Phenylketonuria
(PKU) is a hereditary disorder of the metabolism of amino acids, primarily phenylalanine (PA), which is part of proteins. The substance is involved in protein folding and stabilization of protein structures.
The first symptoms: frequent regurgitation, vomiting, eczema, convulsions, and the smell of mold emanating from the urine and skin. The child may be lethargic or, conversely, hyperactive. He lags behind in psychomotor development, there are signs of mental retardation. The diagnosis can be made in the maternity hospital. All children with phenylketonuria certainly receive the status of “disabled child.”
Treatment of the disease involves following a special low-protein diet that does not contain foods with FA.
Definition of disease
Phenylketonuria is a congenital, genetic pathology that involves impaired hydroxylation of phenylalanine. It is characterized by the accumulation of amino acids and the products of its metabolism in the body, which leads to severe damage to the central nervous system. The disease was first described by the Norwegian doctor I. A. Felling in 1934.
While studying the disease, experts found that a single phenylalanine hydroxylase gene is responsible for the presence of the disease. The first successful treatment was developed and carried out in 1950 in England.
There is no clinic in the neonatal period. The pathology manifests itself in the first six months of a child’s life. Subsequent accumulation of the substance leads to severe developmental disorders. Therefore, it is extremely important to identify the defect immediately after birth and prevent the consumption of foods containing phenylalanine. Later adherence to the diet will not eliminate the resulting disorders, but will not allow the development of new ones.
The pathology is equally common among both sexes. No racial characteristics were identified. A large number of patients are in countries such as China, Türkiye, and Ireland. On average, in Russia every 7 thousandth child is born with phenylketonuria.
Story
Phenylketonuria was discovered in 1934 by the Norwegian doctor Ivar Asbjorn Felling. A positive treatment outcome was first observed in the UK (at the Birmingham Hospital for Children) thanks to the efforts of a team of doctors led by Horst Bickel in the first half of the 50s of the 20th century. However, truly great success in the treatment of this disease was noted in 1958-1961, when the first methods of analyzing the blood of infants for the content of high concentrations of phenylalanine, indicating the presence of the disease, appeared.
It turned out that only one gene, called RAS (phenylalanine hydroxylase gene), is responsible for the development of the disease.
Thanks to this discovery, scientists and doctors around the world were able to identify and describe in more detail both the disease itself and its symptoms and forms. Moreover, completely new, high-tech and modern treatment methods were found and developed, such as gene therapy, which today is a model of effective combat against human genetic pathologies.
Causes of phenylketonuria
There are three types of genetic disorder, the first is considered classic, since it is diagnosed in more than 90% of cases. The second and third are a rarer form of pathology. Symptoms are similar in all types, the disease leads to mental retardation. In the classic form of phenylketonuria, this can be avoided by diet therapy, but atypical variants, unfortunately, cannot be corrected.
- Classic phenylketonuria (type I) is a low production of phenylalanine hydroxylase (PAH), which leads to the accumulation of phenylalanine and its breakdown products in natural body fluids. The pathology is caused by a mutated RAS gene.
- Phenylketonuria type II is a deficiency of dihydropteridine reductase, which prevents the conversion of phenylalanine to tyrosine. Pathology due to mutation of the QDPR gene.
- Phenylketonuria type III is a deficiency of 6-pyruvoyltetrahydropterin synthase, necessary for the synthesis of tetrahydrobiopterin. The pathology is caused by a mutated PTS gene.
All forms of the disease are inherited in an autosomal recessive manner. This means that the genetic defect can be inherited from one of the parents. The gender of the parent and child does not play a role.
Phenylketonuria in children and its treatment
Phenylketonuria (PKU) is a genetic disease characterized by disorders of phenylalanine metabolism. Occurs with a frequency of 1 in 8,000–15,000 newborns. There are four forms of PKU; There are over 400 different mutations and several metabolic phenotypes of PKU [1].
Definition, pathogenesis, classification
Phenylketonuria is a hereditary aminoacidopathy associated with impaired phenylalanine metabolism, resulting in mutational blockade of enzymes leading to persistent chronic intoxication and damage to the central nervous system with a pronounced decrease in intelligence and neurological deficit [1, 2].
Of primary importance in the pathogenesis of classical PKU is the inability of phenylalanine hydroxylase to convert phenylalanine to tyrosine. As a result, phenylalanine and the products of its abnormal metabolism (phenylpyruvic, phenylacetic, phenyllactic acids) accumulate in the body [1–3].
Other pathogenetic factors include disturbances in amino acid transport across the blood-brain barrier, disturbances in the cerebral pool of amino acids with subsequent disruption of the synthesis of proteolipid proteins, disturbances in myelination, and low levels of neurotransmitters (serotonin, etc.) [1–4].
Phenylketonuria I (classic or severe) is an autosomal recessive disease caused by a mutation in the phenylalanine hydroxylase gene (long arm of chromosome 12); 12 different haplotypes were identified, of which about 90% of PKU are associated with four haplotypes. The most common mutations in the phenylalanine hydroxylase gene: R408W, R261Q, IVS10 nt 546, Y414C. The disease is based on a deficiency of phenylalanine 4-hydroxylase, which ensures the conversion of phenylalanine to tyrosine, which leads to the accumulation of phenylalanine and its metabolites in tissues and physiological fluids [1–4].
