In Russia, antiviral drugs are the top sellers in pharmacies. According to a study by DSMGroup, in 2021, two of the ten best-selling drugs at retail are for ARVI: Kagocel (sales volume - 6.7 billion rubles), Ingavirin (6.1 billion rubles). Since the beginning of 2021, Russian hospitals have spent 46,801,712 rubles on the purchase of Ingavirin Among the leaders in government procurement are the antiviral drugs Arbidol (270,265,857 rubles) and Kagocel (30,694,754).
Does Ingavirin cause cancer?
In mid-October, information began to be widely discussed that the drug Ingavirin causes leukemia (blood cancer) . The reason for this was an investigation by the Foundation for the Health of Children and the Elderly , which claims that the now banned drug Dicarbamin and the drug Ingavirin have exactly the same composition (only the dosage differs: the first has a dosage of 100 mg, the second - 90 mg) , were produced in the same way, but were treated for different things: “Dicarbamin” was used for oncology, “Ingavirin” for colds. From the point of view of representatives of the foundation, taking Dicarbamine by a healthy person leads to a sharp increase in white blood cells and can cause cancer, especially in children.
doctor Ivan Rodin also agrees with this opinion , who on October 19 posted a post analyzing the situation on his blog (5 subscribers) on the Yandex.Zen platform.
“The manufacturing company itself revoked the license for Dicarbamin in the early 2010s. Because according to the law, two drugs with the same active substance and method of treatment cannot be produced under different names and treat completely different diseases. After the license was revoked, the company forgot to add side effects of the antitumor drug to the contraindications for Ingavirin. It is not beneficial to inform naive parents that they stimulate the production of white hematopoiesis in their own children from the age of 3 when they give a popular drug for colds,” concludes Dr. Ivan Rodin.
On October 24, a petition was created on the Change.org website addressed to the Ministry of Health of the Russian Federation, with a request to look into it and provide clarification.
“As the father of two daughters, I am seriously concerned about the fact that a cancer cure may be sold under the guise of an antiviral drug. I ask representatives of the Ministry of Health to clarify the situation and, if necessary, take action,” said the author of the petition.
On October 25, a certain group “Grudinin P.N.! People's President! on the social network Odnoklassniki, which calls itself a public organization, reported that it had begun to remove all negativity about the drug from the network.
“is actively clearing the information space after it became known, according to the Foundation for the Health of Children and the Elderly, that their drug Ingavirin can cause blood cancer. Several videos on Youtube and Vkontakte were deleted, and channel administrators are offered money for blocking negative mentions of Ingavirin..,” said the author of the page on the social network.
Today, various blogs and small media outlets have begun to replicate all this information.
Manufacturers of Ingavirin are again being asked uncomfortable questions
Photo by city news agency "Moscow"
The questions asked by consumers after Elena Malysheva’s broadcast on Channel One a few months ago still remain unanswered, and this worries patients and authors of Telegram channels. Well-known blogger and telegrammer Maxim Kononenko drew attention to the situation and decided to once again try to get comments on this topic.
Doctors stated in the program of Dr. Elena Malysheva that the antiviral drug Ingavirin, if used incorrectly, can cause blood cancer, in which drugs related to the fight against COVID-19 were discussed. “Telegram was densely packed with this, and in the program of Russian telemedicine pop star Elena Malysheva, two doctors also made it clear that the drug promotes the growth of white blood cells. And this is a possible reason for a healthy person to meet an oncologist,” comments Kononenko. As stated on air, the antiviral effect of the drug was a side effect, since the drug is intended for those who produce few white blood cells. For people in normal health, an increase in their number may be negatively affected. “If suddenly there is a cell that is atypical, abnormal, potentially cancerous, there is no point in stimulating its growth and proliferation once again, we can thus cause the development of cancer!”, answered Malysheva’s question on air, “What is the danger of increasing the number of such cells in the blood?” immunologist Andrey Prodeus.
Kononenko recalls that “Ingavirin” has a “twin brother” Dicarbamin, which was previously released to restore white blood cells after chemotherapy. Moreover, as the BUSINESS publication writes, “the state registration of the drug Dicarbamin 100 mg (INN Imidazolylethanamide pentanedioic acid) was decided to be canceled on May 12, 2014 (Letter of the Ministry of Health of the Russian Federation No. 20-3/2036504). The decision was made on the basis of the submission by the representative office of the company Valenta Pharmaceuticals OJSC of an application to cancel the state registration of the drug.”
