Nifedipine
Medicines that affect nifedipine
Nifedipine is metabolized with the participation of the CYP3A4 isoenzyme of the cytochrome P450 system, located in the intestinal mucosa and in the liver.
Drugs that inhibit or induce this enzyme system may affect the first-pass effect (after oral administration) or clearance of nifedipine (see section Special instructions
).
The strength of the interaction and the duration of this effect should be taken into account when taking nifedipine simultaneously with the following drugs
Rifampicin
Rifampicin greatly increases the activity of the CYP3A4 isoenzyme of the cytochrome P450 system, which leads to a significant decrease in the bioavailability and effectiveness of nifedipine, therefore their simultaneous use is contraindicated (see section Contraindications
).
During simultaneous use of nifedipine with the following weak or moderate inhibitors of the CYP3A4 isoenzyme, blood pressure should be monitored and, if necessary, the dose of Nifedipine should be adjusted (see section Dosage and Administration).
.
Macrolides (eg, erythromycin)
No interaction studies have been conducted between nifedipine and macrolides. It is known that some macrolides can inhibit the CYP3A4 isoenzyme, which is involved in the metabolism of other drugs. Therefore, a potential increase in the concentration of nifedipine in the blood plasma cannot be excluded when taken simultaneously (see section Special instructions
).
Azithromycin does not inhibit the CYP3A4 isoenzyme, although its structure is a macrolide antibiotic.
HIV protease inhibitors (eg, ritonavir, indinavir, nelfinavir, amprenavir)
Clinical studies of the interaction between nifedipine and HIV protease inhibitors are not presented. It is known that drugs of this class inhibit the CYP3A4 isoenzyme. This class of drugs has also been shown to inhibit the CYP3A4 isoenzyme in vitro,
determining the metabolism of nifedipine.
When taken simultaneously with nifedipine, a significant increase in its concentration in the blood plasma cannot be ruled out due to a decrease in the “first pass” effect and a decrease in its elimination (see section Special instructions
).
Azole antifungals (eg, ketoconazole, fluconazole, itraconazole)
Clinical studies of the interaction between nifedipine and azole antifungals have not yet been presented. It is known that drugs of this class inhibit the CYP3A4 isoenzyme. When taken orally with nifedipine simultaneously, a significant increase in its systemic bioavailability cannot be ruled out due to a decrease in the “first pass” effect (see section Special instructions
).
Fluoxetine
Clinical studies of the interaction between nifedipine and fluoxetine are not presented. Fluoxetine is known to inhibit in vitro
isoenzyme CYP3A4, which determines the metabolism of nifedipine. Therefore, an increase in the concentration of nifedipine in the blood plasma cannot be excluded when taken simultaneously (see section Special instructions).
Nefazodone
Clinical studies of the interaction between nifedipine and nefazodone have not yet been reported. It is known that nefazodone inhibits the CYP3A4 isoenzyme, which determines the metabolism of nifedipine. Therefore, an increase in the concentration of nifedipine in the blood plasma cannot be excluded when taken simultaneously (see section Special instructions
).
Quinupristin/Dalfopristin
Concomitant use of quinupristin/dalfopristin with nifedipine may lead to an increase in the concentration of nifedipine in the blood plasma (see section Special instructions
).
Valproic acid
Clinical studies of the potential interaction between nifedipine and valproic acid are not presented. Since it has been established that valproic acid can increase the concentration of a structurally similar BMCA, nimodipine, in the blood plasma, an increase in the concentration of nifedipine in the blood plasma cannot be excluded and, as a result, an increase in its therapeutic effect (see section Special instructions
I).
Cimetidine
Due to the inhibition of the CYP3A4 isoenzyme, cimetidine increases the concentration of nifedipine in the blood plasma and may enhance the antihypertensive effect (see section Special instructions
).
Other types of interaction
Cisapride
Concomitant use of cisapride and nifedipine may lead to an increase in the concentration of nifedipine in the blood plasma.