A special group consists of atypical variants of PKU, in which the clinical picture resembles the classical form of the disease, but in terms of development indicators, despite dietary therapy, no positive dynamics are noted. These PKU variants are associated with deficiencies of tetrahydropterin, dehydropterin reductase, 6-pyruvoyltetrahydropterin synthase, guanosine 5-triphosphate cyclohydrolase, etc. [1–4].
Phenylketonuria II (atypical) is an autosomal recessive disease in which the gene defect is localized in the short arm of chromosome 4 (section 4p15.3), characterized by deficiency of dehydropterin reductase, leading to impaired restoration of the active form of tetrahydrobiopterin (cofactor in the hydroxylation of phenylalanine, tyrosine and tryptophan) in combined with a decrease in folate in the blood serum and cerebrospinal fluid. The result is metabolic blocks in the mechanisms of conversion of phenylalanine into tyrosine, as well as precursors of neurotransmitters of the catecholamine and serotonin series (L-dopa, 5-hydroxytryptophan). The disease was described in 1974 [1–4].
Phenylketonuria III (atypical) is an autosomal recessive disease associated with deficiency of 6-pyruvoyltetrahydropterin synthase, which is involved in the synthesis of tetrahydrobiopterin from dihydroneopterin triphosphate (described in 1978). Tetrahydrobiopterin deficiency leads to disorders similar to those in PKU II [1–4].
Primapterinuria is an atypical PKU in children with mild hyperphenylalaninemia, in whom primapterin and some of its derivatives are present in the urine in large quantities in the presence of normal concentrations of neurotransmitter metabolites (homovanillic and 5-hydroxyindoleacetic acids) in the cerebrospinal fluid. The enzymatic defect has not yet been identified [1–4].
Maternal PKU is a disease accompanied by a decrease in the level of intelligence (to the point of mental retardation) among the offspring of women suffering from PKU and not receiving a specialized diet in adulthood. The pathogenesis of maternal PKU has not been studied in detail, but the leading role of chronic intoxication of the fetus with phenylalanine and the products of its abnormal metabolism is assumed [1–4].
R. Koch et al. (2008) in an autopsy of the brain of an infant whose mother had PKU (without adequate control of blood phenylalanine levels), found a number of pathological changes: low brain weight, venticulomegaly, white matter hypoplasia and delayed myelination (without signs of astrocytosis); No chronic changes were found in the gray matter of the brain. It is assumed that disturbances in the development of the white matter of the brain are responsible for the formation of neurological deficits in maternal PKU [5].
For practical purposes, medical genetic centers of the Russian Federation use a conditional classification of PKU based on the levels of phenylalanine in the blood serum: classical (severe or typical) - phenylalanine level above 20 mg% (1200 µmol/l); average - 10.1–20 mg% (600–1200 µmol/l), as well as the level of phenylalanine 8.1–10 mg%, if it is stable against the background of the physiological norm of protein consumption in the diet; mild (hyperphenylalaninemia, not requiring treatment) - phenylalanine level up to 8 mg% (480 µmol/l) [2].
Clinical manifestations and diagnosis
At birth, children with PKU I appear healthy, although they often have a specific habit (blond hair, blue eyes, dry skin). In the absence of timely detection and treatment of the disease, during the first two months of life, they develop frequent and intense vomiting and increased irritability. Between 4 and 9 months, a pronounced lag in psychomotor development becomes apparent [1–4].
Patients are distinguished by a specific (“mouse”) odor of the skin. Severe neurological disorders are rare in them, but features of hyperactivity and autism spectrum disorders are characteristic. In the absence of timely treatment, the IQ level is <50. Convulsive seizures, characteristic of children with severe intellectual deficits, often debut before the age of 18 months (they may disappear spontaneously). At an early age, seizures often take the form of infantile spasms, subsequently transforming into tonic-clonic seizures [1–4].
Among the diagnostic methods (in addition to determining the levels of phenylalanine and tyrosine in the blood), the Felling test, Guthrie test, chromatography, fluorimetry, and search for a mutant gene are used. EEG and MRI studies are widely used [1–5].
EEG reveals abnormalities, mainly in the form of a pattern of hypsarthymia (even in the absence of seizures); Single and multiple foci of spike and polyspike discharges are typical [1–4].
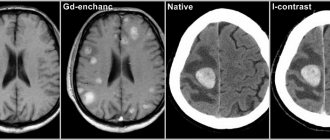
MRI findings are usually abnormal regardless of treatment or lack of treatment for PKU: on T2-weighted images there is increased signal intensity in the periventricular and subcortical white matter of the posterior hemispheres. Although children may have cortical atrophy, there are no detectable signal changes in the brainstem, cerebellum, or cortex. The described changes in MRI studies do not correlate with IQ level, but depend on the level of phenylalanine in the blood [1–4].
With phenylketonuria II, clinical symptoms appear in patients at the beginning of the second year of life. Despite dietary therapy prescribed after detection of elevated levels of phenylalanine in the blood during the neonatal period, a progressive course of the disease is observed. There is severe mental retardation, signs of increased excitability, convulsions, muscular dystonia, hyperreflexia (tendinous) and spastic tetraparesis. Death often occurs by the age of 2–3 years [1–4].
The clinical picture of phenylketonuria III resembles that of PKU II; it includes the following triad of symptoms: profound mental retardation, microcephaly, spastic tetraparesis [1–4].