The blogger draws attention to the unhealthy hype around Ingavirin, citing the opinion of political scientist Sergei Markov, who believes that this whole story related to the drug could be a custom-made company that is spending billions of rubles.
However, Kononenko is most surprised by the reaction of the drug manufacturer, because, according to him, he did not find any refutation of reports about the drug from the Valenta-Pharma company. By the way, as “Life.ru” writes, the publication’s journalists were also unable to get any comments from pharmacists. “Life contacted the external PR service with a request to comment on the benefits of Ingavirin in the treatment of coronavirus. They answered that issues related to the company’s internal processes were not within their area of responsibility. And in general, now the company’s experts are ready to comment only on external processes in the pharmaceutical market,” the publication notes.
“And the questions continue to pile up, regardless of who and how raised this fuss. I'll list three of the most popular ones I found.
1. Dicarbamine was a strictly prescription drug before it was banned. Why is Ingavirin with the same active ingredient and minimal difference in the mass of the active ingredient over-the-counter?
2. Dicarbamine was contraindicated in children and pregnant women. In the updated instructions for the drug, you write that Ingavirin can also be taken by small children. Based on what research did you make this decision?
3. Previously, the instructions for the drug recommended taking 7 tablets per course of treatment, now - 10. However, the dosage of the active substance has not changed. Was it previously “undertreated” or is it now “overtreated”? It would be interesting to hear answers from representatives of Valenta-Pharm,” writes Kononenko.
“Ingavirin” causes cancer: signs of stuffing
Children's and Elderly Health Foundation
The website of the Foundation for the Health of Children and the Elderly, which disseminated information about Ingavirin, as well as its pages on social networks, do not exist. This foundation is not mentioned anywhere except for quotes about Ingavirin causing cancer. There is a YouTube channel called “Children and Elderly Health Foundation,” which has 493 subscribers but does not contain any videos.
“The YouTube channel of the Foundation for the Health of Children and the Elderly was registered on October 4, 2021 . There is no information about the Fund on the tax website,” Tigran Grigoryan, founder of the Tigla digital agency, told BUSINESS in an interview.
In the English-speaking space, there is a certain fund with a similar name: Care Old Age & Child Foundation (COACF). His website careoldageandchildfoundation.org is down. The domain was registered on August 15, 2021, but some changes were made on August 31, 2021 (Updated Date: 2020-08-31T09:09:40Z). The Updated Date indicator at domain registrars indicates the date of the last change in domain data (for example, the domain owner changed his address or ns server), which may mean the domain was transferred to another owner.
In any case, there is no mention of the activities of the Care Old Age & Child Foundation (COACF) on the Internet either. The fund itself is located in Uganda: Ssehab Hotel, Kabalagala District, Bugoma road, Kibanga - Buligo,.,Central,Kampala.
Doctor Ivan Rodin
Doctor Ivan Rodin, who expressed his resonant opinion about “Ingavirin” in Yandex.Zen, has only this one article in his god, 5 subscribers and does not even have a photo on his avatar.
On lead generator sites like ZOON, there are 2 mentions of a certain radiologist Ivan Igorevich Rodin, allegedly working in Zelenograd (Moscow).
“Such a specialist does not work for us,” said an employee of the center in a conversation with BUSINESS.
The group on Odnoklassniki, which claimed that the manufacturer of Ingavirin is hastily deleting negative information about the drug on the Internet, is also anonymous, its authors are unknown. However, the video attached to this post, exposing the side effects of taking the drug, was not deleted in this group.
Verified by BUSINESS
The composition of the drugs Ingavirin and Dicarbamine is actually similar. Minor differences are observed only in the composition of the excipients of the drugs. The active substance is completely identical (the dosage is different).
* The composition of Dicarbamine in the version for children could not be found. The drug has not been produced since 2014.
Both medications are in tablet form.
It was decided to cancel the state registration of the drug “Dicarbamine” 100 mg (INN Imidazolylethanamide pentanedioic acid) on May 12, 2014 (Letter of the Ministry of Health of the Russian Federation No. 20-3/2036504). The decision was made on the basis of the representative office filing an application to cancel the state registration of the drug.
A general practitioner with 40 years of experience interviewed by BUSINESS said that the same active ingredient in different drugs can have different effects on the body depending on the dosage and excipients. Ingavirin is quite popular among her colleagues and she has never received information about the ability of this drug to cause blood cancer.