Inducers of the cytochrome P450 isoenzyme CYP3A4, including antiepileptic drugs such as phenytoin, carbamazette and phenobarbital
Phenytoin induces the CYP3A4 isoenzyme. With simultaneous use of nifedipine with phenytoin, the bioavailability of nifedipine is reduced and, thus, its effectiveness is reduced. When taking these drugs simultaneously, the clinical effect of nifedipine should be assessed and, if necessary, it is recommended to increase its dose. If the dose of nifedipine is increased during concomitant use of both drugs, a reduction in the dose of nifedipine is required after discontinuation of phenytoin. Clinical studies of the potential interaction between nifedipine and carbamazepine or phenobarbital are not presented. Since it was found that, due to enzyme induction, both drugs can reduce the concentration of BMCC, nimodipine, which is similar in structure, in the blood plasma, a decrease in the concentration of nifedipine in the blood plasma and, as a result, a decrease in its therapeutic effect cannot be ruled out.
Effect of nifedipine on other drugs:
Drugs that lower blood pressure
The combined use of nifedipine with other antihypertensive drugs can lead to a mutual enhancement of the antihypertensive effect, such drugs, for example, include:
- Diuretics,
- Beta blockers,
- Angiotensin-converting enzyme inhibitors,
- Angiotensin I receptor antagonists,
- BMKK,
- Alpha blockers,
- Phosphodiesterase type 5 inhibitors,
- Methyldopa,
- Magnesium sulfate.
When using nifedipine and beta-blockers simultaneously, patients should be closely monitored, as in some cases the course of chronic heart failure may worsen.
Digoxin
The simultaneous use of nifedipine and digoxin may lead to a decrease in the clearance of digoxin and, as a result, to an increase in its concentration in the blood plasma. The patient should be carefully monitored for symptoms of digoxin overdose and, if necessary, reduce the dose of digoxin, taking into account its plasma concentration.
Quinidine
When combined with quinidine, its plasma concentration may decrease in some patients, and subsequently, when nifedipine is stopped, it may increase significantly. Therefore, when taking nifedipine concomitantly or discontinuing it after co-administration with quinidine, it is recommended to carefully monitor the concentration of quinidine in the blood plasma and, if necessary, adjust its dose. An increase in the concentration of nifedipine in the blood plasma has also been reported during the simultaneous use of drugs, while at the same time, in some patients there is no change in the pharmacokinetic parameters of nifedipine. When adding quinidine to nifedipine therapy, blood pressure should be monitored and, if necessary, it is recommended to reduce the dose of nifedipine.
Tacrolimus
Tacrolimus is metabolized by the cytochrome P450 isoenzyme CYP3A4. Recently published data indicate that the dose of tacrolimus when coadministered with nifedipine may be reduced in some patients. When taking drugs simultaneously, the concentration of tacrolimus in the blood plasma should be monitored, and if necessary, it is recommended to reduce its dose.
Grapefruit juice
Grapefruit juice inhibits the CYP3A4 isoenzyme of the cytochrome P450 system. Taking nifedipine with grapefruit juice may lead to increased plasma concentrations of nifedipine and prolongation of its action as a result of reduced first-pass metabolism or decreased clearance. This may lead to an increase in the antihypertensive effect of nifedipine. If you take grapefruit juice regularly, this effect can last at least 3 days after your last intake. Grapefruit juice should be avoided while taking nifedipine (see Dosage and Administration).
).
Other forms of interaction
Nifedipine may cause a false increase in the concentration of vanillylmandelic acid in urine when determined by the spectrophotometric method, but does not affect the results of measurements using the high-performance liquid chromatography (HPLC) method.
Nifedipine
Nifedipine
(lat.
