- What is acidosis
- Reasons for development
- Types of acidosis
- Gas acidosis
- Non-gas acidosis
- Mixed acidosis
- Symptoms of acidosis
- Diagnosis of acidosis
- Treatment
Blood acidification is a very common phenomenon of our time.
This is due to the impact of harmful external factors of modern lifestyle and ecology. Violation of the acid-base balance in any direction, either acidification (Acidosis) or alkalization (Alkalosis), leads to a failure of vital systems. The acid-base state is assessed by the value of the hydrogen index (pH). From the point of view of physiology, the hydrogen indicators of the internal environments of the body, such as blood, intercellular fluid, are a rigid constant, because the slightest shifts disrupt the functioning of enzymes, and therefore lead to diseases, and with deviations of 0.5 in any direction, death is likely.
The normal range of blood pH is 7.35-7.45
What is Acidosis, what is the cause and how to deal with it?
Acidosis is a form of acid-base imbalance characterized by an absolute or relative excess of acids in the body.
Normally, the human body produces almost 20 times more acidic products than alkaline ones; therefore, systems that neutralize acidic compounds dominate in the body.
One of the reasons for a decrease in pH in the body is oxidative stress, the process of damage to cells and tissues by reactive oxygen species.
In the area of cell damage by reactive oxygen species, metabolic disturbances occur, inflammation develops, lactic, malic, succinic, a-ketoglutaric acids, under-oxidized products of lipolysis and proteolysis (fatty acids, polypeptides, amino acids, ketone bodies) accumulate.
The accumulation of acidic metabolites underlies the development of metabolic acidosis in the inflammatory zone. Moreover, the more intense the inflammation, the deeper the shifts in the acid-base state at the site of damage.
In the blood with acidosis, there is a decrease in pH below normal (below the average Ph value, taken as 7.39)
Classification according to hydrogen index
The pH value plays a big role in the body. Its norm ranges from 7.25 to 7.44. If this indicator is exceeded or, on the contrary, falls, then the protein loses its natural properties, enzymes begin to function worse and cell destruction occurs. These processes can cause destruction of the body. Based on the pH level, the described state is divided into:
- compensated - the blood pH shifts towards the lower norm - 7.35 (in most cases not accompanied by any special symptoms);
- subcompensated - the acid level increases, the pH reaches 7.29-7.35 (symptoms may include shortness of breath, diarrhea, arrhythmia, vomiting);
- decompensated - the pH level drops below 7.29, and problems arise with the digestive system, heart and brain.
You may be interested in: 5 exercises of Tibetan monks - a simple way to restore health and prolong youth. Benefits, detailed description of the complex, rules of implementation
Causes of acidosis
- Endogenous causes:
the most common and significant in clinical practice. In many life disorders, the functions of buffer systems and mechanisms for maintaining an optimal acid-base state for the body are disrupted - Exogenous causes:
associated with excessive intake of acidic substances into the body. For example, violation of the dosage of medications, the entry of toxic substances into the body. People who use synthetic diets (amino acids with acidic properties) often develop acidosis.
Therapy
Since acidosis occurs due to impaired functioning of the body's systems and organs, the course of treatment is based on the treatment of the disease or dysfunction that led to a shift in the acid-base balance. Any type of acidosis can significantly weaken the body, so at the first suspicion of this pathology, rush to visit a specialist. Typically, treatment of complex forms of acidosis includes the following items:
- improvement of pulmonary ventilation;
- decrease in the amount of protein in the blood plasma;
- strengthening the buffer hydrocarbonate system;
- restoration of blood microcirculation, reduction of its volume;
- normalization of oxidation processes by introducing glucose, ascorbic acid, Riboxin, Pyridoxine, Thiamine, Insulin;
- elimination of the cause of pathology;
- normalization of electrolyte metabolism;
- improving blood flow in the kidneys.
To eliminate symptoms, treatment of acidosis involves the following: ingesting a certain amount of soda (sodium bicarbonate); increased drinking; elimination of additional symptoms such as arrhythmia, nausea, lethargy. If poisoning is detected, medications are prescribed to remove toxic substances from the body; in especially severe situations, it is necessary to cleanse the body. If the acidosis has not become acute, it is worth reducing the consumption of protein foods. An excellent medication is calcium carbonate.
To get rid of metabolic acidosis, glutamic and nicotinic acids, as well as cocarboxylase, are prescribed. Acute forms of acidosis must be treated with rehydration salts. Even if the acid-base balance is disturbed, take Dichloroacetate, which activates enzymes. In addition to medications, the patient should eat a balanced diet and eliminate alcohol and coffee from the diet.
On a note! When treating symptoms of acidosis, the ratio of acids and alkalis must be monitored. For this purpose, an ionogram is constantly taken during therapy.
Gas acidosis
It is characterized by a change in the content of carbon dioxide in the body, and as a result, an increase in the concentration of carbonic acid.
The main reason is a violation of alveolar ventilation of the lungs (during spasm or blockage of the airways) above the body's gas exchange needs for a certain time.
