Instructions for use
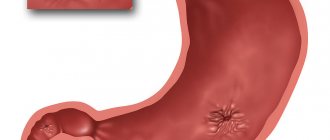
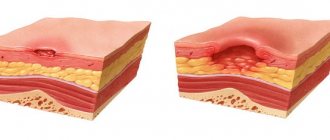
Pilobact AM is a combination drug that is used to treat duodenal ulcers caused by Helicobacter pylori.
Advantages of the medicinal complex:
- high level of eradication (destruction) of the pathogen - up to 97%;
- short course - only 7 days;
- one package is designed for the entire course of treatment;
- few side effects;
- convenient packaging, eliminating overdose, even if the patient is forgetful.
Indications for use
The main pathologies in the treatment of which the drug Pilobact is effective:
ulcers of the stomach and duodenum (during exacerbations or in remission);- atrophic form of gastritis;
- MALT lymphoma;
- postoperative condition after resection (removal of part of an organ) for stomach cancer;
- first-degree relatives of patients with gastric cancer.
Relative indications for which the medicine is prescribed:
- functional dyspepsia;
- gastropathy resulting from taking non-steroidal anti-inflammatory drugs;
- GERD, or gastroesophageal reflux disease.
Release form, composition
Pilobact AM is available in capsule form. The composition of the medicinal complex includes the following drugs:
enteric capsule omeprazole (0.02 g)—2 pcs.;- clarithromycin film-coated tablet (0.5 g) - 2 pcs.;
- amoxicillin capsule (amoxicillin trihydrate - 0.5 g) - 4 pcs.;
- All tablets and capsules are contained in 1 strip, a total of 7 strips in a pack.
The active ingredients produce the following effects:
- omeprazole - inhibits the production of gastric juice, reduces basal and stimulated secretion, acts within an hour after administration, the maximum concentration is observed after 2 hours, the maximum period of action is 24 hours;
- Clarithromycin is a macrolide antibiotic of semi-synthetic origin, a derivative of erythromycin A, acts on bacteria and inhibits protein synthesis, and is characterized by a wide spectrum of action;
- amoxicillin is also a semisynthetic antibiotic made from penicillins, effective against many pathogenic microorganisms.
Clarithromycin and amoxicillin in this combination enhance each other's effect.
Mode of application
Each strip contains a daily dose of medication, which is convenient for the patient, and is divided into two colored zones - morning and evening.
Take in the morning and evening:
- omeprazole and clarithromycin - 1 tablet each;
- amoxicillin - 2 tablets.
Capsules are not opened and tablets are not crushed, as this reduces the effectiveness of the drug.
Interaction with other drugs
The active ingredients of the complex react with some medications. The following combinations are undesirable:
- with theophylline - clarithromycin increases the concentration;
- with terfenadine - the concentration is increased by clarithromycin, which may prolong the QT interval;
- with indirect coagulants - the effect is potentiated by clarithromycin;
- with carbamazepine, cyclosporine, phenytoin, lovastatin, valproate, digoxin, astemizole, disopyramide, cisapride - clarithromycin increases the concentration;
- with phenytoin, warfarin, diazepam - concentration increases with omeprazole;
- with ketoconazole, ketoconazole, iron salts, ampicillin - affects the absorption of omeprazole;
- with oral contraceptives - reduces the effectiveness of amoxicillin.
Pilobact AM can cause adverse events, about which the doctor warns the patient before starting treatment. There are also some contraindications.
Pylobact® AM
Clarithromycin
When used simultaneously with astemizole, cisapride, pimozide, terfenadine, an increase in the concentration of the latter in the blood was reported, which can lead to cardiac arrhythmias (prolongation of the QT interval on the electrocardiogram, ventricular tachycardia, ventricular fibrillation, torsade de pointes). The simultaneous use of clarithromycin with astemizole, cisapride, pimozide, terfenadine is contraindicated.
The simultaneous use of clarithromycin and ergotamine or dihydroergotamine (ergot alkaloids) can lead to acute ergotamine intoxication, accompanied by severe peripheral vasospasm (impaired sensitivity, paresthesia, pain and a marked decrease in pulsation in the extremities, disorders of the central nervous system - dizziness, convulsions, coma). Concomitant use of clarithromycin with ergot alkaloids is contraindicated.
Caution should be exercised when using clarithromycin concomitantly with ototoxic drugs, primarily aminoglycosides, due to increased ototoxicity. During and after treatment, the function of the hearing organ and vestibular apparatus should be monitored.
Concomitant use of zidovudine in HIV-infected adult patients may result in decreased steady-state zidovudine concentrations. Since clarithromycin affects the absorption of concomitantly administered oral zidovudine, it is recommended that these drugs be taken at least 4 hours apart.