Nifedipine
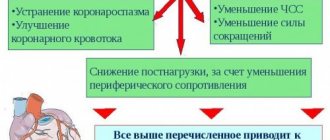
) - a selective blocker of slow calcium channels with a predominant effect on blood vessels, an antianginal, hypotensive agent. In gastroenterology it is sometimes used as a myotropic antispasmodic.
Nifedipine is a chemical compound
Chemically, nifedipine is 1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)-3,5-pyridinedicarboxylic acid dimethyl ester. The empirical formula of nifedipine is C17H18N2O2. Molecular weight - 346.335 g/mol. Yellow crystalline powder. Nifedipine is insoluble in water and poorly soluble in alcohol.
Nifedipine is a medicine
Nifedipine is the international nonproprietary name (INN) of the drug. According to the pharmacological index, nifedipine belongs to the group “Calcium channel blockers”. According to ATC, nifedipine is included in the group “C08 Calcium channel blockers” and has the code C08CA05. In addition, nifedipine is the trade name of the drug.
Indications for use of nifedipine
Nifedipine is indicated for:
- arterial hypertension
- hypertensive crises
- prevention and treatment of angina pectoris
- Raynaud's syndrome
Nifedipine in gastroenterology and proctology
In the 1980–90s, many studies were carried out regarding the effect of nifedipine on the gastrointestinal tract and the following results were obtained (Zimmerman Ya.S., Budnik Yu.B.):
- using manometry of the sphincter of Oddi, it was found that nifedipine causes a significant decrease in the basal pressure of the sphincter of Oddi, the amplitude, duration and frequency of its phase contractions in both healthy people and in patients with biliary dyskinesia, which made it possible to recommend nifedipine for the treatment of biliary dyskinesia
- nifedipine, compared with other calcium channel blockers, such as verapamil and gallopamil, inhibits the secretion of hydrochloric acid by parietal cells to a lesser extent
- after taking nifedipine, the amplitude of contractions in the body of the stomach decreases, indicators of motor activity, motor index and work/rest ratio decrease; in the antrum, in addition, the frequency of gastric contractions decreases
- during electrogastrography (EGG) of gastric motor function, it was found that nifedipine at a dose of 20 mg significantly reduces the initially increased frequency of gastric contractions, the average amplitude and the total power of biopotentials; This dose of nifedipine does not have a noticeable effect on initially low and normal EGG values; at a dose of 10 mg, nifedipine increases the initially reduced frequency of gastric contractions and, conversely, reduces it with an initial increase
- nifedipine significantly increases blood flow in the wall of the stomach and duodenum, and more significantly in the duodenum.
However, nifedipine was not introduced into practical medicine as a treatment for diseases of the intestines and biliary tract, firstly, due to its non-selectivity in relation to the gastrointestinal tract and significant effects on the vascular system, and, secondly, due to the appearance calcium blockers pinaverium bromide and otilonium bromide, selective for smooth muscles of the abdominal organs.
Based on the fact that nifedipine has a moderate antispasmodic effect on the walls of the esophagus and the tone of the lower esophageal sphincter, it is used for esophagospasm (Sheptulin A.A.) and hypermotor dyskinesias of the esophagus (Trukhmanov A.S.).
The use of nifedipine in the treatment of chronic anal fissures is relevant. Nifedipine persistently reduces the tone of the internal anal sphincter both when used sublingually and orally. To achieve a clinical effect, 20 mg of nifepedin is prescribed twice a day or 40 mg of nifedipine retard once. The course of treatment is 8 weeks. Healing of chronic anal fissures is observed in 40–60% of patients. Side effects of nifedipine therapy include headache in 30% of patients, fever, redness of the skin in 60% of patients (Krylov N.N.).
Order of administration (administration) of nifedipine and dose
The dosage and duration of treatment with nifedipine are established by the attending physician individually,
taking into account the patient’s condition and the specific dosage form of the drug. Approximately:
When taken orally
. The initial dose is 10 mg of nifedipine 3-4 times a day, during or after meals, with a small amount of water. If necessary, the dose is gradually increased to 20 mg of nifedipine 3-4 times a day. In special cases, for a short time the dose can be increased to 30 mg 3-4 times a day. The daily dose should not exceed 120 mg.
