcal infections are caused by such strains, and in the United States, about 90 thousand patients die annually from hospital infections caused by drug-resistant bacteria. When recalculated for the world's population, this number is 3-5 million deaths per year!
WHO warns that the world will soon enter a “post-antibiotic” era, when there will be no treatment for common bacterial infections. And against this background, phage therapy looks like a very promising direction, the development of which can lead to the creation of effective personalized methods for treating diseases. For this, there is both the necessary knowledge about phages and the mechanisms of their interaction with bacterial cells, as well as technologies for working with viral agents.
For phage therapy today, only virulent lysis phages are used, mainly “tailed” phages of the order Caudovirales, as well as filamentous phages of the families Leviviridae (with a single-stranded RNA genome) and Inoviridae (with a single-stranded circular DNA genome).
As discussed above, the activity spectra of phages are usually very narrow and limited to one or a few closely related bacterial species. On the one hand, such narrow specificity is good for therapy, since it allows you to eliminate a specific microorganism without disturbing the entire bacterial community of the human body. On the other hand, if emergency treatment is necessary (when there is no time to identify a specific bacterium causing the development of a pathogenic process in a wound or on a burned surface), it is necessary to have a drug that affects several types of bacteria, possible causative agents of infection. To solve this problem, phage cocktails are usually used - preparations containing several phages that differ in specificity.
This approach was also used by d'Herelle. D'Herelle's cocktail, which he brought from Paris back in 1930, is still one of the main phage preparations: it forms the basis of the Georgian pyophage and the Russian intestifage. In Tbilisi, based on phage cocktails, drugs were developed for the treatment of gastrointestinal diseases and purulent wounds for mass use in the event of epidemics or military operations. The results of army trials and a wide experiment on the prevention of childhood gastrointestinal disorders conducted in Tbilisi showed the good effectiveness of such drugs.
Phage cocktails are produced in a standardized manner and target bacterial communities commonly found in specific diseases. Of course, more effective cocktails are obtained when their components are matched to the bacterial community of a particular patient. To obtain such a cocktail, it is necessary to test the patient’s bacteria for sensitivity to phages from the collection in order to select the most effective phage strains. If the required phages are not in the collection, bacteria-specific phages are searched for in natural substrates.
In general, the search for bacteriophages is quite simple: a bacterial culture is exposed to samples from various sources: water bodies, soil, sewage, etc. If the bacteria die, they are separated from the solution by centrifugation, and the remaining solution is tested for activity. The phage is then propagated by growing on an appropriate bacterial culture. Moreover, phages can be lyophilized (vacuum dried) and directly used in capsules. In this form, the drug remains stable for 14 months at temperatures up to 55 °C.
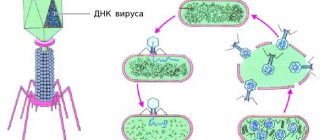
What does a bacteriophage consist of?
A typical phage consists of a “head” with a tightly packed genetic program consisting of nucleic acids (DNA or RNA), and a “tail” with which it “injects” its genes into the bacterial cell. The infected bacterium begins, using its own intracellular systems and resources, to synthesize proteins and nucleic acids necessary for the assembly of new viral particles. Mature phages go out in search of new prey, and the “parent” bacterial cell dies.
Thanks to recent research, it has become clear that bacteriophages play an important role in maintaining the global “microbial balance” in the biosphere: every two days they destroy half of the world’s bacterial population and thereby prevent these rapidly reproducing organisms from covering the earth’s surface with a thick layer.
Bacteriophages appear wherever bacteria live: on land and in the oceans, in soil and water, in plants and animals. Even the human gastrointestinal tract contains about 1012 bacteriophages - an order of magnitude more than the stars in our Galaxy! And although the size of phage particles does not exceed 0.0001 mm, the biomass of phages on the planet reaches a fantastic figure - 1 billion tons. Therefore, these invisible but omnipresent creatures are sometimes called the “dark matter” of the biosphere.