Clarithromycin inhibits the P-glycoprotein transporter, the substrate of which is digoxin. When using clarithromycin and digoxin simultaneously, the concentration of digoxin in the blood plasma should be carefully monitored (its concentration may increase and the development of potentially fatal cardiac arrhythmias may occur).
Interactions due to CYP3A isoenzymes
Drugs that are inducers of the CYP3A4 isoenzyme (for example, rifampicin, phenytoin, carbamazepine, phenobarbital, St. John's wort) can induce the metabolism of clarithromycin. This may result in subtherapeutic concentrations of clarithromycin, resulting in reduced effectiveness. In addition, it is necessary to monitor the concentration of the CYP3A isoenzyme inducer in the blood plasma, which may increase due to the inhibition of the CYP3A isoenzyme by clarithromycin.
The following drugs have a proven or suspected effect on clarithromycin plasma concentrations; if they are used together, dosage adjustments or switching to alternative treatment may be necessary.
Efavirenz, nevirapine, rifampicin, rifabutin, rifapentine increase the metabolism of clarithromycin, reducing its concentration in the blood plasma and increasing the concentration of its biologically active metabolite 14-hydroxyclarithromycin. In patients receiving inducers of CYP3A isoenzymes. Alternative antibiotic therapy options should be considered. The simultaneous use of clarithromycin and rifabutin leads to an increase in the concentration of rifabutin and a decrease in the concentration of clarithromycin in the blood plasma with the risk of developing uveitis.
The concentration of clarithromycin decreases with the use of etravirine, but the concentration of the active metabolite 14-hydroxyclarithromycin increases. Because 14-hydroxyclarithromycin has low activity against Mycobacterium avium complex
(MAC), overall activity against their pathogens may vary, so alternative treatments should be considered for the treatment of MAC.
The simultaneous use of fluconazole leads to an increase in the steady-state concentration and area under the curve of “conconcurrent” ventricular tachycardia. Monitoring of ECG (increased QT interval) and serum concentrations of these drugs is necessary.
Cases of hypoglycemia have been reported with the combined use of clarithromycin and disopyramide. It is necessary to monitor the concentration of glucose in the blood plasma when using these drugs simultaneously.
Concomitant use with HMG-CoA reductase inhibitors (simvastatin, lovastatin) leads to an increased risk of developing myopathy and rhabdomyolysis. Concomitant use of clarithromycin with simvastatin and lovastatin is contraindicated.
Clarithromycin should be used with caution in combination therapy with other statins. If co-administration with statins is necessary, it is necessary to use statins that do not depend on CYP3A metabolism (for example, fluvastatin). It is recommended to take the lowest dose of statin. The development of signs and symptoms of myopathy should be monitored.
When using clarithromycin simultaneously with blockers of “slow” calcium channels that are metabolized by the CYP3A4 isoenzyme (for example, verapamil, amlodipine, diltiazem), caution should be exercised, as there is a risk of arterial hypotension. Plasma concentrations of clarithromycin, as well as slow calcium channel blockers, may increase with simultaneous use. Arterial hypotension, bradyarrhythmia and lactic acidosis are possible when taking clarithromycin and verapamil simultaneously.
Colchicine is a substrate for both CYP3A and P-glycoprotein, which are inhibited by clarithromycin. A single dose of 0.6 mg of colchicine combined with clarithromycin 250 mg twice daily for a week resulted in a 197% increase in the maximum concentration of colchicine and the area under the con-con-con-breakthrough curve. Additional non-hormonal methods of contraception should be used during treatment with amoxicillin.
Omeprazole
Like other drugs that reduce gastric acidity, treatment with omeprazole can lead to decreased absorption of ketoconazole, itraconazole, posaconazole and erlotinib, as well as increased absorption of drugs such as digoxin.
Co-administration of omeprazole 20 mg once daily and digoxin increases the bioavailability of digoxin by 10% (the bioavailability of digoxin increased by up to 30% in 20% of patients).
Omeprazole may reduce the absorption of vitamin B12 with long-term use.
Concomitant use with posaconazole and erlotinib is contraindicated.
Omeprazole should not be used simultaneously with St. John's wort preparations due to pronounced clinically significant interactions.
When omeprazole is taken together with clarithromycin or erythromycin, the concentration of omeprazole in plasma increases.
When used simultaneously with omeprazole, the area under the concentration-time curve of atazanavir decreases by 75%, so their simultaneous use is contraindicated.
When used simultaneously with omeprazole, it is possible to slow down the elimination of warfarin, diazepam, phenytoin, imipramine, clomipramine, citalopram, hexobarbital, disulfiram, since omeprazole is biotransformed in the liver with the participation of the CYP2C19 isoenzyme. Doses of these drugs may need to be reduced.
