Nicorette combines several drugs designed to help former smokers:
- Transdermal patch. Available in the form of rectangular plates on a foil or silicone substrate. The adhesive side of the drug contains about 10 mg. nicotine - dose for 16 hours of action. There are 7 plates in a cardboard package.
- Spray. A clear solution of minty-fruity flavor in plastic spray bottles, one dose contains approximately 13 mg. nicotine
- Pills. White or yellow, 10 pieces in a blister, each containing 2 mg. nicotine A cardboard pack is designed for 30–100 pieces.
- Chewing gum in an assortment: with mint or fruit flavors. White square-shaped lozenges, packed in 15 pieces in cell plates. One serving contains 2-4 mg. nicotine
The entire Nicorette line is not an alternative to smoking, but helps to quit it by supplying the body with small doses of nicotine that do not have a strong toxic effect. Abrupt cessation of cigarettes provokes a withdrawal syndrome, under the influence of which addicts lose self-control, control over their emotional state, experience negative physical symptoms and a strong craving for nicotine. The result may be a relapse and a return to smoking. Nicorette replaces some of the usual neurotoxin, reducing withdrawal symptoms and helping you keep going.
The use of nicotine-containing drugs reduces the desire to puff on a cigarette, improves mood, calms the heart rate, relieves irritation, anxiety, and relieves obsessive thoughts. The drugs do not have any therapeutic effect, but significantly alleviate the suffering of smokers, especially in the first days and weeks after quitting. With their help, it is easier to avoid relapses and get used to life without smoking forever.
How to use Nicorette patch
This remedy is recommended for heavy smokers who find it difficult to tolerate giving up their usual doses. One patch is intended for 1 day, it must be put on in the morning and removed before bed:
- the plate should be carefully cut out of the packaging with scissors;
- detach the backing and apply the adhesive side to the skin: on the inner thigh or other area where there is no thick hair or damage, the skin must be dry and clean;
- Carefully press the patch over the entire surface, avoiding touching the adhesive side.
Nicotine is released from the drug gradually, absorbed through the pores into the blood in small doses and neutralizes negative withdrawal symptoms. A new patch must be used daily. The course of use of the product is up to 8 weeks.
For moderate or slight cravings for smoking, it is recommended to apply the product every other day or every 2 days, each time extending the nicotine-free break until the need for nicotine doping subsides. For intense cravings, you can supplement the action of the patches with another Nicorette product: spray, tablets or chewing gum.
Smoking and men's health
Smoking directly affects erectile dysfunction and increases the risk of impotence by 27% (even in young people) because harmful substances in cigarette smoke reduce blood pressure.
Cigarette smoke slows down the release of testosterone, which leads to decreased libido. Also, sperm may become less and less active, so the ability to conceive decreases. Smoking damages sperm DNA, increasing the risk of pregnancy complications and birth defects.
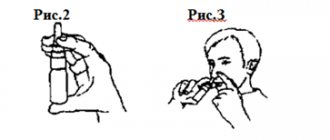
How to apply the spray
In case of moderate dependence, the product is suitable for self-support of former smokers. If the craving is very strong, it is best to fight it with the help of patches, and use the aerosol as an additional drug.
- You need to spray the solution onto your tongue, avoiding getting the liquid on your skin, lips and gums.
- You can inject 1-2 doses at a time, the frequency of use depends on the degree of craving: the longer you can stay without doping, the better. The maximum permissible dose per day is 5 injections.
- After using Nicorette, you must refrain from brushing your teeth, eating and drinking for 10–20 minutes, and try not to swallow saliva so that the dose of nicotine can be absorbed into the blood.
If necessary, the spray can be combined with chewing gum or Nicorette tablets.
Nicorette mint anti-smoking spray for topical use 1 mg/dose, 13.2 ml 1 pc.
pharmachologic effect
A remedy for the treatment of nicotine addiction.
Composition and release form Nicorette mint anti-smoking spray for topical use 1 mg/dose, 13.2 ml 1 pc.
Transdermal therapeutic system (patch) - 1 cm2 nicotine - 0.83 mg excipients: backing film; non-woven polyester, medium molecular weight polyisobutylene, low molecular weight polyisobutylene, polybutene, siliconized polyester in sachet 1 patch 5 mg/16 hours, 10 mg/16 hours or 15 mg/16 hours; in a cardboard pack there are 7 or 14 sachets.
