Pyobacteriophage complex
Treatment of purulent-inflammatory diseases with localized lesions should be carried out simultaneously both locally and through the mouth, for 7-20 days (according to clinical indications).
Depending on the nature of the source of infection, the bacteriophage is used:
1. Locally in the form of irrigation, lotions and tamponing with liquid phage in an amount of up to 200 ml, depending on the size of the affected area. For abscesses, the bacteriophage is injected into the cavity of the lesion after removing the pus using a puncture. The amount of the administered drug should be slightly less than the volume of removed pus. In case of osteomyelitis, after appropriate surgical treatment, 10-20 ml of bacteriophage is poured into the wound.
2. Introduction into cavities - pleural, articular and other limited cavities of up to 100 ml of bacteriophage, after which capillary drainage is left, through which the bacteriophage is reintroduced over several days.
3. For cystitis, pyelonephritis, urethritis, the drug is taken orally. If the cavity of the bladder or renal pelvis is drained, the bacteriophage is injected through the cystostomy or nephrostomy 1-2 times a day, 20-50 ml into the bladder and 5-7 ml into the renal pelvis.
4. For purulent-inflammatory gynecological diseases, the drug is administered into the cavity of the vagina and uterus in a dose of 5-10 ml once daily.
5. For purulent-inflammatory diseases of the ear, throat, nose, the drug is administered in a dose of 2-10 ml 1-3 times a day. The bacteriophage is used for rinsing, washing, instillation, and introducing moistened turundas (leaving them for 1 hour).
6. For conjunctivitis and keratoconjunctivitis, the drug is instilled 2-3 drops 4-5 times a day, for a purulent corneal ulcer - 4-5 drops, for purulent iridocyclitis, the drug is used 6-8 drops every 3 hours in combination with oral administration.
7. In the treatment of stomatitis and chronic generalized periodontitis, the drug is used in the form of rinses in the mouth 3-4 times a day in a dose of 10-20 ml, as well as by introducing turundas impregnated with pyobacteriophage into the periodontal pockets for 5-10 minutes.
8. For intestinal forms of the disease, diseases of internal organs, dysbacteriosis, the bacteriophage is used orally and in an enema. The bacteriophage is given orally 3 times a day on an empty stomach 1 hour before meals. In the form of enemas, they are prescribed once a day instead of once taken by mouth.
Recommended dosage of the drug
| Age | Dose for 1 dose (in ml) | |
| through the mouth | in an enema | |
| Up to 6 months | 5 | 10 |
| From 6 months to 1 year | 10 | 20 |
| From 1 year to 3 years | 15 | 30 |
| From 3 to 8 years | 20 | 40 |
| From 8 years and older | 30 | 50 |
The use of bacteriophages does not exclude the use of other antibacterial drugs. If chemical antiseptics were used to treat wounds before using the bacteriophage, the wound should be thoroughly washed with a sterile 0.9% sodium chloride solution.
Use of bacteriophage in children (up to 6 months)
For sepsis and enterocolitis in newborns, including premature babies, the bacteriophage is used in the form of high enemas (through a gas tube or catheter) 2-3 times a day (see table). In the absence of vomiting and regurgitation, it is possible to use the drug by mouth. In this case, it is mixed with breast milk. A combination of rectal (in enemas) and oral (by mouth) use of the drug is possible. The course of treatment is 5-15 days.
In case of recurrent course of the disease, repeated courses of treatment are possible.
In order to prevent sepsis and enterocolitis during intrauterine infection or the risk of nosocomial infection in newborns, the bacteriophage is used in the form of enemas 2 times a day for 5-7 days.
In the treatment of omphalitis, pyoderma, and infected wounds, the drug is used in the form of applications twice daily (a gauze pad is moistened with a bacteriophage and applied to the umbilical wound or to the affected area of the skin).
Bacteriophage Sextaphage pyobacteriophage polyvalent liquid 20ml 4 bottles
Method of administration and dosage: Treatment of purulent-inflammatory diseases with localized lesions should be carried out simultaneously both locally and by taking the drug orally for 7-20 days (according to clinical indications).