When methotrexate was used concomitantly with proton pump inhibitors, a slight increase in methotrexate plasma levels was observed in some patients. When treating with high doses of methotrexate, you should temporarily stop taking omeprazole.
Concomitant use of omeprazole with amokeicillin or metronidazole does not affect the concentration of omeprazole in the blood plasma.
When omeprazole is used together with caffeine, propranolol, theophylline, metoprolol, lidocaine, quinidine, phenacetin, estradiol, budesonide, diclofenac, naproxen, piroxicam, antacids and ethanol, no clinically significant interaction has been established.
Side effects
The following side effects should be expected from Pilobact AM:
- from the gastrointestinal tract : stomatitis, dry mouth, taste disturbance, vomiting, nausea, dysbacteriosis, stool disorders, impaired liver function and increased activity of enzymes of this organ;
- from the musculoskeletal system : weakness and muscle pain, arthralgia (joint pain);
- circulatory system : changes in the blood formula - leukopenia, neutropenia, thrombocytopenia (decrease in the level of leukocytes, neutrophilic leukocytes, platelets, respectively), thrombocytopenic purpura (Wergolf's disease) - a primary hemorrhagic diathesis caused by a deficiency of the platelet component;
- nervous system : headache, confusion, epileptic seizures, drowsiness, hallucinations, depression, ataxia (impaired coordination of movements), agitation, paresthesia (impaired skin sensitivity);
- possible allergic reactions - angioedema, bronchospasm, urticaria, anaphylactic shock;
- other side effects - visual impairment, tachycardia (heart rhythm disturbance), abnormal sweating, in men - gynecomastia (enlarged mammary glands), increased body temperature, interstitial nephritis, peripheral edema.
Contraindications
Pilobact AM is not used for the treatment of persons with the following diseases:
- intolerance to any component of the drug;
- porphyria is a hereditary disease associated with a disorder of pigment metabolism and a high concentration of porphyrins in the blood;
- insufficient liver or kidney function.
The complex is contraindicated in children.
During pregnancy and breastfeeding
Pilobact AM is prohibited for use by women during pregnancy and breastfeeding . If there is a need to use the medicine during lactation, then breastfeeding is interrupted.
Pilobact AM set of tablets and capsules 6 pcs. strips 7 pcs. in Omsk
Pharmacological action: Triple therapy, including omeprazole, clarithromycin and amoxicillin, allows to achieve a high percentage of Helicobacter pylori eradication (85–94%).
Omeprazole inhibits the secretion of gastric acid due to the specific inhibition of H+ K+-ATPase, an enzyme located in the membranes of parietal cells of the gastric mucosa. Reduces basal and stimulated secretion regardless of the nature of the stimulus. After a single dose of the drug orally, the effect of omeprazole occurs within the first hour and continues for 24 hours, the maximum effect is achieved after 2 hours. After stopping the drug, secretory activity is completely restored after 3-5 days.
Clarithromycin is an antibiotic from the macrolide group, a semi-synthetic derivative of erythromycin A. It has an antimicrobial effect, which is associated with the suppression of protein synthesis by interaction with the 50S ribosomal subunit of the microbial cell. Effective against a large number of gram-positive, gram-negative aerobic and anaerobic microorganisms, including H. pulori. The metabolite formed in the body, 14-hydroxyclarithromycin, also has pronounced antimicrobial activity.
Amoxicillin is a semi-synthetic penicillin, has a bactericidal effect and has a wide spectrum of action. The antimicrobial effect is associated with inhibition of the synthesis of peptidoglycan (the supporting polymer of the cell wall) during division and growth. It has pronounced activity against H. pulori. Resistance of H. pulori to amoxicillin is rare.
The combination of amoxicillin and clarithromycin has a potent antimicrobial effect against H. pulori.
Pharmacokinetics:
All three drugs included in Pilobact® AM have good absorption when taken orally.
Omeprazole is rapidly absorbed after oral administration, and its bioavailability is 30–40%. Food intake does not affect the bioavailability of omeprazole. Cmax of the drug in plasma is achieved after 0.5–1 hour. Plasma protein binding is 90%. Almost completely metabolized in the liver. The main route of excretion is in the urine (80%).