For arterial hypertension, nifedipine is taken 3 times a day, 10 mg, if necessary, the dose is increased to 20–30 mg (3 times a day).
Sublingual
. For hypertensive crisis and angina attack, take 10–20 mg of nifedipine once. If necessary, nifedipine is repeated.
Intravenously
. In case of hypertensive crisis, 5 mg of nifedipine is administered over 4–8 hours. The maximum daily dose is 15–30 mg of nifedipine.
Intracoronary
when relieving acute spasms of the coronary arteries - 100-200 mcg.
Taking the maximum daily dose, depending on the dosage form and the patient’s condition, should be limited to several days. In elderly patients and those with other diseases, the maximum daily dose should be reduced.
In case of acute myocardial infarction, severe cerebrovascular accidents, impaired liver or kidney function, diabetes mellitus, nifedipine is used only in a clinical setting under the supervision of a physician.
It is necessary to stop taking nifedipine gradually, reducing the daily dose.
Problems of interaction of nifedipine with acid-lowering drugs
Nifedipine is metabolized by the cytochrome P450 isoenzyme CYP3A4 located in liver hepatocytes and apical membranes of small intestinal enterocytes.
With the help of this isoenzyme, the biotransformation of a large group of drugs occurs (about 60% of oxidized drugs). When two or more drugs metabolized by the same type of isoenzymes are taken simultaneously, “competition for resources” occurs and the metabolic process changes. In addition, various substances may inhibit or stimulate metabolism and other interactions, thereby altering the effects of drugs. Many of these mechanisms are not well understood. Proton pump inhibitors and nifedipine
. Metabolism of proton pump inhibitors is carried out with the participation of cytochrome P-450 isoforms. During metabolism, their monooxidase activity decreases, which underlies drug-drug interactions. The metabolism of omeprazole and, partially, lansoprazole slow down the metabolism of nifedipine. Among proton pump inhibitors, pantoprazole has the lowest affinity for the cytochrome P-450 system (Bordin D.S.). Therefore, if it is necessary to take nifedipine and a proton pump inhibitor for the treatment of synchronous diseases, pantoprazole should be prescribed, and not another PPI (Standards for the diagnosis and treatment of acid-dependent and Helicobacter pylori-associated diseases).
H2 blockers and nifedipine.
Cimetidine and ranitidine slow down biotransformation and increase the level of nifedipine in plasma.
Interaction of nifedipine with some other drugs
Nifedipine affects or is affected by many drugs. The following are just some of the interactions:
- The simultaneous use of nifedimin with verapamil
enhances the effect of both drugs. - Octreotide
modifies the effects of nifedipine. When octreotide and nifedipine are co-administered, monitoring of blood pressure is necessary. It may be necessary to change the dose. - Rifampicin
accelerates the biotransformation of nifedipine and does not allow the creation of effective plasma concentrations of nifedipine. The simultaneous use of rifampicin and nifedipine is contraindicated. - Fluconazole
may increase the systemic exposure of nifedipine. Patients taking fluconazole and nifedipine at the same time have a significantly increased risk of side effects.
When combined with nifedipine, inhibitors of the cytochrome P450 CYP3A isoenzyme, such as fluconazole, itraconazole, clarithromycin, erythromycin, fluoxetine, saquinavir, indinavir, nelfinavir, may lead to increased nifedipine exposure. Caution should be exercised, patients' condition should be carefully monitored and the dosage of medications should be adjusted if necessary. When co-administered with CYP3A4 inhibitors, the dose of nifedipine should be selected as low as possible.