Prevention
Timely detection of PKU using appropriate screening tests in maternity hospitals, as well as genetic counseling, is necessary. Expectant mothers with PKU are advised to strictly follow a low phenylalanine diet, maintaining phenylalanine levels < 4 mg% (< 242 µmol/L), to prevent fetal damage before conception and throughout pregnancy. The offspring of mothers with mild PKU (phenylalanine < 6.6 mg% or < 400 μmol/L) are not affected [1–4].
New treatments
Currently, several types of alternative therapy for PKU are being intensively developed. Among them: the so-called “large neutral amino acids” method, enzyme therapy with phenylalanine hydroxylase, phenylalanine ammonia lyase; treatment with tetrahydrobiopterin (Sapropterin) [6–11].
There is evidence of successful treatment of patients with moderate or mild PKU using tetrahydrobiopterin (10–20 mg/kg/day) [7, 9, 10].
D. M. Ney et al. (2008) showed that the use of dietary glycomacropeptides in PKU (with limited supply of essential acids) reduces phenylalanine concentrations in the blood plasma and brain, and also promotes adequate physical development [8]. An experimental treatment for PKU is to inject the phenylalanine hydroxylase gene directly into the affected liver cells. In the Russian Federation, these methods are not currently used.
Diet therapy
It is the therapeutic diet that is most effective in preventing intellectual deficits in severe (classical) PKU. The age of the patient at the time of starting diet therapy is of greatest importance (IQ decreases by approximately 4 points for each month from birth to the start of treatment). Approaches to dietary therapy for PKU vary somewhat in different countries, but their principles themselves are consistent [1–4].
Dietary restrictions are not indicated for infants whose blood phenylalanine levels are between 2 and 6 mg% (120 and 360 µmol/L). The basis of the diet for PKU is the prescription of diets low in phenylalanine, the source of which is protein foods. This diet is prescribed to all patients in the first year of life. It should be prescribed to children with diagnosed PKU before 8 weeks of age; its use at a later age is much less effective [1–4].
General characteristics of the diet for PKU. The therapeutic diet for PKU is represented by three main components: medicinal products (mixtures of amino acids without phenylalanine), natural foods (selected), low-protein starch-based products.
Animal products high in protein (meat, poultry, fish, dairy products, etc.) are excluded from the diet for PKU. Breast milk is limited in the first year of life (previously it was completely abolished). Among formulas (breast milk substitutes), preference is given to those containing less protein [1–4].
Diet therapy in the first year of life. Equivalent replacement for protein and phenylalanine is made using the “portion” method of calculation: 50 mg of phenylalanine is equal to 1 g of protein (for adequate replacement of products for protein and phenylalanine). Since phenylalanine is an essential amino acid, a minimum requirement must be met to ensure normal development of a child with PKU. During the first year of life, the permissible amount of phenylalanine ranges from 90 to 35 mg/kg of a child [1–4].
For children with PKU under the age of 12 months, the following medicinal products of foreign and domestic production are currently available in the Russian Federation: Aphenilac (Russian Federation), MD mil PKU-0 (Spain) and HR Analogue LCP (Netherlands-UK).
Diet therapy begins when the level of phenylalanine in the blood is from 360–480 mmol/l and above. It is the indicator of its content in the blood that is considered the main criterion for diagnosis and evaluation of the effectiveness of treatment.
Introduction of complementary foods and additional foods. After three months, the diet begins to expand through the use of juices (fruit and berry), prescribing them from 3-5 drops, with a gradual increase in volume to 30-50 ml, and by the end of the first year of life - up to 100 ml. Basic juices: apple, pear, plum, etc. Fruit purees are prescribed, increasing their amount in the diet in the same way as that of the administered juice [2].
In the period from 4–4.5 months, the first complementary food is introduced into the diet in the form of vegetable puree prepared independently (or canned fruits and vegetables for feeding infants - the latter without the addition of milk). Next, the 2nd complementary food is sequentially prescribed - porridge (10%) from ground sago or protein-free grains. Industrially produced dairy-free porridges based on corn and/or rice flour can be used, containing no more than 1 g of protein per 100 ml of ready-to-eat product.
After 6 months, you can introduce jelly and/or mousses (protein-free), which are prepared using amylopectin swelling starch and fruit juice, a protein-free drink with milk flavor Nutrigen or a low-protein milk drink PKU “Loprofin” into the diet.
From 7 months, a child with PKU can receive low-protein Loprofin products, for example, spirals, spaghetti, rice or protein-free noodles, and from 8 months - special protein-free bread [2].
Diet therapy in children over one year of age. Features of the preparation of therapeutic diets for patients older than 12 months are the use of products based on mixtures of amino acids without phenylalanine and/or protein hydrolysates with a small amount of it (exceeding that in products for children with PKU in the first year of life), which contain complexes of vitamins, macro - and microelements. The proportion of protein equivalent gradually increases as children grow, and the quota of fat and carbohydrate components, on the contrary, decreases (later completely eliminated), which subsequently makes it possible to significantly expand the patient’s diet through selected natural products [1–4].
The amount of phenylalanine that children of different ages are allowed to receive through nutritional intake when following a therapeutic diet gradually decreases from 35 to 10 mg/kg/day [1–4].
In diet therapy for children over one year old, it is customary to use specialized medicinal products (based on mixtures of amino acids without phenylalanine): Tetrafen 30, Tetrafen 40, Tetrafen 70, MD mil PKU-1, MD mil PKU-3 (Spain).
Nutricia products (Netherlands–Great Britain) are distinguished by their special variety and proven quality over the years: P-AM 1, P-AM 2, P-AM 3, Isifen (ready-to-use product), as well as XP Maxamade and XP Maxamum with neutral and orange flavors.