"Counters" of bacteria
Bacteriophages serve not only as a versatile therapeutic and “disinfectant” agent, but also as a convenient and accurate analytical tool for a microbiologist. For example, due to their high specificity, they are natural analytical reagents for identifying bacteria of a certain type and strain.
In the simplest version of such a study, various diagnostic bacteriophages are added dropwise to a Petri dish with a nutrient medium seeded with a bacterial culture. If the bacterium turns out to be sensitive to the phage, then in this place of the bacterial “lawn” a “plaque” is formed - a transparent area with killed and lysed bacterial cells.
By analyzing the reproduction of phages in the presence of target bacteria, it is possible to quantify the number of the latter. Since the number of phage particles in a solution will increase in proportion to the number of bacterial cells contained in it, to estimate the number of bacteria it is enough to determine the titer of the bacteriophage.
The specificity and sensitivity of such an analytical reaction are quite high, and the procedures themselves are simple to perform and do not require complex equipment. It is important that diagnostic systems based on bacteriophages signal the presence of a living pathogen, while other methods, such as PCR and immunoanalytical methods, only indicate the presence of biopolymers belonging to this bacterium. This type of diagnostic methods is especially convenient for use in environmental studies, as well as in the food industry and agriculture.
reference species are used to identify and quantify different strains of microorganisms
phages.
Very fast, almost real-time analytical systems can be created based on genetically modified bacteriophages, which, when they enter a bacterial cell, trigger the synthesis of reporter fluorescent (or luminescent) proteins, such as luciferase
. When the necessary substrates are added to such a medium, a luminescent signal will appear in it, the value of which corresponds to the content of bacteria in the sample. Such “light-labeled” phages were developed to detect dangerous pathogens such as plague, anthrax, tuberculosis, and plant infections.
It is likely that with the help of modified phages it will be possible to solve a long-standing problem of global importance - to develop cheap and fast methods for detecting tuberculosis pathogens at an early stage of the disease. This task is very difficult, since the mycobacteria that cause tuberculosis are characterized by extremely slow growth when cultivated in laboratory conditions. Therefore, diagnosing the disease using traditional methods can be delayed for up to several weeks.
Phage technology makes this task easier. Its essence is that bacteriophage D29, which is capable of infecting a wide range of mycobacteria, is added to the blood samples being analyzed. The bacteriophages are then separated and the sample is mixed with a fast-growing, non-pathogenic culture of mycobacteria that is also sensitive to this bacteriophage. If the blood initially contained mycobacteria that were infected with phages, then the production of bacteriophage will also be observed in the new culture. In this way, single mycobacterial cells can be detected, and the diagnostic process itself is reduced from 2–3 weeks to 2–5 days (Swift & Rees, 2016).
Advantages of bacteriophages
| Bacteriophages – antibacterial agents and natural antiseptics | Safe and non-toxic, no side effects, used in newborns, pregnant and lactating women |
| The action of bacteriophages does not affect the beneficial microflora of the body, unlike antibiotics | Bacteriophages are compatible with all medications. The use of bacteriophages does not limit the use of other drugs and does not affect their effectiveness |
| Affects only pathogenic bacteria that are sensitive to them and cause infectious disease, destroying them from the inside | Bacteriophages are eliminated from the body naturally |
Bacteriophages, what do we know about them? Modern possibilities of phage therapy in the practice of a pediatrician
THEM. SHCHERBENKOV
, PhD,
CELT, Moscow Bacteria resistant to most or all of the known antibiotics are causing increasingly serious problems.
This increases the risk of the medical community returning to the problems of the period when antibiotics were unknown and incurable infections and epidemics were widespread. Despite the intensive work of leading chemists and pharmacists around the world, over the past 30 years the synthesis of new classes of antibiotics has sharply decreased, and fundamentally new representatives of antibacterial agents are not expected to enter clinical practice in the near future. It is hoped that the newfound ability to completely sequence microbial genomes and determine the molecular basis of pathogenicity will open new ways to treat infectious diseases, but the search for other approaches to this problem is increasingly being pursued. One of the results of this search is a renewed interest in the possibilities of the therapeutic use of bacteriophages (from bacteria and Greek phagos eater; literally eaters of bacteria) specific viruses that attack only bacteria and kill pathogenic microorganisms. Bacteriophages have the ability to penetrate bacterial cells, reproduce in them and cause their lysis.