Professional medical articles addressing the use of nifedipine in gastroenterology and proctology
- Zimmerman Ya.S., Budnik Yu.B. Prerequisites for the use of calcium antagonists in the treatment of diseases of the digestive system // Russian Journal of Gastroenterology, Hepatology, Coloproctology. – 1995. – No. 3. – p. 22–28.
- Krylov N.N. Chronic anal fissure // Bulletin of surgical gastroenterology. - 2008, - No. 1, - p. 5-11.
- Clinical recommendations for the diagnosis and treatment of adult patients with anal fissure // Association of Coloproctologists of Russia. Moscow. 2013 17 p.
- Fomina L.A. Comorbid course of gastroduodenal ulcers in terms of calcium imbalance and slow calcium channel blockers in their treatment // Therapeutic archive. 2021. No. 2. pp. 28–34.
- Fomina L.A. Calcium imbalance in the pathogenesis of symptomatic gastoduodenal ulcers associated with non-steroidal anti-inflammatory drugs, and the effectiveness of slow calcium channel blockers in their treatment // Experimental. and clinical gastroenterology. 2021. Issue. 151. No. 3. pp. 58–63.
On the website in the literature catalog there is a section “Anspasmodics”, containing articles on the use of antispasmodics in the treatment of diseases of the gastrointestinal tract.
Use of nifedipine in pregnant women, nursing mothers and children
Nifedipine should not be used by pregnant, breastfeeding women and children.
Nifedipine is FDA Category C (animal studies have shown adverse effects on the fetus and there have been no adequate studies in pregnant women, but the potential benefits associated with the use of this drug in pregnant women may justify its use despite the risks ).
A certain amount of nifedipine is excreted into mother's milk and therefore breastfeeding should be suspended while the mother is being treated with nifedipine.
The safety and effectiveness of nifedipine in children has not been established.
Contraindications to the use of nifedipine
Arterial hypotension, tachycardia, the first 8 days of myocardial infarction, collapse, cardiogenic shock, severe heart failure, severe aortic stenosis, hypersensitivity to nifedipine.
Trade names of drugs containing the active ingredient nifedipine
The following drugs with the active substance nifedipine are (were) registered in Russia: Adalat, Adalat SL, Vero-Nifedipine, Calcigard retard, Cordafen, Cordaflex, Cordaflex, Cordipin, Cordipin XL, Cordipin retard, Corinfar, Corinfar retard, Corinfar UNO, Nicardia, Nicardia SD retard, Nifadil, Nifebene, Nifehexal, Nifedex, Nifedicap, Nifedicor, Nifedipine, Nifecard, Nifecard HL, Nifelat, Nifelat Q, Nifelat R, Nifesan, Osmo-Adalat, Sanfidipine, Sponif 10, Phenigidine.
In the United States, nifedipine is sold under the trade names: Adalat CC, Afeditab CR, Nifediac CC, Nifedical XL, Procardia, Procardia XL.
Instructions
Official instructions for the use of various drugs with the active substance nifedipine (pdf):
- instructions, tablets 10 mg
- Bayer Pharma instructions:
- “Instructions for use of the drug for medical use Adalat”, solution for infusion, dated January 17, 2013.
- “Instructions for use of the drug for medical use Osmo-Adalat”, tablets 30 and 60 mg, dated September 11, 2012.
- “Instructions for medical use of the drug Corinfar”, extended-release film-coated tablets, 10 mg, dated June 24, 2009.
- “Instructions for medical use of the drug Corinfar Retard,” extended-release film-coated tablets, 20 mg, dated May 14, 2009.
- “Instructions for medical use of the drug Corinfar UNO”, modified-release film-coated tablets, 40 mg
- "Procardia (nifedipine) Capsules For Oral Use", August 2011
- "Procardia XL (nifedipine) Extended Release Tablets For Oral Use", August 2011
general information
Nifedipine is a prescription medicine.
Nifedipine has contraindications, side effects and application features; before taking it, consultation with a specialist is necessary.
Back to section