It is recommended to make a gradual transition from a specialized formula (for infants) to products for older children gradually (over 1–2 weeks). In this case, the volume of the previous mixture is reduced by 1/4–1/5 part and an amount of the new product equivalent in protein is added. It is preferable to give the new medicinal product (the amount of which is calculated depending on body weight and age-appropriate amounts of phenylalanine) to the child in small doses, 3-4 times a day, offering to wash it down with juices, water or other drinks [2].
The range of products for children with PKU is significantly limited. During the period of maximum strict adherence to the diet (infancy and early childhood), the use of specialized medicinal products is mandatory. The purpose of their use in PKU is to replace protein sources in full compliance with the standards for consumption of basic nutrients by children (taking into account age and specific clinical situation). Some medicinal products contain polyunsaturated fatty acids (omega-6 and omega-3) in a ratio of 5:1–10:1; such food sources are preferred [1–4].
Among special products, dry amino acid mixtures are used, devoid of phenylalanine, with a subsidy of protein equivalent - its artificial analogue (in quantities corresponding to the age of patients with PKU).
Other low-protein products available in the Russian Federation for dietary therapy of PKU include sago, special bread, vermicelli and other types of therapeutic foods [2, 3]. These medicinal products (amylophens) are based on starches that do not contain difficult-to-digest carbohydrates and minerals. They are represented by pasta, cereals, sago, special flour, bakery products, instant jelly, mousse, etc. Vitamin supplements increase the nutritional value of low protein foods [2].
There are also foreign-made low-protein products, Loprofin (Netherlands-Great Britain), based on starches (wheat, rice, potato, corn, etc.), including pasta, cereals for making porridges, special types of bread (tapioca, wheat and rice starch), cookies, crackers, crackers, as well as flour, various desserts, seasonings and sauces with an attractive taste, a significant range of drinks (including milk, cream and coffee substitutes), etc. [3].
Calculation and preparation of diet. The following formula is used: A = B + C, where A is the total protein requirement, B is the protein of natural food, C is the protein provided by medicinal foods [1–4].
Enrichment of diet with tyrosine. Some researchers suggest supplementing a low-phenylalanine diet with tyrosine, although there is no statistically significant evidence of improved intellectual development with a PKU diet [11].
Organoleptic properties of the diet. The taste properties of almost all artificial medicinal products for patients with PKU are specific. To mask the organoleptically unpleasant qualities of a therapeutic diet for PKU, various flavoring additives (devoid of protein) and special formulations are used. The sweetener aspartame should not be used as it breaks down into phenylalanine, methanol and aspartate [2].
Monitoring the effectiveness of diet therapy. It is based on regular monitoring of the phenylalanine content in the blood (it should be in the average range of 3–4 mg% or 180–240 μmol/l) [1–4].
In the Russian Federation, the following scheme is used to monitor the level of phenylalanine in the blood of patients with PKU: up to 3 months of age - once a week (until stable results are obtained) and then at least 2 times a month; from 3 months to 1 year - 1 time per month (if necessary - 2 times per month); from 1 year to 3 years - at least 1 time every 2 months; after 3 years - once every 3 months [2].
The nutritional status of the patient, his physical and intellectual, emotional and speech development are constantly monitored. If necessary, medical specialists are involved in examining the patient, psychological and defectological testing and a number of studies are carried out (ultrasound of internal organs, ECG, EEG, MRI of the brain, general blood and urine analysis, blood proteinogram, according to indications - glucose, cholesterol, creatinine, ferritin , serum iron, etc.). A general blood test is performed once a month, and a biochemical blood test is performed as indicated [1–4].
Nutrition for infectious diseases. In case of intercurrent diseases with hyperthermia, intoxication and/or dyspeptic symptoms, it is possible to temporarily stop diet therapy (for several days) with the replacement of medicinal products with natural ones (with a low protein content). At the end of the acute period of the disease, the medicinal product is reintroduced into the diet, but for a shorter period than at the beginning of diet therapy [2].
Discontinuation of diet therapy. The age at which diet therapy can be discontinued in patients with PKU continues to be controversial.
There is evidence that when diet therapy was discontinued at 5 years of age, one third of children with PKU experienced a decrease in IQ level by 10 points or more over the next 5 years. In patients over 15 years of age, breaks in diet therapy are often accompanied by progressive changes in the white matter of the brain (according to MRI data) [1].
Diet therapy for patients with classic PKU should be lifelong [1–4].
In the Russian Federation, in accordance with the law, special dietary therapy must be provided to the patient free of charge, regardless of the degree of disability and the patient’s age. Strict, mandatory dietary treatment for PKU is usually carried out until the age of 18, followed by an expansion of the diet. Adult patients are recommended to avoid consuming high-protein foods of animal origin (the total amount of protein should not exceed 0.8–1.0 g/kg/day).
Literature
- Blau N. et al. Phenylketonuria // Lancet. 2010. No. 376. P. 1417–1427.
- Therapeutic nutrition for hereditary metabolic disorders (E70.0-E74.2). In the book: Clinical dietetics of childhood / Ed. Borovik T. E., Ladodo K. S. M.: “MIA”. 2008. pp. 330–383.
- Harding CO et al. Advances and challenges in phenylketonuria // J. Inherit. Metab. Dis. 2010. V. 33. P. 645–648.
- Lord B. et al. Implications of resolving the diagnosis of PKU for parents and children // J. Pediatr. Psychol. 2008. V. 33. P. 855–866.