History of the study and use of bacteriophages
In 1896, Ernest Hankin reported that the waters of the Ganges and Jumna rivers in India had significant antibacterial activity, which remained after passing through a porcelain filter with very small pores, but was eliminated by boiling. He studied in most detail the effect of an unknown substance on Vibrio cholerae and suggested that it was responsible for preventing the spread of cholera epidemics caused by drinking water from these rivers. However, he did not subsequently explain this phenomenon.
In 1898, the first transplantable lysis of bacteria (anthrax bacillus) was observed by the Russian microbiologist N.F. Gamaleya.
Bacteriophages were officially discovered almost 20 years later, independently of each other, by F. Twort, together with A. Londe and F. d'Herelle, as filterable, transmitted agents of destruction of bacterial cells. The English scientist F. Twort in 1915 described the phenomenon of lysis in purulent staphylococcus and discovered the first “virus that devours bacteria” when he observed a curious degenerative change - lysis in cultures of staphylococci from calf lymph. The name “Twort phenomenon” is associated with his name. In 1917, Felix d'Herelle made a similar discovery; it was he who gave them the name "bacteriophages", using the suffix "phage" not in its direct sense of "is", but in the sense of development due to something.
In the 1980s The effectiveness of antibiotic treatment has decreased significantly; bacteria are actively developing drug resistance. To create a new powerful antibiotic, pharmaceutical companies today must spend an average of 10 years and $800 million. This has led to increased interest in phage therapy. In the early 2000s. Glenn Morris, an employee of the University of Maryland (USA), together with the Research Institute of Bacteriophages, Microbiology and Virology in Tbilisi, set up tests of phage preparations to obtain a license for their use in the USA. And already in July 2007, bacteriophages were approved for use in the United States. Over the past few years, research into the properties of bacteriophages has been carried out in Russia, Georgia, Poland, France, Germany, Finland, Canada, USA, Great Britain, Mexico, Israel, India, and Australia. Characteristics of phages
The use of modern electron microscopes, as well as improved methods for preparing preparations for electron microscopy, have made it possible to study the fine structure of phages in more detail. It turned out that it is very diverse and in many phages it is more complex than the structure of plant viruses and a number of human and animal viruses. Bacteriophages, like other viruses, carry their genetic information in the form of DNA or RNA. Most bacteriophages have tails, the tips of which are attached to specific receptors, such as carbohydrate, protein, and lipopolysaccharide molecules on the surface of the host bacterium. The bacteriophage injects its nucleic acid into the host, where it uses the host's genetic machinery to replicate its genetic material and reads it to form new phagocapsular material to create new phage particles. The number of phages produced during a single infection cycle (yield size) varies between 50 and 200 new phage particles.
Phages have strict specificity, i.e. they are capable of parasitizing only a certain type of microorganism: streptococci, staphylococci, etc. Phages with more strict specificity, which parasitize only certain representatives of a given species, are called type phages. Phages that lyse microorganisms of related species, for example, species included in the genus of dysentery pathogens (Shigella), are called polyvalent.
Lysogenization of bacteria is accompanied by changes in their morphological, cultural, enzymatic, antigenic and biological properties. For example, non-toxigenic strains of corynebacteria diphtheria become toxigenic as a result of lysogenization.
Practical use of phages
Phage therapy (the use of bacterial viruses to treat bacterial infections) was a problem of great interest to scientists 60 years ago. Discovery of penicillin and other antibiotics in the 1940s. provided a more effective and multifaceted approach to suppressing viral diseases and provoked the closure of work in this area.
Due to the catastrophically increasing antibiotic resistance and the absence of new antibacterial agents in the near future, active interest in phage therapy has been revived.