Depending on the nature of the source of infection, the bacteriophage is used:
1. Locally in the form of irrigation, lotions and tamponing with liquid phage in an amount of up to 200 ml, depending on the size of the affected area. For abscesses, the bacteriophage is injected into the cavity of the lesion after removing the pus using a puncture. The amount of the administered drug should be slightly less than the volume of removed pus. In case of osteomyelitis, after appropriate surgical treatment, 10-20 ml of bacteriophage is poured into the wound.
2.Introduction into cavities - pleural, articular and other limited cavities of up to 100 ml of bacteriophage, after which capillary drainage is left, through which the bacteriophage is reintroduced over several days.
3. For cystitis, pyelonephritis, urethritis, the drug is taken orally. If the cavity of the bladder or renal pelvis is drained, the bacteriophage is administered through a cystostomy or nsphrostomy 1-2 times a day, 20-50 ml into the bladder and 5-7 ml into the renal pelvis.
4. For purulent-inflammatory gynecological diseases, the drug is administered into the cavity of the vagina and uterus in a dose of 5-10 ml once daily.
5. For purulent-inflammatory diseases of the ear, throat, nose, the drug is administered in a dose of 2-10 ml 1-3 times a day. The bacteriophage is used for rinsing, washing, instilling, introducing moistened turundas (leaving them for 1 hour).
6. For conjunctivitis and keratoconjunctivitis, the drug is instilled 2-3 drops 4-5 times a day, for corneal ulcers - 4-5 drops, for purulent iridocyclitis, the drug is used 6-8 drops every 3 hours in combination with oral administration.
7. In the treatment of stomatitis and chronic generalized periodontitis, the drug is used in the form of rinses in the mouth 3-4 times a day in a dose of 10-20 ml, as well as by introducing turundas impregnated with iiobacteriophage into the periodontal pockets for 5-10 minutes.
8. For intestinal forms of the disease, diseases of internal organs, dysbacteriosis, the bacteriophage is used orally and in the form of enemas for 7-20 days. The bacteriophage is given orally 3 times a day on an empty stomach 1 hour before meals. In the form of enemas, they are prescribed once a day instead of once taken by mouth.
Recommended dosages of the drug Patient's age Dose per 1 dose (ml) Orally In enema 0 - 6 months. 5 10 6 - 12 months. 10 20 From 1 year to 3 years 15 20-30 From 3 to 8 years 20 30-40 From 8 years and older 20-30 40-50
If chemical antiseptics were used to treat wounds before using the bacteriophage, the wound should be thoroughly washed with a sterile 0.9% sodium chloride solution.
Use of bacteriophage in children (up to 6 months). For sepsis and enterocolitis in newborns, including premature babies, the bacteriophage is used in the form of high enemas (through a gas tube or catheter) 2-3 times a day (see table). In the absence of vomiting and regurgitation, it is possible to use the drug by mouth. In this case, it is mixed with breast milk. A combination of rectal (in enemas) and oral (by mouth) use of the drug is possible. The course of treatment is 5-15 days. In case of recurrent course of the disease, repeated courses of treatment are possible. In order to prevent sepsis and enterocolitis during intrauterine infection or the risk of nosocomial infection in newborns, the bacteriophage is used in the form of enemas 2 times a day for 5-7 days.
In the treatment of omphalitis, pyoderma, and infected wounds, the drug is used in the form of applications twice daily (a gauze pad is moistened with a bacteriophage and applied to the umbilical wound or to the affected area of the skin).
Today, one of the pressing problems of modern medical science is antibiotic resistance of pathogenic microorganisms. This circumstance determines the recurrent course of pyoderma, torpidity to traditionally used drugs. At the same time, the increased virulence of microorganisms, coupled with a violation of the barrier function of the skin, which is observed in a number of common chronic dermatoses (for example, atopic dermatitis), creates conditions for secondary infection of the lesions, which leads to the development of a “vicious” circle and the chronicization of the process. Thus, expanding the arsenal of agents that have an antibacterial effect against pathogenic bacteria that have etiological significance both in pyoderma and in secondary infected dermatoses is very relevant. Such alternative drugs include bacteriophages.
The main conditions necessary for the development of pyoderma and secondary infection of foci of chronic dermatoses include the presence of an “entry gate” (impaired skin barrier function), a decrease in human immune reactivity and nonspecific resistance, and sufficient virulence of the pathogen itself.