Description of the dosage form
5 mg/16 h patch: rectangular patch with rounded edges with an area of 10 cm2; One side is light brown with the inscription “nicorette®”, the other is adhesive, silver-white, covered with a transparent synthetic protective film. 10 mg/16 h patch: rectangular patch with rounded edges with an area of 20 cm2; One side is light brown with the inscription “nicorette®”, the other is adhesive, silver-white, covered with a transparent synthetic protective film. 15 mg/16 h patch: rectangular patch with rounded edges with an area of 30 cm2; One side is light brown with the inscription “nicorette®”, the other is adhesive, silver-white, covered with a transparent synthetic protective film.
Directions for use and doses
For application to the oral mucosa.
The patient should do everything possible to permanently quit smoking while being treated with Nicorette spray.
Adults over 18 years old
Nicorette spray should be used at the moment when an irresistible desire to smoke arises. After preparing the spray for use, place the tip of the spray as close to your open mouth as possible. Press the dispenser from above, thus releasing one dose of the drug into the oral cavity; Avoid getting the spray on your lips. To prevent the substance from entering the respiratory tract, do not inhale when pressing the dispenser. For best results, do not swallow saliva for several seconds after injection.
While using the spray, eating and drinking is not recommended.
If symptoms of overdose appear, use of the drug should be stopped immediately.
Complete smoking cessation Nicorette Spray should be used in all cases of craving for smoking or to prevent cravings in situations that may provoke it.
Smokers who want or are able to quit smoking immediately should immediately replace smoking cigarettes with Nicorette spray and, as soon as possible, reduce the number of injections until they stop completely.
If you completely quit smoking, take 1 or 2 injections during the period of time when you usually smoked a cigarette, as well as if you have a craving for smoking.
If after a single injection the craving for smoking does not decrease within a few minutes, a second injection should be made. If two doses are required, subsequent application of the spray may consist of 2 consecutive injections.
Every hour you are allowed to take no more than 4 dosed injections of the spray.
Do not inject more than 2 doses of spray at a time or use more than 64 doses per day (or 4 doses per hour for 16 hours).
Each bottle contains at least 150 doses.
The average course of use of the spray at the indicated dose is 6 weeks. Then you should begin to reduce the number of injections so that by the end of the 9th week the number of doses is half the average number of doses per day received in the first 6 weeks, and during the 12th week - no more than 4 doses per day.
When the daily dose drops to 2-4 injections, use of the spray should be discontinued.
After completion of therapy, to prevent a return to smoking, patients can use Nicorette spray if they have an irresistible urge to smoke. In such situations, you can make 1 injection, and if after a single injection the craving for smoking does not decrease within a few minutes, you should make a second injection. In this case, you should not exceed 4 dosed injections per day.
Regular use of the spray for more than 6 months is usually not recommended, but some patients may require longer therapy to avoid relapse into smoking.
Reducing the number of cigarettes smoked
Smokers wishing to reduce the number of cigarettes they smoke should use the spray as needed between smoking episodes to increase the time between smoking and to reduce smoking as much as possible.
Once readiness is felt, smokers should aim to quit completely.
After quitting smoking, you should follow the recommendations for therapy and gradual dose reduction indicated above for complete smoking cessation.
Behavioral therapy and psychological support usually increase treatment success. Those who have managed to quit smoking but find it difficult to give up the spray are advised to consult a doctor for medical help.
Temporary smoking cessation The spray can be used during periods when it is necessary to abstain from smoking, for example, when in places where smoking is prohibited, or in other situations when it is necessary to abstain from smoking. The maximum daily dose for temporary smoking cessation is 64 doses.
In combination with a transdermal patch For smokers with a severe nicotine addiction (more than 20 cigarettes per day) or experiencing an irresistible craving for smoking, or smokers who have not been able to quit smoking using only one type of nicotine replacement therapy, it is possible to use a spray for the mucous membrane Nicorette oral membranes in combination with the Nicorette transdermal patch for rapid relief of smoking cravings.