Scientific data of recent decades prove that, unlike antibiotics, bacteriophage preparations have the following positive qualities:
• when multiplying, they independently regulate their numbers (increasing or decreasing them), since they multiply only as long as there are sensitive bacteria, and then are gradually eliminated from the body and the environment; • they are much more specific than most antibiotics; By targeting specific problem bacteria, they cause much less damage to the body's normal microbial balance. The bacterial imbalance, or “dysbiosis,” caused by treatment with many antibiotics can lead to serious secondary infections involving sufficiently resistant bacteria, increasing treatment costs and mortality. Specific problems resulting include infections with Pseudomonas, which are difficult to treat, and Clostridium difficile, a cause of severe diarrhea and pseudomembranous colitis; • phages have the ability to use receptors on the bacterial surface involved in pathogenesis as targets, which means that the virulence of any mutants resistant to them is weakened; • few side effects have been described regarding phage therapy; • phage therapy would be particularly useful for individuals with allergies to antibiotics; • properly selected phages can be easily used prophylactically, helping to prevent bacterial diseases in humans or animals upon contact with microbes, or for the sanitation of hospitals and the fight against hospital-acquired infections; • phage can be used either independently or in combination with other antibiotics to reduce the likelihood of bacterial resistance developing; • phages do not affect the normal intestinal flora and preparations of eubiotics and protobiotics, which makes it possible to use them together.
Possessing a wide range of antibacterial activity and clinical effectiveness, bacteriophages are effective against drug-resistant organisms, which makes it possible to regard them as analogues or substitutes for antibiotics and antiseptic therapy.
Phage therapy can be used prophylactically to control the spread of an infectious disease where the source is identified early, or where outbreaks occur within relatively closed organizations such as schools or daycare centers.
Activity of therapeutic and prophylactic bacteriophages in infectious diseases of the digestive system, purulent-septic diseases of the skin, circulatory system, respiratory system, musculoskeletal system, genitourinary system (more than 180 nosological units of diseases caused by bacteria Klebsiella, Escherichiae, Proteus, Pseudomonas, Staphylococcus, Streptococcus, Serratia, Enterobacter) is quite high - from 72 to 90% - and is often the only effective treatment. This also applies to strains of hospital origin characterized by multiple resistance to antibiotics.
Bacteriophage preparations
Therapeutic and prophylactic preparations of bacteriophages are composed of polyclonal pathogenic bacteriophages with a wide range of action, effective against antibiotic-resistant bacteria. Based on their composition, they distinguish between polyvalent (active against various species and serovars of one pathogen) and combined (containing phages for several pathogens) bacteriophages, which makes it possible to obtain a therapeutic effect in the presence of microbial associations. FSUE NPO Microgen of the Russian Ministry of Health produces a wide range of medicinal bacteriophages: staphylococcal, streptococcal, coli, proteus, pseudomonas, klebsiella, typhoid, dysentery, salmonella. There are also their combined forms: coliproteus bacteriophage, intesti bacteriophage (a mixture of sterile filtrates of phagolysates of bacteria: Shigella Flexneri 1-6 serogroup B, Sonnei serogroup D; Salmonella paratyphi A, B, Typhimurium, Choleraesuis, Oranienburg, Enteritidis, the most common serological groups E. coli – 0111, 055, 026, 125, 0119, 0128, 018, 044, 025, 020, Proteus (vulgaris, mirabilis), Staphylococcus, Pseudomonas, Enterococcus – phage titer of at least 1 x 106).
Bacteriophage preparations are a sterile filtrate of bacterial phagolysates; they are prescribed for oral use, topically for irrigation of lesions and mucous membranes, introduction into the cavities of the uterus, bladder, ear, paranasal sinuses, as well as into drained cavities - abdominal, pleural, and also into abscess cavities and ulcers after removal of exudate. Bacteriophages are able to quickly penetrate the bloodstream and lymphatic system, and are removed from the body along with urine. The correspondence of bacteriophage preparations to the current atiological structure of pathogens is achieved by the production of strains, or producer strains, or synthesized material that is not subject to any transformations. This plasticity of bacteriophage preparations ensures a long-lasting effect of primary phage resistance of pathogens. The use of bacteriophages for the treatment of infectious diseases initiates factors of specific and nonspecific immunity, which is especially effective for the treatment of long-term infectious diseases that arise as a result of weakened immunity against the background of depressive disorder due to bacterial carriage. Scientific research, during clinical observations, and experimental methods have revealed the inability of plasmids to transfer antibiotic immunity to toxigenicity to prophylactic and therapeutic drugs of bacterial carriage, because they are polyclonal complexes of virulent bacteriophages.