The barrier function of the skin is ensured by many factors: first of all, the mechanical protection of the stratum corneum and granular layer, the acidic pH of 5.5 on the surface of the skin, the antibacterial properties of sebum, the antibiotic properties of normal microflora, the factors of innate and adaptive immunity. Violations in any of the listed links can lead to the development of skin infections.
Another predictor of the occurrence of pyoderma and contamination of lesions with pathological bacteria in inflammatory skin diseases is a violation of local and/or general immunity. In cases with pyoderma, disturbances in the adaptive immune system prevail, largely due to the presence of concomitant somatic pathology (diabetes mellitus, metabolic syndrome, endocrinopathies, immunodeficiency states, etc.). The addition of a secondary infection in dermatoses is mainly due to disturbances in the innate immunity of the skin itself, and immune-mediated inflammation causes disturbances in the barrier function of the skin.
However, one of the main predictors of the development of bacterial infection is still the virulence of bacteria, which is ensured by the following factors:
1. the presence of a microcapsule that protects bacteria from absorption by phagocytes;
2. cell wall components that stimulate the development of inflammatory reactions, enhance the synthesis of IL-1 by macrophages, activate the complement system and are powerful chemoattractants for neutrophils;
3. enzymes produced by bacteria that destroy β-lactam antibiotic molecules facilitate the adhesion and penetration of microorganisms into tissues.
In the development of pustular skin diseases, the leading role belongs to staphylococci and streptococci, although other microorganisms can also cause a purulent process: Staphylococcus aureus
occurs in 85-90%;
Streptococcus pyogenes
(groups A, C, G) - in 10%;
St.
epidermidis at 5%.
The etiological significance in the development of secondary infection in eczema, atopic dermatitis and dermatophytoses is primarily Staphylococcus aureus and/or group A streptococci, and in chronic ulcers (varicose, traumatic) - Escherichia coli, Proteus, Pseudomonas, Bacteroides, Clostridium perfringens
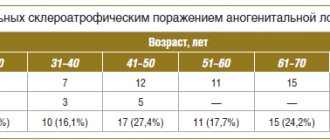
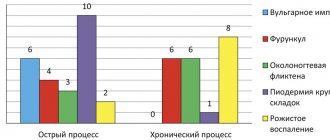
(see figure ).
The main causative agents of pyoderma.
The principles of therapy for infectious (bacterial) skin diseases include the use of complex methods of etiopathogenetic therapy, primarily aimed at eliminating the pathogen. At the same time, the main drugs still remain antibiotics, the range of effective drugs of which tends to decrease, which is associated with a global increase in the resistance of microorganisms to antibacterial drugs [1]. This problem is designated by WHO as the most urgent, since it can lead to the fact that most infectious diseases can get out of control [2, 4-6].
The main reasons for the development of antibiotic resistance
in bacteria are:
- lack of a structure on which the antibiotic acts (for example, bacteria of the genus Mycoplasma are insensitive to penicillin because they do not have a cell wall);
- impermeability to antibiotics (most gram-negative bacteria are immune to penicillin G, since the cell wall is protected by an additional membrane);
- the ability of a microorganism to convert an antibiotic into an inactive form (many staphylococci contain the enzyme β-lactamase, which destroys the β-lactam ring of most penicillins);
- gene mutations, as a result of which the metabolism of the microorganism is changed in such a way that the reactions blocked by the antibiotic are no longer critical for the life of the microorganism;
- the ability of a microorganism to “pump” an antibiotic out of a cell.
To date, resistance to a number of antibiotics has already developed in such causative agents of skin infections as Staphylococcus aureus
(MRSA - methicillin-resistant
S. aureus
),
Escherichia coli
and
Klebsiella pneumoniae
, producing broad and extended spectrum β-lactamases,
Pseudomonas aeruginosa
and
Acinetobacter baumannii
, resistant to carbapenems,
Enterococcus faecium
and
Enterococcus faecalis
(VRE - vancomycin-resistant enterococci) and a number of other microorganisms [2].
In this regard, one of the primary tasks of modern medical science is the development and use of additional means of combating pathogenic bacterial microorganisms, which can be bacteriophages.