- Koch R. et al. Neuropathology of a 4-month-old infant born to a woman with phenylketonuria // Dev. Med. Child. Neurol. 2008. V. 50. P. 230–233.
- Van Spronsen FJ et al. Large neutral amino acids in the treatment of PKU: from theory to practice // J. Inherit. Metab. Dis. 2010. V. 33. P. 671–676.
- Harding CO New era in treatment for phenylketonuria: pharmacologic therapy with sapropterin dihydrochloride // Biologics. 2010. V. 9. P. 231–236.
- Ney DM et al. Dietary glycomacropeptide supports growth and reduces the concentrations of phenylalanine in plasma and brain in a murine model of phenylketonuria // J. Nutr. 2008. V. 138. P. 316–322.
- Singh RH et al. BH4 therapy impacts the nutrition status and intake in children with phenylketonuria: 2-year follow-up // J. Inherit. Metab. Dis. 2010. V. 33. P. 689–695.
- Trefz FK et al. Sapropterin dihydrochloride: a new drug and a new concept in the management of phenylketonuria // Drugs Today. 2010. V. 46. P. 589–600.
- Webster D. et al. Tyrosine supplementation for phenylketonuria // Cochrane Database Syst. Rev. 2010. V. 8: CD001507.
V. M. Studenikin, Doctor of Medical Sciences, Professor T. E. Borovik, Doctor of Medical Sciences, Professor T. V. Bushueva, Candidate of Medical Sciences
SCCD RAMS, Moscow
Contact information for authors for correspondence
Classification
Phenylketonuria currently does not have a generally accepted worldwide classification. There is ongoing debate over this issue, as well as research into the disease. A little earlier, before deciphering genes, it was believed that the degree of damage to intellectual abilities depended on the degree of enzyme activity. Therefore, the current qualification is considered outdated. It also does not take into account other symptomatic factors.
When diagnosing they put:
- Type I (PAH deficiency) - FA concentration is more than 20 mg/dl.
- The average form of PKU is FA from 8.1 to 20 mg/dl.
- Mild form of HPA level - FA from 2.1 to 8.0 mg/dl.
At levels up to 8.0 mg/dL, phenylketonuria is considered benign. It does not require special treatment, but observation is necessary for the first year of the child’s life. The condition is monitored by a pediatrician, neurologist, and geneticist.
There is also another form of phenylketonuria that does not require correction. This is a transient form of HPA during the neonatal period. Occurs, as a rule, with prematurity, which is due to the functional immaturity of the body. Transient phenylketonuria is a temporary increase in FA levels that can rise to critical values. In this case, there is no clinical manifestations or the manifestations are very minor. After a few months, biochemical parameters returned to normal.
How to treat phenylketonuria
The only effective method of treating phenylketonuria is considered to be a specially designed diet organized from the first days of life, the principle of which is to limit the phenylalanine contained in food products, for which such foods as are excluded:
- cereals,
- legumes,
- eggs,
- cottage cheese,
- bakery products,
- nuts,
- chocolate,
- fish, meat, etc.
The therapeutic diet of patients with phenylketonuria consists of specialized products of both foreign and domestic production. Children of the first year of life are shown products that are close in composition to breast milk, these are mixtures such as “Lofenilak” and “Aphenilak”. For slightly older children, such mixtures as “Tetrafen”, “Maxamum-HR”, “Phenyl-Free” have been developed. Pregnant women and older children (after six years) suffering from phenylketonuria are advised to take the Maxamum-HR mixture. In addition to specialized medicinal products, the patient’s diet includes juices, fruits and vegetables.
Timely initiation of diet therapy most often allows one to avoid the development of characteristic clinical manifestations of classical phenylketonuria. Treatment is mandatory until puberty, and sometimes longer. Due to the fact that a woman with phenylketonuria is not able to bear a healthy fetus, special treatment is carried out, starting before conception and continuing until birth, aimed at preventing damage to the fetus by phenylalanine from the sick mother.
Children undergoing treatment must be under the constant supervision of a psychoneurologist and a local pediatrician. At the beginning of treatment for phenylketonuria, phenylalanine levels are monitored weekly; when the levels are normalized, they are switched to once a month during the first year of life, and once every two months in children older than one year.
In addition to diet therapy, doctors can prescribe the following for children with phenylketonuria:
- mineral compounds;
- nootropics;
- B vitamins;
- anticonvulsants.
Complex therapy should include physical therapy, acupuncture and massage.
Please note: for the atypical form of phenylketonuria, which cannot be corrected by diet therapy, doctors prescribe hepatoprotectors and anticonvulsants. This treatment will help alleviate the child’s condition.
Pathogenesis
The mechanism of the origin and development of phenylketonuria is associated with a violation of the metabolism of an organic compound - the amino acid phenylalanine. The metabolic block prevents the conversion of phenylalanine to tyrosine. The amino acid not only is not converted, but accumulates in the form of toxic metabolites:
- phenyllactic acid;
- phenylpyruvic acid;
- phenylacetic acid;
- phenylethylamine and so on.
The accumulation of phenyl substances has a toxic effect on the central nervous system. At the moment, the mechanism has not yet been fully studied; doctors do not know the pathogenesis of brain dysfunction.
There are suggestions that damage to the nervous system is the result of a number of factors. Among them are both the direct toxic effects of phenylalanine and disruption of the metabolism of proteins, lipoproteins and glycoproteins, failure of homonal metabolism and membrane transport of amino acids. All this together is important for the maturation and proper functioning of the central nervous system.