When using bacteriophages in large clinics, it is advisable to include hospital strains of pathogens of purulent-inflammatory diseases characteristic of a given hospital in the production strains on which commercial drugs are prepared. Domestic neonatologists have shown the high effectiveness of phage therapy for purulent-septic infections in young children. In addition to the lytic effect on microbes, their importance in the mechanism of antitoxic, cellular and humoral immunity is noted. A study of the possibility of using bacteriophages as an alternative to antibiotic therapy for the treatment of acute intestinal infection (AIE) in children under 3 years of age, carried out at the Department of Children's Infectious Diseases of the KNMU, showed the high effectiveness of the polyvalent Intesti-bacteriophage. It was concluded that it is possible to carry out etiotropic therapy with a polyvalent Intesti-bacteriophage without the inclusion of antibiotics in patients with mild and moderate-severe acute intestinal infections, even in the conditions of a general intestinal department.
Dysbiosis as a pressing problem in children
In recent years, rational pharmacotherapy of dysbiosis of various origins remains an urgent task in pediatrics. The problem of intestinal dysbiosis in young children is especially relevant. The results of modern studies indicate the presence of intestinal dysbiosis of I-II degree in 50% of healthy infants, III-IV degree - in 20-25% of children. Disturbances of intestinal microbiocenosis are observed in almost all childhood diseases. With the formation of dysbiosis, the patient’s general condition worsens, the body’s resistance to infectious and antigenic agents, and tolerance to food products decreases. All this creates the background for a more severe course of the disease, the occurrence of complications, and the transition of acute forms to chronic ones. Children in the first six months of life are especially susceptible to dysbiosis, which is caused by transient deficiency of enzymes (mainly lactase), immaturity of the autonomic nervous system (ANS), which regulates intestinal motility, and immaturity of immune mechanisms.
The main causes of intestinal dysbiosis in childhood are:
• untimely onset and improper management of lactation; • early transition and irrational artificial feeding in the first year of a child’s life and violation of the diet in older age; • acute intestinal infections and non-infectious diseases of the digestive canal; • irrational use of antibiotics and other chemotherapeutic drugs; • allergic predisposition; • reduction of the body's natural resistance.
Treatment of patients with intestinal dysbiosis should be carried out differentially and begin with identifying the underlying disease, without treatment of which the signs of dysbiosis recur. The duration of one course of treatment for children is individual and ranges from 10 days. up to 1.5–2 months. Repeated courses are carried out after intermediate bacteriological control (stool examination) no earlier than 2 weeks later. after completion of the course of therapy. The total duration of recovery (to the level of stable clinical compensation) depends on many associated factors and is 6–9 months.
In modern pediatric gastroenterology, a wide arsenal of drugs is used to correct impaired intestinal microbiocenosis. In clinical practice, pediatricians and gastroenterologists are increasingly using bacteriophages to correct dysbiosis. They use coli-proteus, staphylococcal, pseudomonas, polyvalent dysenteric, salmonellosis, combined (a mixture of staphylococcal, streptococcal, coli, pseudomonas, proteus bacteriophages), polyvalent pyobacteriophage, intestifage, etc. The use of specific bacteriophages allows for optimal selective decontamination carried out in a number of pathological conditions. conditions for the purpose of a sanitizing effect, as well as to restore normal microbiocenosis. As a harmless biological treatment method, bacteriophage therapy can be used in young children. To obtain positive results from the use of bacteriophages, a preliminary study of the sensitivity of microorganisms to them is necessary.