The patch is applied to an intact area of skin immediately after waking up in the morning and removed before going to bed. The patch should be applied to dry, clean, intact, hair-free skin, such as the thighs, upper limbs or chest. It is necessary to change the application site every day: do not use the same area for two consecutive days. After applying the patch, wash your hands thoroughly to avoid eye irritation from possible nicotine contact.
Initial therapy:
Treatment should begin with a 25 mg/16 hour patch (stage 1) in combination with a 1 mg/dose spray. Usually 13 doses of spray per day are sufficient. The maximum daily dose of the spray is 32 doses.
Patients should completely stop smoking during therapy. Usually the general course of treatment lasts for 8 weeks. After this, the dose of nicotine should be gradually reduced.
Cancellation of combination therapy:
Combination therapy can be discontinued in two ways.
Method 1: Over the next 2 weeks, switch from the 25 mg/16 hour patch (Stage 1) to the 15 mg/16 hour patch (Stage 2), and then, over the next 2 weeks, to the 10 mg/16 hour patch ( stage 3), while maintaining, if necessary, the number of doses of Nicorette spray used, as with Initial therapy. Next, the number of doses of the spray is gradually reduced until complete withdrawal for the time that the patient needs depending on his needs, but no later than 12 months after the start of combination therapy.
Method 2: involves completely removing the patch immediately after completing the Initial Therapy phase. Next, the number of doses of Nicorette spray is gradually reduced until complete withdrawal for the time required by the patient depending on his needs, but no later than 12 months after the start of combination therapy.
Recommended dose:
- Initial therapy: First 8 weeks - 1 patch 25 mg/16 hours (1 stage) daily. If necessary. 5-6 chewable gums/sublingual tablets/13 doses of topical spray per day are recommended.
- Cancellation (1st method): The next 2 weeks - 1 patch of 15 mg/16 hours (stage 2) daily. Continue use of gummies/sublingual tablets/topical spray as needed. The next 2 weeks - 1 patch 10 mg/16 hours (stage 3) daily. Continue use of gummies/sublingual tablets/topical spray as needed. Up to 12 months after starting combination therapy. Gradually phase out chewing gum/sublingual tablets/topical spray.
- Cancellation (2nd method): Up to 12 months after starting combination therapy. Gradually phase out chewing gum/sublingual tablets/topical spray.
Children and teenagers under 18 years of age
The drug is not recommended for use by persons under 18 years of age. There is no experience in treating adolescents under 18 years of age with the spray.
How to open the spray dispenser/unlock the nozzle
- Using your thumb, gently lower the dispenser button down until you can press the button inward.
- While pressing the button, lift the dispenser up. Release the button.
First use of Nicorette spray
When using the spray for the first time, you should check the operation of the sprayer.
Take the bottle so that the stream is directed away from you, other people, children and pets. Open the spray nozzle and press 3 times until fine spray appears.
If you do not use the spray within 2 days, you must repeat this procedure.
How to use Nicorette spray
- Apply the spray bottle to your open mouth and hold it as close as possible.
- Press the dispenser to release a dose of spray into the oral cavity. When spraying, avoid getting the spray on the back of the throat and lips.
Do not inhale the spray while spraying to prevent ingestion.
For best results, do not swallow within a few seconds of spraying.
How to close the spray dispenser/lock the nozzle
- Smoothly lower the dispenser button down until the button can be pressed inward.
- Press the dispenser button and lower the dispenser down. Then release the button. The spray is now closed.
For next use, repeat the above steps.
Close the spray bottle each time after use to prevent children from using the spray or accidental spraying.
If the spray accidentally gets into your eyes, rinse your eyes with water.
Pharmacodynamics
Nicotine is an agonist of nicotinic receptors in the peripheral and central nervous system (CNS), and has a pronounced effect on the central nervous system and the cardiovascular system.
Abrupt cessation of smoking causes the development of a characteristic withdrawal syndrome, including cravings for smoking.
Clinical studies have shown that nicotine replacement therapy drugs help smokers abstain from smoking by easing withdrawal symptoms. A single-dose study in 200 healthy smokers demonstrated that using two doses of a 1 mg spray reduced the desire to smoke from the first minute after using the spray and to a significantly greater extent than using lozenges containing nicotine. Compared to nicotine gum or lozenges, nicotine absorption from an oral spray is faster and, based on accumulated experience with nicotine replacement therapy, results in a faster reduction in cravings and other symptoms.
