Symbicort Turbuhaler por. 160mcg+4.5mcg/dose-120d d/ing
Method of administration and dosage Bronchial asthma Symbicort Turbuhaler is not intended for the initial treatment of intermittent and mild persistent bronchial asthma. The dose of the drugs included in Symbicort is selected individually and depending on the severity of the disease. This must be taken into account not only when starting treatment with combination drugs, but also when changing the maintenance dose of the drug. In the event that individual patients require a different combination of doses of active components than in Symbicort Turbuhaler, α2-adrenergic agonists and/or glucocorticosteroids should be prescribed in separate inhalers. The dose should be reduced to the lowest dose that maintains optimal control of asthma symptoms. Patients should be under constant medical supervision to ensure adequate dosage adjustment of Symbicort Turbuhaler. Once complete control over the symptoms of bronchial asthma is achieved on the background of the minimum recommended dose of the drug, at the next stage you can try prescribing monotherapy with inhaled glucocorticosteroids. There are two approaches to prescribing therapy with Symbicort Turbuhaler: A. Symbicort Turbuhaler as maintenance therapy: Symbicort Turbuhaler is prescribed for continuous maintenance therapy in combination with a separate short-acting beta2-agonist agonist to relieve attacks. B. Symbicort Turbuhaler as maintenance therapy and for the relief of attacks: Symbicort Turbuhaler is prescribed both for continuous maintenance therapy and on demand when symptoms appear. A. Symbicort Turbuhaler as maintenance therapy The patient must always have with him a separate inhaler with a short-acting beta2-adrenergic stimulant to relieve attacks. Adults (18 years and older): Symbicort Turbuhaler 80/4.5 mcg/dose and 160/4.5 mcg/dose: 1-2 inhalations twice daily. If necessary, the dose can be increased to 4 inhalations twice a day. Adolescents (12-17 years): Symbicort Turbuhaler 80/4.5 mcg/dose and 160/4.5 mcg/dose: 1-2 inhalations twice a day. Children over 6 years of age: Symbicort Turbuhaler 80/4.5 mcg/dose: 1-2 inhalations twice a day. After achieving optimal control of asthma symptoms with twice daily dosing, it is recommended to titrate the dose to the lowest effective dose, up to once daily dosing in cases where, in the opinion of the physician, the patient requires maintenance therapy in combination with a long-term bronchodilator actions. An increase in the frequency of use of short-acting beta2-agonists is an indicator of deterioration in overall disease control and requires a review of anti-asthmatic therapy. B. Symbicort Turbuhaler as maintenance therapy and for the relief of attacks Symbicort Turbuhaler can be prescribed both as continuous maintenance therapy and as on-demand therapy when attacks occur. The patient must always have Symbicort with him to relieve attacks. Symbicort as a maintenance therapy and for the relief of attacks is especially indicated for patients with: • insufficient control of bronchial asthma and the need for frequent use of drugs to relieve attacks; • a history of exacerbations of bronchial asthma that required medical intervention. Careful monitoring of dose-related side effects is required in patients using large numbers of inhalations to relieve attacks. Adults (18 years and older): Symbicort Turbuhaler 80/4.5 mcg/dose and 160/4.5 mcg/dose: recommended dose for maintenance therapy 2 inhalations per day, taken 1 inhalation in the morning and evening, or 2 inhalations once only in the morning or only in the evening. For some patients, a maintenance dose of Symbicort Turbuhaler 160/4.5 mcg/dose 2 inhalations twice a day may be prescribed. If symptoms occur, 1 additional inhalation is necessary. With a further increase in symptoms within a few minutes, 1 additional inhalation is prescribed, but no more than 6 inhalations to relieve 1 attack. Usually it is not necessary to prescribe more than 8 inhalations per day, but you can increase the number of inhalations to 12 per day for a short time. Patients receiving more than 8 inhalations per day are advised to seek medical help to review therapy. Children and adolescents under 18 years of age: Symbicort Turbuhaler is not recommended for children and adolescents as maintenance therapy and for the relief of attacks.
COPD Adults: 2 inhalations of Symbicort Turbuhaler 160/4.5 mcg/dose twice daily. Special groups of patients: there is no need for special selection of the drug dose for elderly patients. There is no data on the use of Symbicort in patients with renal or hepatic impairment. Since budesonide and formoterol are primarily eliminated by hepatic metabolism, a slower rate of elimination of the drug can be expected in patients with severe cirrhosis. Children under 6 years of age: Symbicort Turbuhaler is not recommended for children under 6 years of age.