We use liquid coli-proteus bacteriophage in the treatment of children with dysbiosis caused by enteropathogenic Escherichia coli (Escherichia) and Proteus (mirabilis or vulgaris). We administer the bacteriophage orally or as an enema. Daily dose of the drug for oral use: children under 6 months of age. 5 ml 3 times a day orally and 10 ml 1 time a day in an enema instead of one of the oral doses; from 6 months up to 1 year: 1015 ml 2 times a day orally and 20 ml 1 time a day as an enema; at the age of 13 years, 1520 ml 2 times a day orally and 40 ml 1 time a day as an enema; over 3 years: 20 ml 2-3 times a day orally and 40-60 ml 1 time a day as an enema. The bacteriophage is administered orally 1-1.5 hours before meals. For children in the first month of life, the bacteriophage is diluted with boiled water 2 times. Children over 6 months. 5-10 minutes before administration of the drug, give 10-20 ml (depending on age) of 2-3% sodium bicarbonate solution to neutralize gastric juice. The course of treatment is 5-10 days. depending on the severity of dysbiotic disorders.
It is advisable to use the drug as an enema in the absence of malabsorption syndrome: for children under 6 months. — 20 ml, from 6 months. up to 3 years - 30-40 ml, over 3 years - 40-50 ml. The drug is administered once a day in 2-3 courses lasting 3-4 days. With an interval between courses of 3 days. There are no contraindications to the use of the drug. Prescribing a bacteriophage does not exclude the use of other drugs.
We prescribe liquid staphylococcal bacteriophage orally in a daily dose: for children up to 6 months. — 20 ml, 6 months. - 3 years - 40 ml, over 3 years - 100 ml. Administered in 2 doses, on an empty stomach, 1.5-2 hours before meals. In an enema, the same doses should be administered once a day according to the same scheme.
Since in real clinical practice with dysbiosis we encounter the simultaneous growth of various representatives of pathogenic microflora, it is important to prescribe in such cases, taking into account the data of bacteriological studies of combined bacteriophages - a mixture of staphylococcal, streptococcal, coli, Pseudomonas, Proteus bacteriophages. They are prescribed to children under 3 years of age, 3-5 ml 3 times a day orally and 10 ml 1 time a day as an enema; over 3 years - 5-10 ml 3 times a day orally and 10 ml 1 time a day as an enema. Orally administered 1 hour before meals. It is possible to additionally administer the combined phage in a high enema of 5-20 ml. The course of treatment is 5-15 days.
Intestifag contains phagolysates of Escherichia coli, Salmonella shigellosis, and UPM. Prescribed orally 1 hour before meals for children under 3 years of age, 3-5 ml 3 times a day orally and 10 ml 1 time a day as an enema; for children over 3 years old - 5-10 ml 3 times a day orally and 10 ml 1 time a day as an enema. The course of treatment is 5-6 days.
Polyvalent pyobacteriophage, or sextyphage, is a mixture of phagolysates of Escherichia coli, Klebsiella, Pseudomonas aeruginosa, Staphylococcus, Streptococcus, Proteus. This drug is distinguished by the highest degree of purification from bacterial metabolites, which significantly improves its taste and makes it the first choice for children under one year of age. Prescribed: for children under 3 years of age - 3-5 ml 3 times a day orally and 10 ml 1 time a day as an enema; over 3 years - 5-10 ml 3 times a day orally and 10 ml 1 time a day as an enema. Use internally 1 hour before meals. The course of treatment is 5-15 days.
The use of phages precedes the administration of acid-forming drugs (prebiotics, probiotics, etc.). Conclusion
Bacteriophage preparations are effective in the treatment of diseases caused by antibiotic-resistant strains of microorganisms, in particular in the treatment of peritonsillar ulcers, inflammation of the sinuses, as well as purulent-septic infections, intensive care patients, surgical diseases, cystitis, pyelonephritis, cholecystitis, gastroenterocolitis, intestinal dysbiosis, inflammatory diseases and neonatal sepsis.