Clarithromycin is rapidly absorbed from the gastrointestinal tract. The absolute bioavailability of 250 mg clarithromycin is approximately 50%. Eating slightly slows down the onset of absorption of clarithromycin and the formation of 14-hydroxyclarithromycin, but does not affect bioavailability. When taken on an empty stomach, Cmax in serum is achieved within 2 hours after oral administration and is 0.6 and 0.7 mcg/ml for clarithromycin and its main metabolite. T1/2 of clarithromycin is 3–4 hours. Clarithromycin is widely distributed in the body. The concentration of clarithromycin in tissues exceeds that in serum. Protein binding ranges from 42 to 70%. It is excreted by the kidneys and in feces (20–30% in unchanged form, the rest in the form of metabolites). The simultaneous administration of clarithromycin and omeprazole improves the pharmacokinetic properties of clarithromycin: the average Cmax increases by 10%, the minimum concentration by 15% compared with the same indicators with clarithromycin monotherapy. The concentration of clarithromycin in the gastric mucosa is also increased when administered simultaneously with omeprazole.
Amoxicillin is rapidly absorbed from the gastrointestinal tract. Eating does not affect the absorption of amoxicillin. The bioavailability of amoxicillin is 75–90%. The drug is quickly distributed in the tissues of the body. T1/2 is 1–1.5 hours. Protein binding is 20%. About 60% of the dose taken is excreted unchanged in the urine, and a small amount is excreted in feces.
Reviews
Patients complain of poor tolerability of the drug complex. Most common complaints:
- bitterness in the mouth;
- diarrhea;
- pain in different parts of the abdomen;
- weakness;
- dizziness.
Increased hair loss, rumbling in the stomach, and mood swings are also possible.
In view of the described side effects, those who have already been treated with Pilobact AM are advised to issue a sick leave certificate for the duration of the course of therapy.
Read detailed reviews at the end of the article.
Pilobact AM
Pilobact AM (omeprazole + clarithromycin + amoxicillin) is a combination drug from the Indian pharmaceutical used for the treatment of helicobacteriosis against the background of duodenal ulcer. The inclusion of three independent drugs in Pilobact AM provides a high percentage of Helicobacter eradication (85-94%). Omeprazole suppresses the secretion of hydrochloric acid in the stomach by inhibiting the so-called proton pump - the enzyme H+ K+-ATPase. Reduces stimulated and unstimulated (basal) secretion, and in the first case - regardless of the nature of the irritating agent. After oral administration of a single dose of omeprazole, the effect is noted already in the first hour and continues throughout the day. The peak effect of omeprazole occurs 2 hours after its administration to the body. When the drug is discontinued, secretory function is completely restored after 3-5 days. Clarithromycin is an antibiotic belonging to the group of macrolides and is a semi-synthetic derivative of erythromycin. Its antibacterial effect is based on the ability to suppress protein synthesis through interaction with the 50S ribosomal subunit of the bacterial cell. Active against a wide range of gram-positive and gram-negative microorganisms, aerobes and anaerobes, including Helicobacter. It is metabolized to form 14-hydroxyclarithromycin, which is also endowed with pronounced antibacterial activity. Amoxicillin is a synthetically produced penicillin. Has a bactericidal effect against a wide range of microorganisms. The antimicrobial activity of the drug is due to the suppression of the synthesis of peptidoglycan (a structure-forming component of the cell wall) in the most important period of a cell’s life - when it divides and grows. At the same time, amoxicillin is especially hostile to Helicobacter. Resistance of the latter to the drug is extremely rare. If we talk about the combination of clarithromycin with amoxicillin, then it is truly destructive for Helicobacter, i.e.
because each of its components potentiates the action of the other. All three drugs included in Pilobact AM are well absorbed when taken orally. Omeprazole is rapidly absorbed after oral administration. Food intake does not affect the bioavailability of the drug, which averages 30-40%. The peak concentration of omeprazole in the blood is reached after 0.5-1 hour. It is excreted mainly by the kidneys. Clarithromycin is ahead of omeprazole in terms of bioavailability, which is 50%. The maximum concentration of the active substance in the blood is achieved after 2 hours. The half-life of clarithromycin ranges from 3 to 4 hours. Excreted in urine and feces. The uncontested leader in bioavailability among all three drugs is amoxicillin - 75-90%. Its half-life is 1-1.5 hours. It is excreted mainly by the kidneys.
Pilobact AM is available in the form of kits containing tablets and capsules and placed in strips. Each strip is designed for one day of treatment and consists of two multi-colored parts: red (morning) and blue (evening). One kit contains two amoxicillin capsules, one clarithromycin tablet and one omeprazole capsule. All ingredients of the kit are taken before meals. Tablets and capsules should be swallowed whole without chewing. The duration of the medication course is 1 week. Before starting pharmacotherapy, it is necessary to make sure that there is no cancer: otherwise, treatment may mask malignant symptoms and delay the diagnosis of a much more serious disease. Pilobact AM is used with caution in combination with drugs metabolized by the liver. The combination of Pilobact AM with indirect anticoagulants (for example, warfarin) requires monitoring of the prothrombin time (blood clotting time). Amoxicillin is poorly compatible with oral contraceptives (when taken together, the effect of the latter weakens).