Increased appetite is a well-known symptom of nicotine withdrawal, and weight gain often occurs after smoking cessation. An important symptom of withdrawal syndrome is also the desire to smoke.
Pharmacokinetics
The pharmacokinetics of nicotine have been extensively studied; it was found that the method of delivery of the substance to the body has a significant impact on the rate and extent of absorption.
The pharmacokinetics of the oral mucosal spray were studied in four studies involving 141 subjects.
Suction
The dosage form of the spray for the oral mucosa assumes that the dose of nicotine is delivered immediately and, as a result, its absorption from the oral cavity is rapid.
The maximum concentration of 5.3 ng/ml is achieved within 13 minutes after administration of 2 mg of nicotine. 10 minutes after application of the 1 and 2 mg spray, the area under the concentration-time curve (AUC) exceeded those obtained in studies of chewing gum and tablets containing 4 mg nicotine (0.48 and 0.64 h×ng /ml, compared with 0.33 and 0.33 h×ng/ml).
AUC∞ values indicate that the bioavailability of nicotine when applied as a spray to the oral mucosa is similar to the bioavailability when using tablets and is slightly higher than when using nicotine chewing gum. The AUC∞ for the 2 mg spray was 14.0 h×ng/mL compared with 23.0 h×ng/mL for the 4 mg chewing gum and 26.7 h×ng/mL for the 4 mg tablets.
The mean steady-state plasma nicotine concentration achieved after administration of the maximum dose (i.e., 2 sprays of 1 mg spray every 30 minutes) was approximately 28.8 ng/ml compared to 23.3 ng/ml with chewable nicotine. gum at a dosage of 4 mg (1 tablet every hour) and 25.5 ng/ml for nicotine tablets at a dosage of 4 mg (1 tablet every hour).
Given the rapid absorption and similar high relative bioavailability, most of the nicotine released from the spray is apparently absorbed through the buccal mucosa.
Distribution
The volume of distribution after intravenous administration of nicotine ranges from 2–3 l/kg.
The binding of nicotine to blood plasma proteins is less than 5%. For this reason, changes in nicotine binding due to the use of concomitant drugs or changes in plasma protein levels in a number of diseases should presumably not have a significant effect on the pharmacokinetics of nicotine.
Biotransformation
The metabolism and elimination of nicotine are independent of the dosage form, and therefore the results of studies of nicotine administered intravenously are suitable for their description.
The main organ that eliminates nicotine is the liver. However, nicotine is also metabolized in the kidneys and lungs. More than 20 nicotine metabolites are known, all of which appear to be less active than the parent compound.
The plasma half-life of the main metabolite of nicotine, cotinine, is 15–20 hours, and its concentration is 10 times higher than that of nicotine.
Removal
The average plasma clearance of nicotine is 70 l/hour, the half-life is 2–3 hours.
The main nicotine metabolites found in urine are cotinine (12% of the administered dose) and trans-3-hydroxycotinine (37% of the administered dose).
Approximately 10% of nicotine is excreted unchanged in the urine.
At high filtration rates and urine pH below 5, the amount of nicotine excreted unchanged in urine can reach 30%.
Linearity/nonlinearity
At 1, 2, 3, and 4 sprays of the 1 mg/dose spray, only minor deviations from linearity were detected in AUC∞ and Cmax.
Special patient groups
Renal dysfunction
The progression of renal failure is accompanied by a decrease in the overall clearance of nicotine. Nicotine clearance in patients with severe renal impairment is reduced by an average of 50%. Increased nicotine concentrations were observed in hemodialysis patients.
Liver dysfunction
The pharmacokinetics of nicotine in patients with mild liver dysfunction (5 points on the Child-Pugh scale) did not change, but in the presence of moderate liver dysfunction (7 points on the Child-Pugh scale) it decreased by 40–50%.
There are no data on the pharmacokinetics of nicotine in liver dysfunction of more than 7 points on the Child-Pugh scale.
Elderly patients
In healthy elderly patients, a slight decrease in the total clearance of nicotine was noted, which did not require a change in the dose of the drug.
Indications for use Nicorette mint anti-smoking spray for topical use 1 mg/dose, 13.2 ml 1 pc.