Instructions for correct use of Turbuhaler:
The mechanism of action of Turbuhaler: when inhaled by the patient through the mouthpiece, the drug enters the respiratory tract. It is necessary to instruct the patient: • carefully study the instructions for use of Turbuhaler • inhale strongly and deeply through the mouthpiece to ensure that the optimal dose of the drug enters the lungs • never exhale through the mouthpiece • rinse the mouth with water after inhaling maintenance doses to reduce the risk of developing candidiasis of the oral mucosa and throats. It is also necessary to rinse your mouth with water after inhalation to relieve symptoms in case of candidiasis of the oral mucosa and pharynx. The patient may not taste or feel the drug after using Turbuhaler, which is due to the small amount of the substance delivered.
Symbicort® Turbuhaler®
Bronchial asthma
Symbicort Turbuhaler is not intended for the initial treatment of intermittent and mild persistent bronchial asthma. The dose of the drugs included in Symbicort is selected individually and depending on the severity of the disease. This must be taken into account not only when starting treatment with combination drugs, but also when changing the maintenance dose of the drug. In the event that individual patients require a different combination of doses of active components than in Symbicort Turbuhaler, β2-adrenergic agonists and/or glucocorticosteroids should be prescribed in separate inhalers.
Patients should visit their doctor regularly to monitor the optimal dose of Symbicort Turbuhaler. The dose should be reduced to the lowest dose that maintains optimal control of asthma symptoms. Once optimal control of asthma is achieved with twice daily dosing, it is recommended to titrate the dose to the lowest effective dose, up to once daily dosing, in cases where, in the opinion of the physician, the patient requires maintenance therapy in combination with a long-acting bronchodilator . Adults (18 years and older): Symbicort Turbuhaler 320/9 mcg/dose: 1 inhalation twice daily. If necessary, the dose can be increased to 2 inhalations twice a day. After achieving optimal control of asthma symptoms while taking the drug twice a day, it is possible to reduce the dose to the lowest effective dose, up to once a day. Adolescents (12-17 years): Symbicort Turbuhaler 320/9 mcg/dose: 1 inhalation twice daily. Children under 12 years of age: Symbicort Turbuhaler 320/9 mcg/dose is not recommended for children under 12 years of age due to the lack of clinical data. Symbicort Turbuhaler 320/9 mcg/dose is intended for maintenance therapy only. COPD Adults: 1 inhalation of Symbicort Turbuhaler 320/9 mcg/dose twice a day. Special groups of patients: there is no need for special selection of the drug dose for elderly patients. There is no data on the use of Symbicort Turbuhaler 320/9 mcg/dose in patients with renal or hepatic impairment. Since budesonide and formoterol are eliminated primarily by the kidneys, with the participation of hepatic metabolism, a slower rate of elimination of the drug can be expected in patients with severe cirrhosis.
Instructions for correct use of Turbuhaler:
The mechanism of action of Turbuhaler: when inhaled by the patient through the mouthpiece, the drug enters the respiratory tract. The patient must be instructed:
- carefully study the instructions for use of Turbuhaler
-inhale strongly and deeply through the mouthpiece to ensure that the optimal dose of the drug reaches the lungs
-never exhale through the mouthpiece
- rinse your mouth with water after inhaling maintenance doses to reduce the risk of developing candidiasis of the oral mucosa and pharynx. It is also necessary to rinse your mouth with water after inhalation to relieve symptoms in case of candidiasis of the oral mucosa and pharynx.
The patient may not taste or feel the drug after using Turbuhaler, which is due to the small amount of the substance delivered.
INSTRUCTIONS FOR USE OF TURBUHALER
Turbuhaler is a multi-dose inhaler that allows you to dose and inhale the drug in very small doses (Fig. 1).
When you inhale, Turbuhaler powder is delivered to your lungs. Therefore, it is important that you take a strong and deep breath
through the mouthpiece.
Preparing Turbuhaler for first use:
Before the first
using Turbuhaler, it must be prepared for work.
Unscrew and remove the cover.
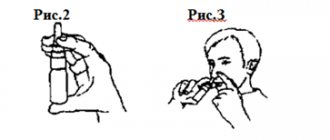
Hold the inhaler vertically with the red dispenser facing down (Fig. 2). Do not hold the inhaler by the mouthpiece when turning the dispenser.
Turn the dispenser all the way in one direction
(no matter clockwise or counterclockwise),
and then also all the way in the opposite direction.
You will hear a click as you turn the dispenser. Follow the described procedure twice.