Consequences and life prognosis
The effect of excessive amounts of phenylalanine on the child’s nervous system leads to persistent psychological disorders. By the age of 4, without proper treatment, children with phenylketonuria are considered weak-minded and physically underdeveloped members of society. They join the ranks of disabled children and the colors of life fade for them.
The life of the parents of a sick child does not sparkle with happiness either. The baby requires constant care, and with limited financial resources this results in a general deterioration in the well-being of the family. The pain experienced by mom and dad from the inability to change the child’s existence for the better is depressing and oppressive, but one cannot despair. Help yourself, help your child pass these tests with fewer losses in love and mercy.
Science is in a hurry, it is making leaps and bounds towards eliminating the disease from the rank of severe. Diagnosis of phenylketonuria in the womb is of great importance, but such a method has not yet been invented. “yet” does not mean “never”, let’s wait and believe
Symptoms
Type I The first signs in a child appear between the ages of 2 months and six months.
- Apathy or, conversely, increased irritability.
- Lack of interest in the environment, people, objects, settings.
- Frequent regurgitation.
- Allergic dermatitis.
- Violation of muscle tone.
- Low pressure.
- Cramps.
- Sometimes microcephaly (small size of the skull relative to other parts of the body) and hydrocephalus (excess fluid surrounding the brain) develop.
Characteristic symptoms include hypopigmentation of the skin, hair, and iris. Urine has a specific musty smell or is also called a “mouse” smell. Epileptic seizures are observed in half of the patients and are often the first pronounced clinical symptom. The attack is characterized by “salaam” convulsions, reminiscent of nodding. They occur frequently and do not respond well to anticonvulsant treatment.
If PA concentrations are not adjusted, the disease progresses. As a rule, the IQ level in such children does not exceed 20, with the norm being 85. Mental retardation is so severe that there are no emotional reactions, psychopathy and schizophrenia-like disorders are observed.
Type II The first symptoms appear in the first year of life.
- Increased excitability.
- Developmental delay.
- Profuse drooling.
- Reduced blood pressure.
- Frequent increase in body temperature.
- Tendon hyperreflexia (increased reflexes) or spastic tetraparesis (weakness of all four limbs).
- Myoclonic epilepsy (generalized seizures, predominantly occurring upon awakening).
- Microcephaly.
A distinctive feature of the second type is the death of neurons, disruption of folate metabolism, as well as calcification in various parts of the brain. The disease progresses rapidly and can lead to the death of a child within 2 to 3 years.
III type. Symptoms of pyruvoyltetrahydropterin synthetase deficiency are similar to those of Parkinson's disease:
- Postural instability and gait difficulties. It is difficult or impossible to maintain a certain position of the whole body or limbs.
- Hypokinesia (low motor activity, limited tempo and range of movements).
- Hypersalivation (increased salivation).
- Swallowing disorders.
- Oculogyric crisis (symmetrical deviation of both eyes, usually directed upward).
In 80% of cases, this type of disease is accompanied by a decrease in the amount of biogenic amines in the cerebrospinal fluid. Treatment is complicated by the fact that an early decrease in FA concentration can cause serious pathological changes. Failure to comply with diet therapy will lead to slower speech development, low intelligence, and memory problems.
Phenylketonuria and motherhood
It is very important for pregnant women with PKU to keep phenylalanine levels low before and throughout pregnancy to ensure a healthy baby. And although the developing fetus may only be a carrier of the PKU gene, the intrauterine environment may have very high levels of phenylalanine, which has the ability to penetrate the placenta. As a result, the child may develop a congenital heart defect, developmental delay, microcephaly and mental retardation are possible. As a rule, women with phenylketonuria do not experience any complications during pregnancy.
In most countries, women with PKU who are planning to have children are advised to reduce phenylalanine levels (usually to 2-6 µmol/L) before pregnancy and monitor them throughout pregnancy. This is achieved by conducting regular blood tests and following a strict diet, and constant supervision by a dietitian. In many cases, once the fetal liver begins to produce PAH normally, the level of phenylalanine in the mother's blood drops, and accordingly it is “necessary” to increase it to maintain a safe level of 2-6 µmol/L.
This is why a mother's daily intake of phenylalanine may double or even triple by the end of pregnancy. If the level of phenylalanine in the mother’s blood is below 2 µmol/l, then sometimes women may experience various complications associated with a deficiency of this amino acid, such as headache, nausea, hair loss and general malaise. If low levels of phenylalanine in patients with PKU are maintained throughout pregnancy, then the risk of giving birth to an affected child is no higher than in those women who do not have PKU.
Diagnostics
Phenylketonuria can be detected in the first days after birth before any symptoms appear. To determine the concentration of phenylamine in the blood, carry out:
- microbiological test;
- chromatography;
- fluorimetry;
- mass spectrometry.
In all cases, the biological material is dry spots of capillary blood from the baby.
Recently, testing for phenylketonuria has been included in the neonatal screening program. It is carried out on all newborns; the study is especially important for premature babies. The diagnostic criterion is an increased concentration of phenylalanine at a rate of 0 - 2 mg/dl. An elevated level requires further diagnostic testing. It will be necessary to establish the very fact of the presence of phenylketonuria and identify its cause.
- If the screening test shows high PA level results, the following may additionally be prescribed:
- Phenylalanine load diagnostics to identify the nosological form of the disease.