Nicorette spray relieves and/or prevents cravings for smoking and withdrawal symptoms that occur with tobacco addiction. Indicated as support for smokers who intend to quit smoking or reduce the number of cigarettes smoked before quitting completely; in order to assist smokers who do not want to smoke or do not have such an opportunity; and also as a safer alternative to smoking.
Contraindications
Hypersensitivity to nicotine or other components of the drug. Children under 18 years of age.
Application of Nicorette mint anti-smoking spray for topical use 1 mg/dose, 13.2 ml 1 pc. during pregnancy and breastfeeding
Pregnancy
Smoking during pregnancy is associated with risks such as intrauterine growth restriction, premature birth, or stillbirth. Smoking cessation is the single most effective intervention for improving the health of both the pregnant woman and her baby. Quitting smoking early is the best option.
Nicotine penetrates the placental barrier and affects the respiratory activity and blood circulation of the fetus. The effect on blood circulation is dose-dependent.
Therefore, ideally, smoking cessation during pregnancy should be carried out without nicotine replacement therapy. However, if a woman is unable (or expected) to quit smoking without pharmacological support, then nicotine replacement therapy is used, since continued smoking carries a greater risk to the fetus compared to nicotine replacement therapy. The best option is to quit smoking completely, but if this is not possible, Nicorette spray can be used as a safer alternative to smoking during pregnancy. Due to the potential for nicotine-free periods, intermittent dosage forms are preferred, but patches may be required if nausea and/or vomiting is significant. A pregnant woman should begin using Nicorette spray only after consulting a doctor.
Breast-feeding
Nicotine passes into breast milk in quantities that can affect the baby even when the drug is used in therapeutic doses.
Therefore, you should refrain from using Nicorette spray during breastfeeding. If you are unable to quit smoking, use of the drug should only be started after consultation with your doctor.
Dosage forms for periodic use minimize the nicotine content in breast milk and allow breastfeeding with the lowest nicotine concentration in it. In order to reduce the negative effects of nicotine on a child, the drug should be used immediately after feeding. The periods between the use of the drug and the next feeding should be as long as possible (at least 2 hours are recommended).
Smoking increases the risk of infertility in men and women. In vitro studies have shown that nicotine negatively affects sperm quality in men.
special instructions
The use of Nicorette is associated with less risk than smoking.
An assessment of the risk-benefit ratio should be made by a physician of the appropriate specialty in patients with the following conditions:
Concomitant cardiovascular diseases
With a stable course of cardiovascular diseases, Nicorette spray causes less harm than continued smoking. However, smokers with a recent myocardial infarction, unstable or worsening angina, including Prinzmetal angina, severe arrhythmia, recent cerebrovascular disease, and/or patients with uncontrolled hypertension should be advised to stop smoking without pharmacological intervention. If such attempts are unsuccessful, the use of Nicorette spray may be considered, but since safety data in this category of patients is limited, such treatment should only be initiated under strict medical supervision.
Diabetes
In patients with diabetes mellitus, it is recommended that blood glucose concentrations be monitored more closely after cessation of smoking and from the start of nicotine replacement therapy, since a decrease in catecholamines, the release of which is induced by nicotine, may affect carbohydrate metabolism.
Allergic reactions
The drug should be used with caution in patients predisposed to angioedema and urticaria.
Diseases of the gastrointestinal tract
Ingested nicotine can aggravate the symptoms of esophagitis, gastritis, or peptic ulcers, so oral nicotine replacement therapy should be used with caution in these conditions.
Liver and kidney dysfunction
In patients with moderate to severe hepatic impairment and/or severe renal impairment, the drug should be used with caution as the clearance of nicotine and its metabolites may be reduced, which may increase the risk of adverse events.
Danger for small children
Doses of nicotine that are easily tolerated by adult and adolescent smokers can cause severe toxicity in children, which can lead to death. It is important not to leave nicotine-containing products unattended, as this may lead to their misuse and ingestion by children.
Pheochromocytoma and uncontrolled hyperthyroidism
In patients with uncontrolled hyperthyroidism and pheochromocytoma, the drug should be used with caution, since nicotine causes the release of catecholamines.
Formation of addiction
Dependence on the drug may develop, but it is less dangerous to health and more easily overcome than addiction to smoking.