The inhaler is now ready for use and you do not have to repeat this procedure
preparing Turbuhaler for work before each use. To take the drug, follow the instructions below.
How to use SYMBICORT® TURBUHALER®
To take one dose, follow the procedure described below.
1. Unscrew and remove the cover.
2. Hold the inhaler vertically with the red dispenser facing down (Fig. 2). Do not hold the inhaler by the mouthpiece when turning the dispenser. In order to measure the dose, turn the dispenser all the way in one direction (no matter clockwise or counterclockwise), and then also all the way in the opposite direction. You will hear a click as you turn the dispenser.
3. Exhale. Do not exhale through the mouthpiece.
4. Gently place the mouthpiece between your teeth, purse your lips and inhale forcefully and deeply through your mouth (Figure 3). Do not chew or squeeze the mouthpiece with your teeth.
5. Before exhaling, remove the inhaler from your mouth.
6. If inhalation of more than one dose is required, repeat steps 2-5.
7. Close the inhaler with the cap and check that the inhaler cap is screwed on tightly.
8. Rinse your mouth with water without swallowing.
IMPORTANT!
Do not attempt to remove the mouthpiece as it is attached to the inhaler and cannot be removed.
The Turbuhaler's mouthpiece rotates, but do not turn it unless necessary.
Because the amount of powder inhaled is very small, you may not feel the taste of the powder after inhalation. However, if you followed the instructions, you can be sure that you inhaled (inhaled) the required dose of the drug.
If you mistakenly repeat the procedure for loading the inhaler more than once before taking the drug, you will still receive one dose of the drug when inhaling. While the dose indicator will show the total number of doses measured.
The sound you hear when you shake the inhaler is made by the drying agent, not the medicine.
How do you know when your inhaler needs to be changed?
The dose indicator (Fig. 4) shows the approximate number of doses remaining in the inhaler; the count of doses of filled Turbuhaler begins with the 60th or 120th dose (depending on the total number of doses of Turbuhaler you purchased).
The indicator shows an interval of 10 doses, so it does not show every dispensed (loaded) dose. You can be sure that Turbuhaler delivers the required dose of the drug, even if you do not notice a change in the dose indicator window.
The appearance of a red background in the dose indicator window means that there are 10 doses of the drug left in Turbuhaler. When the number 0 appears on a red background in the middle of the dose indicator window (Fig. 5), the inhaler must be replaced with a new one.
Note that even when the dose indicator window shows 0, the dispenser continues to rotate. However, the dose indicator stops recording the number of doses (stops moving) and the number 0 remains in the dose window of the inhaler.
Cleaning
Clean the outside of the mouthpiece regularly (once a week) with a dry cloth.
Do not use water or other liquids to clean the mouthpiece.
Disposal
Be careful with the used inhaler and remember that some medication may remain inside the inhaler.
Symbicort - a new drug for the treatment of bronchial asthma
About the article
16716
0
Regular issues of "RMZh" No. 12 dated June 17, 2001 p. 503
Category: General articles
For quotation:
Symbicort is a new drug for the treatment of bronchial asthma. RMJ. 2001;12:503.
Currently, when the inflammatory nature of bronchial asthma (BA) is beyond doubt, inhaled glucocorticoids along with b2-agonists occupy a central place in all guidelines for the treatment of this disease. Inhaled glucocorticoids have an anti-inflammatory effect, and b2-agonists relieve bronchospasm, relieving asthma symptoms. Combining long-acting β2-agonists with inhaled glucocorticoids produces greater improvements in pulmonary function and asthma control than doubling the dose of inhaled glucocorticoids. This publication, based on materials from foreign studies, is devoted to the combination drug Symbicort. In a study by Pauwels et al. [1] showed the benefits of adding the long-acting b2-agonist formoterol (Oxis Turbuhaler) to treatment with the inhaled glucocorticoid budesonide (Pulmicort Turbuhaler). Patients receiving formoterol along with budesonide were less likely to experience both severe and mild exacerbations of asthma. In the group of patients who inhaled budesonide at a dose of 200 mcg per day and formoterol (18 mcg/day), the improvement in respiratory function was more pronounced than in patients who inhaled only budesonide at a dose of 800 mcg/day. The combination of budesonide and formoterol generally made it possible to better control the course of asthma, improve the quality of life of patients and reduce the costs of treating exacerbations. In a study by Kips et al. [2] it was shown that the combination of formoterol and budesonide in small doses is not inferior to large doses of budesonide not only in clinical effectiveness, but