- Molecular genetic analysis to determine the form: classical, type II or III.
- Sequencing of the RAS gene, if molecular genetic diagnostics gave a negative result for the PAH gene.
- Analysis for pterins in urine to exclude pterin-dependent forms.
Differential diagnosis of phenylketonuria is carried out with such pathologies as liver dysfunction, galactosemia and other diseases.
Symptoms of phenylketonuria
Phenylketonuria has a fairly pronounced clinical picture, including symptoms such as:
- delayed physical development from 6 months of age;
- microcephaly;
- autonomic dysfunctions;
- increased excitability and motor hyperactivity;
- eczema or dermatitis , possibly with papular skin rashes (as in the photo of a child with phenyluketonuria);
- muscle hypertension;
- ataxia;
- hyperkinesis;
- unsteady gait;
- seizures;
- heart defects are often detected ;
- sensitivity to trauma and lightening of the skin, hair and iris of the eyes ( depigmentation
), especially if the necessary diet is not followed, causing a deficiency of melanin in the body, which is a derivative of tyrosine.
Eczema with phenylketonuria
Mental disorders
Phenylketonuria causes significant pathological changes in metabolic processes in the brain, which entails the following disorders:
- deep degree of mental retardation, up to idiocy or imbecility ;
- learning difficulties;
- echopraxia phenomena - repetition of movements after others;
- echolalia (repetition of speech);
- lethargic behavior may be replaced by rare outbursts of anger and irritability .
As you can see in the photos of patients with phenylketonuria, their physique is usually dysplastic, the size of the skull is reduced, hypogenitalism and nanism .
Treatment
Symptomatic therapy for any form of phenylketonuria is ineffective. There is only one way to prevent the negative consequences of the disease - diet therapy. High-protein foods and phenylalanine-containing foods are excluded from the diet. The missing amount of protein is replenished with specialized therapeutic nutrition, with the lowest possible content of the amino acid FA or completely devoid of it. It should be taken into account that the effectiveness of therapy directly depends on the time the correction began and the pathological changes that have already occurred.
The goal of therapeutic nutrition in the classic form of the disease is to prevent the development of disorders of the central nervous system, physical and mental development. A mild form of HFA allows for an expansion of the diet under the strict supervision of a doctor over the child’s condition and biochemical parameters. Prohibited: meat, fish, nuts, chocolate and legumes, all types of eggs, cottage cheese and cheeses. You should also avoid foods that contain the artificial sweetener aspartame.
The criterion for the effectiveness of treatment is the level of FA in the blood.
Pathogenesis of phenylketonuria
Deficiency of the enzyme phenylalanine-4-hydroxylase underlies the classical form of the disease; it leads to impaired oxidation of phenylalanine ingested from food. Its concentration in the blood and cerebrospinal fluid increases significantly, and the level of tyrosine decreases. This leads to impaired myelination of nerve fibers, decreased formation of neurotransmitters and triggers pathogenetic mechanisms of mental retardation and causes progressive dementia.
Symptoms of phenylketonuria
The manifestation of Felling's disease occurs at the age of 2–6 months. The first nonspecific symptoms develop: lethargy, anxiety and hyperexcitability, muscle dystonia, regurgitation, convulsive syndrome. Persistent vomiting is considered a pathognomonic symptom of phenylketonuria.
After 6 months There is a delay in the child's psychomotor development. This manifests itself as a decrease in activity, indifference to the environment, the child ceases to recognize his relatives, does not make attempts to sit down or stand on his feet. Skin peeling, eczema, dermatitis, and scleroderma may appear.
Children with phenylketonuria who do not receive treatment suffer from microcephaly, prognathia, delayed teething, and enamel hypoplasia. They are characterized by delayed speech development; by the age of three to four years, mental retardation develops with an almost complete absence of speech.
Patients have a dysplastic physique and are distinguished by light skin, hair and eyes. The upper and lower limbs are bent at the joints (the so-called “tailor’s pose”), the gait is unsteady, mincing, there may be tremor of the hands, hyperkinesis.
Prognosis and prevention
Carrying out mass screening in maternity hospitals allows for timely identification of genetic abnormalities. Start following diet therapy in a timely manner and, as a result, prevent serious consequences. Otherwise, the prognosis for mental development is unfavorable.
Classic PKU has a favorable prognosis if it is diagnosed in the first weeks of a child’s life and all doctors’ requirements are met. Such children go to regular schools, are able to obtain higher education and lead a normal life.
When preparing for pregnancy, a couple should undergo preliminary genetic testing for the presence of mutations in the RAS gene. If one of the parents has a defective gene, the chance of having a child with PKU is 1:4 and 100% if both parents are sick.
Women with established phenylketonuria should follow a strict diet during pregnancy and breastfeeding. High concentrations of the amino acid in the blood and amniotic fluid have a serious teratogenic effect on the fetus.
Prevention
Since we are talking about a genetic disease, there is simply no specific prevention of phenylketonuria. Parents can take a special test before conception and see what the risks are that they will have a child with this disease. But regardless of whether a genetic test was passed, all newborn babies in the maternity hospital are still checked.
The only preventive measure concerns those parents who, for some reason, decided to give birth at home or in countries where testing for phenylketonuria is not mandatory. They need to take blood tests during the first three weeks of the baby’s life and check the newborn for the presence of the distorted 12th gene.
Types of phenylketonuria
There are three types of disease:
- phenylketonuria type 1 (classical) - characterized by a mutation of the gene responsible for the synthesis of the enzyme phenylalanine-4-hydroxylase. This type of disease accounts for 98% of all diagnosed cases of fermentopathy.