To give up smoking
Polycyclic aromatic hydrocarbons contained in tobacco smoke induce the metabolism of drugs metabolized by the CYP1A2 isoenzyme (and possibly CYP1A1). Quitting smoking can cause a slowdown in metabolism and, as a result, an increase in the concentration of these drugs in the blood.
This has potential clinical implications for drugs with a narrow therapeutic index, such as theophylline, tacrine, clozapine and ropinirole.
Excipients
Nicorette spray contains a small amount of ethanol (alcohol) - less than 10 mg per dose.
When using Nicorette spray, you should be careful not to let the spray get into your eyes.
The warnings and precautions for combination therapy of Nicorette transdermal patch spray are the same as the warnings and precautions for using either drug alone.
If the medicine has become unusable or has expired, do not throw it into wastewater or onto the street! Place the medication in a bag and place it in the trash. These measures will help protect the environment!
Impact on the ability to drive vehicles and operate machinery
The profile of adverse reactions of the drug (dizziness, changes in behavior, etc.) should be taken into account, which may impair the ability to drive vehicles and operate machinery.
Overdose
When used in accordance with the instructions for use, symptoms of nicotine overdose may occur in patients with a low pre-treatment nicotine intake or when using multiple nicotine sources simultaneously.
Symptoms
The minimum lethal dose for an acute overdose for an unaccustomed adult is 40–60 mg of nicotine. In case of an overdose, the same symptoms are observed as in acute nicotine poisoning, namely: nausea, vomiting, increased salivation, abdominal pain, diarrhea, sweating, headache, dizziness, hearing loss and severe general weakness. At higher doses, they may be accompanied by arterial hypotension, weak and irregular pulse, respiratory failure, impaired consciousness, collapse and generalized convulsions.
Doses of nicotine that are well tolerated during treatment in adult smokers can cause symptoms of severe poisoning in young children and can even be fatal. Suspicion of nicotine poisoning in children should be regarded as an emergency condition requiring immediate hospitalization.
Treatment
Nicotine use should be stopped immediately and symptomatic treatment should be initiated. If necessary, artificial ventilation should be started.
Taking activated charcoal prevents the absorption of nicotine in the gastrointestinal tract.
Side effects Nicorette mint anti-smoking spray for topical use 1 mg/dose, 13.2 ml 1 pc.
Regardless of the dosage form of the nicotine product used, some symptoms may be due to nicotine withdrawal due to smoking cessation. These include the following: dysphoria or depressed mood; insomnia; irritability, dissatisfaction or anger; anxiety; difficulty concentrating, restlessness, or impatience; decreased heart rate; increased appetite or weight gain; cough; aphthous ulcers; nasopharyngitis. A cause-and-effect relationship has not been established.
In addition, users of the oral mucosal spray also reported other symptoms associated with smoking cessation: dizziness, near-syncope, cough, constipation and bleeding gums.
Nicotine craving, considered a clinically significant symptom, is an important manifestation of nicotine withdrawal after smoking cessation.
Nicorette spray may cause nicotine-related adverse reactions similar to those observed with other nicotine-containing drugs; these reactions are predominantly dose-dependent.
Allergic reactions (such as angioedema, urticaria and anaphylactic shock) may occur in susceptible individuals.
Most adverse reactions to Nicorette spray were observed in the early phase of treatment and are similar to those for drugs taken orally. In the first few days of treatment, irritation of the mucous membrane of the oral cavity and pharynx may occur, and hiccups are common. Continued treatment leads to adaptation.
Daily data collection from study subjects showed that very common adverse events occurred in the first 2-3 weeks of use of the spray and subsequently disappeared.
Criteria for assessing the frequency of side effects: very common (≥1/10); frequent (≥1/100,
According to clinical studies:
Gastrointestinal disorders: Common: Nausea, vomiting.
General and administration site conditions: Uncommon: Fatigue.
Immune system disorders: Uncommon: Hypersensitivity reactions.
Nervous system disorders: Common: Headache; Uncommon: Paresthesia.
Skin and subcutaneous tissue disorders: Very common: Itching.
According to post-marketing studies:
Cardiac disorders: Uncommon: Palpitations, tachycardia; Rare: Atrial fibrillation.
Gastrointestinal disorders: Rare: Gastrointestinal discomfort.