Ingavirin® (Ingavirin®)
Antiviral drug.
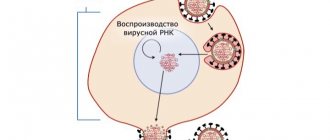
Preclinical and clinical studies have shown the effectiveness of the drug Ingavirin® against influenza viruses type A (A(H1N1), including “swine” A(H1Nl)pdm09, A(H3N2), A(H5N1)) and type B, adenovirus, parainfluenza virus, respiratory syncytial virus; in preclinical studies: coronavirus, metapneumovirus, enteroviruses, including Coxsackie virus and rhinovirus.
The drug Ingavirin® promotes accelerated elimination of viruses, reduces the duration of the disease, and reduces the risk of complications.
The mechanism of action is realized at the level of infected cells due to the stimulation of innate immune factors suppressed by viral proteins. In experimental studies, in particular, it was shown that the drug Ingavirin® increases the expression of the first type interferon receptor IFNAR on the surface of epithelial and immunocompetent cells. An increase in the density of interferon receptors leads to an increase in the sensitivity of cells to endogenous interferon signals. The process is accompanied by activation (phosphorylation) of the transmitter protein STAT1, which transmits a signal to the cell nucleus to induce antiviral genes. It has been shown that under conditions of infection, the drug stimulates the production of the antiviral effector protein MxA, which inhibits the intracellular transport of ribonucleoproteins of various viruses, slowing down the process of viral replication.
The drug Ingavirin® causes an increase in the level of interferon in the blood to the physiological norm, stimulates and normalizes the reduced α-interferon producing ability of blood leukocytes, stimulates γ-interferon producing ability of leukocytes. Causes the generation of cytotoxic lymphocytes and increases the content of NK-T cells, which have high killer activity against virus-infected cells.
The anti-inflammatory effect is due to the suppression of the production of key pro-inflammatory cytokines (tumor necrosis factor (TNF-α), interleukins (IL-1β and IL-6)), and a decrease in the activity of myeloperoxidase.
Experimental studies have shown that the combined use of the drug Ingavirin® with antibiotics increases the effectiveness of therapy in a model of bacterial sepsis, including that caused by penicillin-resistant strains and staphylococcus.
Experimental toxicological studies conducted indicate a low level of toxicity and a high safety profile of the drug.
According to the parameters of acute toxicity, the drug Ingavirin® belongs to toxicity class 4 - “Low toxic substances” (when determining LD50 in acute toxicity experiments, lethal doses of the drug could not be determined).
The drug does not have mutagenic, immunotoxic, allergenic or carcinogenic properties, and does not have a local irritant effect. The drug Ingavirin® does not affect reproductive function and does not have embryotoxic or teratogenic effects.
Efficacy in children
In a double-blind, randomized, placebo-controlled, multicenter study assessing the clinical efficacy and safety of the drug Ingavirin® at a daily dose of 60 mg for the treatment of influenza and other acute respiratory viral infections in 180 children aged 13-17 years, it was shown that the drug Ingavirin® was significantly superior to placebo, faster normalizing body temperature, relieving intoxication, fever, and catarrhal symptoms.
A double-blind, randomized, placebo-controlled, multicenter study assessing the clinical efficacy and safety of the drug Ingavirin® at a daily dose of 60 mg for the treatment of influenza and other acute respiratory viral infections in 310 children aged 7-12 years showed that the drug Ingavirin® is significantly more effective than placebo and provides a faster (on average 18 hours) decrease in body temperature and disappearance of intoxication symptoms (sore throat, sore throat, pain when swallowing, nasal congestion, runny nose).
Valenta Farm
Russian, founded in 1997. The scientific and production complex "Valenta" is located in the city of Shchelkovo, Moscow region.
Valenta Pharm. Photo from the company website.
The company's drug portfolio is represented by such well-known brands as Ingavirin , Grammidin, Trimedat, Teraligen, Phenazepam, Pantocalcin, Aminazine and others.
At the time of publication of the article, it was not possible to reach Valenta Pharm at the telephone numbers listed on their website. The editors of BUSINESS sent a journalistic request to the press service of the pharmaceutical company. There has been no response yet.
UPD: the editors have the results of studies on Ingavirin at their disposal.
More: Bill Gates on “conspiracy theories” around coronavirus and vaccination.