Bacteriophage - “bacteria eater” (phagos - “I devour”, Greek) - belongs to a group of viruses that selectively absorbs bacteria. In nature, bacteriophages are widespread and are natural limiters of the spread of bacteria, while phages are characterized by high specificity for a certain type of bacteria. Despite the fact that the history of active study of the effectiveness of phages in the treatment of bacterial infections dates back to the beginning of the twentieth century, after the widespread introduction of antibiotics into clinical practice due to their greater effectiveness, at that time interest in phages somewhat weakened, and they faded into the background. However, today, in light of the problem of antibiotic resistance, interest in them is growing again.
It should be noted that the use of bacteriophages, like any other antibacterial drugs, should be based on the principles of evidence-based medicine. The main condition for the effectiveness of bacteriophages should be their sufficiently high virulence against etiologically significant bacteria. Thus, bacteriophages are tested in laboratory conditions to determine whether their lytic activity is sufficient, after which they can be recommended for use in clinical practice [3, 7–9].
In order for a bacteriophage to be recommended for use in clinical practice, it must comply with the following parameters:
— high virulence, causing complete lysis of bacteria;
— preservation of activity in the host cell;
— possibility of long-term storage while maintaining lytic activity;
— lack of activity against representatives of the resident microbiota.
The interaction of a phage with a target cell occurs in several stages and ends with the lysis of the bacterial cell, the reproduction of new full-fledged phages and their release into the environment. Therefore, it is very important that the lysis is complete (complete), and this aspect can only be provided by highly virulent bacteriophages.
Insufficient lysis activity of phages can lead to the appearance of virulence genes in bacteria, which, for example, in cases of hospital infection contributes to the epidemic spread of infection of clonal lines of bacteria. Therefore, the use of non-virulent or moderately virulent bacteriophages is unacceptable, and microbiological control is necessary when using phages for medicinal purposes.
There are certain rules for using bacteriophages in practice. Before prescribing the drug, it is necessary to assess the spectrum and degree of its virulence to resolve the issue of the sensitivity of the pathogen to it, since in some cases lytic inertness of phages may be observed, which may be associated with a narrow spectrum of lytic activity of the bacteriophage itself or with atypical properties of the bacterial culture. Based on the results of a bacteriological study, a conclusion is made about the presence or absence of sensitivity of a certain microorganism isolated from the biomaterial of a particular patient to a given bacteriophage.
Modern bacteriophage preparations are a complex of polyclonal highly virulent bacterial viruses, specially selected against the most common groups of pathogens of bacterial infections. Bacteriophages are produced in the form of tablets, solutions, and gels. Numerous studies have proven comparable, and in some cases even superior, effectiveness of phages to antibiotics against infections caused by antibiotic-resistant pathogens, while bacteriophages do not cause adverse toxic and allergic reactions and have no contraindications [10-12]. In addition to the antibacterial effect, bacteriophages increase the adaptive capabilities of the body, positively influencing the factors of specific and nonspecific immunity, which can be especially valuable in the treatment of immune-mediated inflammatory skin diseases complicated by secondary infection. Equally important is the speed of their action and the depth of penetration, which is a distinctive feature of phages [13].
Today, bacteriophage preparations in the form of mono- and combined preparations are produced in Russia by two enterprises: FSUE NPO Microgen of the Russian Ministry of Health (liquid and tablet forms) and LLC NPC MicroMir (gels).
One of the first combined therapeutic and prophylactic preparations of bacteriophages that appeared in the arsenal of doctors is “Piobacteriophage polyvalent” (produced by NPO Microgen). The high therapeutic and preventive effectiveness of pyobacteriophage has been proven in numerous clinical studies. Thus, in the work of I.N. Khairullina et al. reported the successful use of pyobacteriophage in surgical practice in patients with infection in the surgical area, when the use of phage reduced the healing time of a wound defect by more than 2 times [14]. According to L.P. Zueva et al. [15], pyobacteriophage causes not only a high therapeutic effect against bacterial infections, but also has preventive potential, which was shown by the example of the frequency of nosocomial infections with Pseudomonas aeruginosa infection, which decreased by 5 times.