General and administration site disorders: Uncommon: Application site reactions, asthenia, chest pain and discomfort, malaise.
Immune system disorders: Rare: Allergic reactions, including angioedema and anaphylactic shock.
Musculoskeletal and connective tissue disorders: Uncommon: Myalgia; Unknown: Pain in limbs.
Mental disorders: Uncommon: Unusual dreams.
Respiratory, thoracic and mediastinal disorders: Uncommon: Dyspnea.
Skin and subcutaneous tissue disorders: Common: Skin rashes, urticaria; Uncommon: Increased sweating; Rare: Erythema.
Vascular disorders: Uncommon: Hyperemia, hypertension.
Adverse reactions observed with combination therapy of transdermal patch with chewable gum/sublingual tablets/topical spray differ from those observed with each drug alone only with respect to local adverse events associated with specific dosage forms. The frequency of these adverse reactions is comparable to the frequency given in the instructions for use for each drug.
Drug interactions
There are no clear clinically significant interactions between nicotine replacement therapy and other drugs. However, theoretically, nicotine may enhance the hemodynamic effects of adenosine, i.e., lead to an increase in blood pressure and heart rate, and also enhance the pain response (angina-type chest pain) provoked by the administration of adenosine.
Contraindications to the use of Nicorette
To quit smoking without the help of nicotine-containing aids you need to:
- for diseases of the heart and blood vessels: atherosclerosis, pre-infarction, acute cerebrovascular accident, hypertension;
- for stomach ulcers, internal bleeding, oncological processes;
- with severe diabetes mellitus, liver, kidney failure;
- with thyroid dysfunction.
Pregnant and lactating women also need to choose a different method of therapy. It is also advisable for minors to find another way to quit smoking, since even tiny doses of nicotine continue to harm their health.
Smoking and women's health
Smoking can cause hormonal changes and reduce your fertility by about a third. On average, smokers take twice as long to conceive as non-smokers.
Smoking while using birth control pills contributes to the formation of blood clots, damage and narrowing of the walls of blood vessels, and also significantly increases the risk of developing cardiovascular complications, even strokes. Cigarettes sometimes disrupt women's menstrual cycles, making them irregular and more painful.
Lactation
If you are breastfeeding, you should try to quit smoking without NRT. But if you can't manage it yourself, NRT, taken intermittently, may be a good option. In any case, you should contact your doctor or healthcare professional for advice.
Currently, there are a huge number of different products on the medical goods market that help overcome nicotine addiction and once again feel “easy breathing”. Nicorette products, including spray, can become reliable assistants in quitting cigarette addiction and receive positive reviews from smokers.
Side effects
The nicotine in Nicorette spray differs from the similar substance in cigarettes in its purity, as well as the inability to control the dose received. This is the reason for possible side effects. All smokers remember typical symptoms of an overdose from their first smoking experience:
- dizziness;
- nausea;
- dry mouth;
- abdominal pain;
- change in taste.
Data from post-marketing studies show that patients may experience discomfort from the cardiovascular system: a feeling of heartbeat, tachycardia.
All those smokers who have tried Nicorette spray say that it is necessary to carefully read the instructions so that there are no side effects in the form of an irritated throat, dizziness or hiccups. When used correctly and in the absence of chronic diseases, side effects and discomfort are minimal.
Is it harmful to health?
The use of the drug is accompanied by less risk than smoking, since during the treatment the patient does not receive carcinogenic substances contained in cigarette smoke. However, nicotine itself is harmful to health. Its toxic properties manifest themselves primarily on the cardiovascular system. Nicotine damages blood vessels, accelerating the process of sclerotization of their walls. It leads to increased blood pressure.
- disrupts normal metabolic processes;
- increases the risk of developing diabetes;
- impairs the nutrition of organs and tissues, which leads to diseases;
- has a stimulating effect on the nervous system;
- accelerates skin aging.
Can it be used during pregnancy?
If a woman has not given up smoking before pregnancy and cannot do it on her own during pregnancy, then the spray can be used. The absence of thousands of other toxic substances that are present in tobacco makes Nicorette more preferable than smoking.
However, a woman should first try to quit smoking without using nicotine replacement therapy. Regardless of whether nicotine from a cigarette comes from a spray, it has a negative vasoconstrictor effect, including on the blood vessels of the placenta.