With the widespread development of antibiotic resistance in pathogenic bacteria, the need for new antibiotics and alternative technologies for the control of microbial infections is becoming increasingly important. Bacteriophages likely have yet to fulfill their role in the treatment of infectious diseases, either when used independently or in combination with antibiotic therapy. Literature
1. Killer antibiotics: [history of discovery, benefits and harms, contraindications, looking for a replacement when there is no way out]. M.: Eksmo, 2007. 2. Privorotsky V.F., Lupova N.E., Shilnikova O.V. The logic of constructing corrective drug programs for impaired intestinal microbiocenosis in children // RMZh. 2007. No. 1. pp. 6–9. 3. Belmer S.V. Antibiotic-associated intestinal dysbiosis // Breast Cancer. 2004. T. 12. No. 3. pp. 148–151. 4. Methods for normalizing digestion in children with dysbiosis: a manual for doctors / ed. Academician of the Russian Academy of Medical Sciences A.A. Baranova. M., 2005. pp. 38–39. 5. Intestinal diseases. Directory for practicing doctors "Remedium Doctor". M.: LLC Publishing House "Remedium". pp. 74–76. 6. State register of medicines. M.: MZiSR (Internet version www.drugreg.ru). 7. Nizhevich A.A., Khasanov R.Sh., Nurtdinova N.M., Ochilova R.A., Loginovskaya V.V., Kalmetyeva L.R. Antibiotic-associated intestinal dysbiosis in children // RMZh. 2007. No. 1. pp. 12–15. 8. Shcherbakov P.L., Tsvetkov P.M., Nechaeva L.V. Prevention of diarrhea associated with taking antibiotics in children // Issues of modern pediatrics. 2004. T. 3. No. 2. 9. Korman D.B. Basics of antitumor chemotherapy. M.: Practical Medicine, 2006. 10. Zelenin K.N. The emergence and development of chemotherapy. 11. Ursova N.I. Intestinal dysbiosis in children: a guide for practitioners / ed. G.V. Rimarchuk. M.: BORGES Company, 2006. 12. Larcini D., Parenti F. Antibiotics / trans. from English Yu.V. Angelica. M.: Mir, 1985. 13. Clinical and immunological effectiveness of immunobiological preparations / ed. M.P. Kostinova and I.V. Medunitsyna. M.: Miklos, 2004. pp. 195–206. 14. Stent G. Molecular biology of bacterial viruses / trans. from English M., 1965. 15. Hayes W. Genetics of bacteria and bacteriophages / trans. from English M., 1965. 16. Schlegel G. General microbiology / trans. with him. M., 1987. P. 142.
Application of bacteriophages
Immediately after the discovery of bacteriophages, drugs based on them began to be used to combat human infectious diseases. However, as a result of the invention of antibiotics and lack of knowledge about bacteriophages, their therapeutic potential was not realized.
Half a century later, molecular biologists became interested in bacteriophages. They found that these simple “nanodevices” with short genetic programs are convenient objects for experimental studies to study the structure and operation of the genome. Further study of phages and the mechanisms by which bacteria protect themselves from enemies has revealed to science one of the most effective genome editing tools - CRISPR-CAS, based on the “bacterial immunity” system.
Phages have found application in various areas of human activity, including bio- and nanotechnology. For example, as simple systems for producing proteins with given properties or as a basis for creating materials with a given architecture in catalytic chemistry.
As “smart” molecular devices, they are used to transport drugs in the body and as diagnostic sensors, for example, to detect pathogenic bacteria in food. Phage preparations are used for disinfection in agriculture and the food industry. This increases the environmental friendliness of the products.
But still, medicine, like a century ago, remains the main area of application of these bacterial enemies. With the increase in drug resistance of bacteria to chemical antibiotics, the importance of phage therapy for the prevention and treatment of human infectious diseases has increased.