also in anti-inflammatory effect. Data from these and other studies served as the basis for the creation of a new drug by the pharmaceutical company AstraZeneca - Symbicort. Symbicort is a combination of the glucocorticoid budesonide and the long-acting b2-agonist formoterol in one inhaler (Turbuhaler). Symbicort has potential advantages over the administration of its components through separate inhalation devices and represents a new approach to the treatment of bronchial asthma. One inhalation dose of Symbicort contains 160 or 80 mcg of budesonide and 4.5 mcg of formoterol. The variability of drug dosing (from 1 to 4 inhalations per day) provides flexibility of therapy depending on the severity of asthma and allows you to titrate the dose to the minimum required using the same inhaler. Symbicort has undergone a number of clinical trials abroad. Compared to inhaled corticosteroids alone, Symbicort has been shown to significantly reduce asthma symptoms, the frequency and duration of exacerbations, and improve pulmonary function. Therapy with Symbicort gives the patient 2 additional months a year without daytime and nighttime asthma symptoms compared to budesonide. When comparing treatment with Symbicort with therapy with budesonide and formoterol through separate inhalers, a trend towards a more rapid increase and stabilization of peak flow measurements in the Symbicort group was revealed [5]. In terms of the speed of onset and severity of the bronchodilator effect, Symbicort is superior to the combination of fluticasone (250 mcg) with salmeterol (50 mcg) [4]. Research shows that many asthma patients do not adhere to their doctor's prescribed treatment regimens. Patients take the recommended dose of inhaled glucocorticosteroid for maintenance therapy only 20–73% of the time [8]. One of the reasons why patients do not comply with doctor’s prescriptions is that when symptoms weaken, many stop taking further maintenance therapy. Many patients do not understand the importance of using maintenance medications that, when inhaled, do not provide immediate relief from asthma symptoms. When treated with Symbicort, patients experience immediate relief after inhalation, since the formoterol contained in Symbicort acts as quickly as traditionally used short-acting bronchodilators. The rapid relief of symptoms experienced by the patient may be more likely to motivate their continued use of Symbicort as prescribed; this should ensure regular inhalation of the corticosteroid included in Symbicort. The latter, in turn, ensures control of the disease, which leads to an improvement in the health of asthma patients using Symbicort [5]. Treatment with Symbicort for asthma in children aged 4-17 years is well tolerated and improves pulmonary function more effectively than budesonide monotherapy [6]. The tolerability of Symbicort when the recommended doses are exceeded was studied in patients with asthma who, against the background of regular treatment with Symbicort at a dose of 160/4.5 mcg, 2 inhalations 2 times a day, received an additional 10 doses of Symbicort within 1 hour (total dose 1600/45 mcg) [7]. Although an increase in heart rate characteristic of b2-agonists was noted (by 5.4 beats/min, etc.), this did not threaten the patients’ condition. The drug Symbicort Turbuhaler was approved for use as a treatment for asthma in Europe in 2000. In Russia, the drug has been submitted for registration and its appearance on the Russian pharmaceutical market is planned for the fall of 2001. Literature:
1. Pauwels RA, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. // N Engl J Med, 1997; 337:1405-11.
2. Kips JC, et al. A long-term study of the antiinflammatory effect of low-dose budesonide plus formoterol versus high-dose budesonide in asthma. // Am J Respir Crit Care Med, 2000; 161:996-1001.
3. Ellul-Micallef R., Johansson SA Acute dose-response studies in bronchial asthma with a new corticosteroid, budesonide. // Br J Clin Pharmacol, 1983; 15: 419-22.
4. Palmqvist M., et al. Onset of bronchodilation of budesonide-formoterol vs salmeterol/fluticasone in single inhalers. // Pulmonol Pharmacol Ther, 2001; 14:29-34.
5. Zetterstrom O., et al. Efficacy and safety of a new single inhaler product, containing both budesonide and formoterol, in adult asthma. // Eur Respir J, 2000; 16 (suppl 31): 455s.
6. Tal A., et al. The benefit of a new single inhaler product, containing both budesonide and formoterol, in asthmatic children. // Eur Respir J, 2000; 16 (suppl 31): 384s.
7. Ankerst J., et al. A high-dose of budesonide/formoterol in a single inhaler was well-tolerated by asthmatic patients. // Eur Respir J, 2000; 16 (suppl 31): 33s.
8. Cochrane MG, et al/ Inhaled corticosteroids for asthma therapy – Patient compliance, devices and inhalation technique. // Chest, 2000; 117(2): 542–50
Content is licensed under a Creative Commons Attribution 4.0 International License.
Share the article on social networks
Recommend the article to your colleagues