- phenylketonuria type 2 (atypical) - occurs when the 4th chromosome, which is responsible for the synthesis of dihydropyridine reductase, breaks down.
- phenylketonuria type 3 (atypical) - develops with tetrahydrobiopterin deficiency.
Based on the level of phenylalanine in the blood, three forms of this disease are conventionally distinguished:
- light;
- average;
- heavy.
Clinical picture
Patients with phenylketonuria are characterized by light hair color and blue irises (children are always lighter than their parents and healthy brothers and sisters). In the first 2-3 months of life, most children look completely healthy. Only some of them experience lethargy, drowsiness, and less often anxiety. An early symptom is vomiting. There is a peculiar smell of the child’s urine (musty, mousey). By the end of the first six months of life, 20-50% of sick children develop eczematous skin changes, sometimes significant, and some develop scleredema (see Scleredema of newborns). At the same age, mental retardation begins to emerge, which, as it grows, further leads to severe dementia (see) - imbecility or idiocy. Only in a small number of patients the damage to the intellectual sphere is mildly expressed. There is a lag in physical development. The formation of motor skills is delayed. Many patients experience convulsions, which can gradually disappear even without treatment, and hilereflexia and involuntary movements are detected. In most patients, paroxysmal activity is recorded on the EEG. Some children experience muscle spasms in the extremities (mainly legs). All noted changes are typical for patients who have not received pathogenetic treatment since birth.
Diet for phenylketonuria
Protein-free diet
- Efficacy: therapeutic effect after 2 weeks
- Time frame: until effect is achieved
- Cost of products: 1200-1300 rubles. in Week
It is extremely important for individuals with phenyleketonuria to adhere to a strict diet that limits protein foods, including meat, fish, eggs, dairy products, sausages and other foods containing animal or plant proteins. To compensate for protein deficiency, amino acid mixtures without phenylalanine are used. Typically, protein hydrolysates such as Lofenalac , Phenylfree , Berlofen , Aponti , Hypophenate for children under 4-5 years of age and Nophelan for children over 5 years of age are recommended.
The diet is usually based on vegetable and fruit juices, as well as purees, low protein foods - sago, bread, pasta, starch-based cereals, sugar and jam.
Some modern carbonated drinks, chewing gums and medications contain phenylalanine in the form of aspartame , which the manufacturer is required to warn about on the packaging, but they do not always adhere to this rule, so you need to be extremely careful when choosing products. On some soft drinks, for example, after information about the composition and nutritional value of 100 ml of the product, the following note is written: “The drink contains a source of phenylalanine. Contraindicated in case of phenylketonuria."
Acceptable values of phenylalanine for phenylketonuria at different ages of children
Attention! A diet for patients with phenylketonuria must be prepared by a qualified specialist who can take into account all the body’s needs for phenylalanine and its permissible amount of consumption.
Treatment of phenylketonuria
Timely treatment gives a good effect. A child with PKU is not hospitalized. Parents are provided with qualified advisory assistance, the baby is given free formulas that do not contain animal proteins as part of a specialized diet - the only effective method of treating PKU. The child receives special protein hydrolysates with a minimum of PA or without it, but with sufficient levels of vitamins and calcium.
During treatment, breastfeeding is limited (previously it was completely excluded). Milk is expressed and given according to calculations. When the level of phenylalanine is normalized, the child begins to be fed animal protein to avoid the breakdown of its own tissues and the depletion of the baby. The inclusion of fruits, vegetables, fats, and carbohydrates is carried out with strict consideration of the level of phenylalanine.
Today it is not a problem to find a dietary product or protein mixture with a certain concentration of FA. According to Russian law, children suffering from PKU receive free special meals. The following compositions are allowed in the Russian Federation:
- Afenilak.
- XP Analogous to LCP.
- MD mil PKU-0.
A special diet is strictly followed until 6 years of age. Excluded from the diet: bread, dairy products, eggs, fish, meat, as well as Aspartame, an artificial sweetener that is used in the manufacture of carbonated drinks, candies and chewing gum. Over time, neurons become less sensitive to excess PA, and after 16-18 years of age, adolescents switch to a regular diet. But stopping treatment can provoke fear. Returning to dietary therapy improves the mental state of patients.
Adults with phenylketonuria experience mood lability and depression. At high doses of PA, they suffer from deconcentration and insomnia, lack of motivation, schematic decision-making, impulsiveness, and loss of the ability to rationally assess situations.
The treatment improves cognitive function even in those with severe mental retardation. If the disease was diagnosed late, then adherence to a strict diet can reduce aggressiveness and improve the patient’s contact with the environment. In some cases, lifelong adherence to the diet is required.
Treatment is supervised by a psychiatrist and medical geneticist. If necessary, you may need the help of a speech therapist. A greater effect is achieved when using exercise therapy, massage, and acupuncture.
During pregnancy
Women with phenylketonuria who have been successfully treated with diet should be extremely careful about their condition while carrying a child, because even with genetically normal fetal development, there is a high risk of disorders due to the increased level of phenylalanine and its metabolites in the mother’s bloodstream as a serious teratogenic factor with neurotropic toxicity. Therefore, before conceiving, such patients are advised to return to a strict diet and stick to it throughout the pregnancy.
A special diet should limit intake to 15-20 mg/kg of phenylalanine per day, while ensuring that essential amino acid .