Phage antibiotics
Phages do not need to be used directly for therapeutic purposes. Over millions of years of evolution, bacteriophages have developed an arsenal of specific proteins - tools for recognizing target microorganisms and manipulating the biopolymers of the victim, on the basis of which antibacterial drugs can be created. The most promising proteins of this type are endolysin enzymes, which phages use to destroy the cell wall when leaving the bacterium. These substances themselves are powerful antibacterial agents that are non-toxic to humans. The effectiveness and direction of their action can be increased by changing their addressing structures - proteins that specifically bind to certain bacteria.
Most bacteria are divided according to the structure of their cell wall into gram-positive, whose membrane is covered with a very thick layer of peptidoglycan, and gram-negative, in which a layer of peptidoglycan is located between two membranes. The use of natural endolysins is especially effective in the case of gram-positive bacteria (staphylococci, streptococci, etc.), since their peptidoglycan layer is located on the outside. Gram-negative bacteria (Pseudomonas aeruginosa, Salmonella, Escherichia coli, etc.) are a less accessible target, since the enzyme must penetrate the outer bacterial membrane to reach the inner peptidoglycan layer.
To overcome this problem, so-called artilisins were created - modified versions of natural endolysins containing polycationic or amphipathic peptides that destabilize the outer membrane and ensure the delivery of endolysin directly to the peptidoglycan layer. Artilisins have high bactericidal activity and have already shown their effectiveness in the treatment of otitis media in dogs (Briers et al., 2014).
An example of a modified endolysin that selectively acts on certain bacteria is the drug P128 from the Canadian company GangaGen Inc.
. It is a biologically active fragment of endolysin combined with lysostaphin, a targeting protein molecule that binds to the surface of staphylococcal cells. The resulting chimeric protein has high activity against various strains of staphylococcus, including those with multidrug resistance.
Modern history
To date, specialists from Tbilisi and the specialized center of the Institute of Immunology and Experimental Therapy named after. L. Hirschfeld (Wroclaw, Poland), where bacteriophage preparations for testing are produced in small quantities.
Polish researchers initially focused on personalized therapy. They used phage therapy to experimentally treat patients with chronic diseases that did not respond to antibiotics. Thousands of patients have already passed through the center, many of whom were completely cured.
The results of these clinical trials have proven the high effectiveness of phages in the treatment of infectious pulmonary diseases: a single intranasal administration of the drug is sufficient to suppress infection in the throat, nose and lungs. Phages are no less effective in eliminating pathogenic bacteria from the gastrointestinal tract. The high efficiency of bacteriophages has also been demonstrated in almost all cases of pyogenic diabetic foot ulcers, lung diseases, mastitis, and urogenital infections. The list of such diseases goes on, but it is important to note that none of the trials observed any side effects caused by bacteriophages.
As specific agents that destroy bacteria, bacteriophages today are used in the treatment of diseases not only in humans, but also in animals, as well as for plant protection and food preservation. Thus, in 2006, the FDA approved the use of bacteriophage cocktails for processing meat and other agricultural products. In this case, phages received the status of food additives. They have also been approved for use as a disinfectant. Phage preparations (in the form of aerosols) have been successfully tested in experiments to protect poultry on large farms, as well as in fish farms
In England, phage preparations were successfully tested for the treatment of chronic otitis media, a difficult-to-treat disease due to the formation of so-called bacterial biofilms - drug-resistant microbial films. In France, the cradle of phage therapy, research in this area is now almost non-existent, although until recently the Pasteur Institute made phage cocktails to order.
Today, phage preparations are produced on an industrial scale by the Russian company Microgen. Similar medications can be bought in pharmacies in Russia, Belarus and Ukraine. Phage preparations produced by Microgen and the Tbilisi Center for the treatment of burn infections were successfully tested in Belgium.
However, the use of bacteriophages in therapy is still not officially permitted in most countries: this applies to both the FDA, the American Food and Drug Administration, and similar European agencies. In the European Union, phages are used to treat patients only at the aforementioned Polish Institute of Immunology and Experimental Therapy.
Therefore, treatment of interested patients is carried out in the mode of medical tourism. (California, USA) refers patients from different countries suffering from chronic diseases caused by drug-resistant bacteria either to the Phage Therapy Center in Tbilisi or to its clinic in Mexico.