As described above, one of the factors in the development of antibiotic resistance is the formation of biofilms by bacteria. There is evidence of the destructive effect of phages on biofilm, which may be another predictor of the effectiveness of combined techniques using bacteriophages [16, 17].
Of course, rational treatment regimens for pyoderma and secondarily infected dermatoses, as well as the prevention of recurrence of the process, should include specific antibacterial methods using drugs whose action is aimed at suppressing pathogenic microorganisms and restoring normal skin microbiota [18, 19]. In dermatology, the most promising and in demand due to the etiological significance of these pathogens are bacteriophages that are active against staphylococci and streptococci. Such bacteriophages include Sextaphage
(polyvalent pyobacteriophage) (FSUE NPO Microgen, Ministry of Health of Russia).
This drug is a sterile filtrate of phagolysates of bacteria Staphylococcus, Streptococcus, Proteus
(
P. vulgaris, P. mirabilis
),
Pseudomonas aeruginosa
, enteropathogenic
Escherichia coli, Klebsiella pneumoniae
.
Sextaphage
indicated for the treatment of various clinical types of pyoderma, including deep ones, as it is characterized by deep penetration and high bioavailability.
Sextaphage
can be used in a complex of therapeutic measures or as monotherapy, for example, for single elements of superficial pyoderma.
An important advantage of pyobacteriophage is the possibility of its administration per os
and externally, which, of course, helps to increase therapeutic effectiveness due to the general effect on the state of the body's microbiota. Locally, the drug can be used in the form of irrigation and lotions. In case of deep pyoderma, the bacteriophage is punctured into the cavity of the lesion after removal of the pus, but it must be remembered that the amount of the injected drug should be slightly less than the volume of the removed pus.
Prophylactic use of the drug Sextafag
indicated for patients with recurrent pyoderma, which is most typical for patients with reduced immune reactivity (diabetes mellitus, metabolic syndrome, endocrinopathies).
With regard to inflammatory skin diseases, when contamination with pathological bacteria not only complicates the course of dermatosis, but can also be one of the pathogenetic links, the use of a polyvalent bacteriophage is indicated from various positions. A classic example: atopic dermatitis, in which S. аureus
and its toxins, acting as superantigens, so lysis of these bacteria leads to clinical remission. Thus, in chronic dermatoses complicated by secondary infection, the feasibility of using polyvalent pyobacteriophage is determined by the pathogenetic direction of the drug’s action, and the absence of contraindications and side effects allows it to be recommended to pediatric patients and pregnant women, which is especially important when the arsenal of therapeutic agents is extremely limited.
In case of pyoderma (especially in chronic diseases), the preventive use of bacteriophages and the possibility of external use are of particular importance, which opens up broad prospects for dermatological practice. Gel for external use Fagoderm
(LLC NPC "MikroMir") allows for prevention with bacteriophages.
The gel contains a complex consisting of 47 types of virulent bacteriophages, differing in morphological structure and receptor specificity, active against the vast majority of pathogenic and opportunistic microorganisms that cause bacterial infections of the skin, while Fagoderm is characterized by tolerance
towards resistant microflora of the skin biota. There is already evidence of the effectiveness of its use in dermatology for bacterial skin infections [20, 21].
Results of clinical trials of Fagoderma
showed a pronounced anti-inflammatory and wound-healing effect of the gel. The use of the gel for acne contributed to the elimination of pathogens on the treated surface [21], the sanitation of the inflammation site from pathogens in 45% of cases, and the reduction of the number of pathogens to the level of normal skin flora in 55% of cases [20].
Gel Fagoderm
is especially effective as a prophylactic agent in patients with recurrent pyoderma, as well as for the prevention of infection of lesions in chronic dermatoses, after various cosmetic and surgical procedures, when there is a risk of contamination with pathogenic bacteria.
Thus, today one of the promising areas of modern dermatovenerology can be considered the use of systemic and topical bacteriophages, which determines the relevance of this therapy both for pyoderma and for secondary infected dermatoses. The high effectiveness of the drugs, pathogenetic orientation, lack of toxicity and side effects determine the feasibility of using these drugs in the complex treatment of patients with pyoderma and chronic dermatoses, in which contamination with pathogenic microorganisms is pathogenetically significant.