Characteristic manifestations are:
- Spasm of small bronchi and bronchioles, increased intracranial pressure
- Impairment of microcirculation and blood supply to tissues, which leads to the development of cell hypoxia, metabolic disorders and the formation of ATP (the body’s universal source of energy)
- Ion imbalance
Scientists have found that patients with chronic obstructive pulmonary disease are more likely to develop respiratory acidosis (COPD is known to develop due to oxidative stress, as free radicals cause damage to the alveoli)
Non-gas acidosis.
The main causes are metabolic disorders resulting from the development of oxidative stress, directly related to the increased formation of free radicals and a decrease in the effectiveness of the antioxidant system.
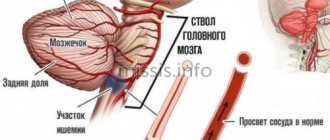
The most characteristic manifestations of non-gas acidosis are: decreased blood flow in the brain, kidneys and myocardium, edema, increasing depression of the nervous system (this is manifested by drowsiness, lethargy, and in severe cases - coma, for example in patients with diabetes mellitus)
Non-gas acid-base disorders (ABS) are, in turn, divided into three types: metabolic, excretory and exogenous.
MECHANISM OF DEVELOPMENT AND TREATMENT OF ACIDOSIS IN PATIENTS WITH UREMIA
Endogenous formation of acids
Fats and carbohydrates are metabolized to produce water and carbon dioxide, which is eliminated through the lungs.
As a result of protein metabolism, in addition to carbon dioxide, various non-volatile acids are formed, which are normally excreted by the kidneys. Catabolism of sulfur-containing amino acids, cysteine and methionine, leads to the formation of 40 - 70 mmol of sulfuric acid per day. As a result of the metabolism of cationic amino acids, histidine, lysine, arginine, 138 mmol of free anions are released per day. At the same time, the catabolism of anionic amino acids causes the intake of 100 mmol of bases per day. In addition, food contains organic anions, such as acetate, citrate, lactate and others, which are metabolized to form bicarbonate in an amount of 60 mmol/day. In general, protein metabolism leads to the formation of about 500 mmol of acids, which are normally excreted by the kidneys. High consumption of meat products leads to an increase in the production of acids and acid-forming cationic amino acids. On the contrary, plant foods contain large amounts of organic bicarbonate-forming anions, such as citrate, lactate, etc. Thus, the daily excretion of acids can vary significantly depending on the diet. Renal failure leads to acid retention and a decrease in plasma HCO-3 and total CO2 levels. With moderate acidosis, a decrease in the level of HCO-3 occurs in parallel with an increase in the content of chloride anions, resulting in the formation of hyperchloremic acidosis. As renal failure progresses, the concentration of unmeasured anions increases and acidosis develops with an increased anion gap. Hyperventilation and limited supply of bases from bone tissue partially compensate for the metabolic acidosis of uremia. Measuring the pH of the extracellular fluid allows us to judge the acid-base state inside the cell, since the pH of the intracellular fluid, both in the interdialytic period and during dialysis, changes similar to the change in plasma pH. Possible complications of acidosis in patients with uremia Cardiovascular system
A decrease in pH from 7.4 to 7.2 leads to an increase in the secretion of norepinephrine, which causes tachycardia, arrhythmia, and ventricular fibrillation. Hydrogen ions disrupt the transport of calcium ions inside the myocardial cells, as a result of which its contractile function suffers. Norepinephrine also makes it difficult to transport calcium into the cell. At a pH in the range of 7.4 - 7.2, the negative inotropic effect of acids and the positive inotropic effect of catecholamines compensate each other, and myocardial contractility remains virtually unchanged. Only when the pH drops below 7.2 does the negative inotropic effect predominate. Based on this, it can be assumed that acute or chronic heart disease makes the myocardium more susceptible to the effects of acidosis, and beta blockers and calcium antagonists may enhance the negative inotropic effect of acidosis. Gastrointestinal tract
As in the myocardium, in the muscle cells of the gastrointestinal tract, acidosis leads to a decrease in calcium content, as a result of which muscle tone and contractility decrease.
In vitro, an acidic environment reduces the sensitivity of taenia coli to cholinergic stimulation. Electrolytes
As acidosis increases, protons compete with calcium for protein binding sites, which leads to an increase in the content of ionized calcium in the plasma. A decrease in pH for every 0.1 leads to an increase in potassium concentration by 0.6 μeV/L.
Thus, correction of hyperkalemia by protein restriction also leads to a decrease in the degree of acidosis. In renal failure, especially associated with diabetic nephropathy, a decrease in renin and aldesterone levels is often observed. Hyperkalemia in the setting of diabetes may be a particularly serious problem because ketoacidosis further lowers pH and hypoaldosteronism reduces potassium transport into the cell. Protein metabolism
Acidosis increases protein catabolism without affecting their synthesis, which forms a negative nitrogen balance.
An excess of protein breakdown over their synthesis by only 2% will lead to a loss of protein mass - 168 g/month or 2 kg per year. In patients on dialysis, when the concentration of HCO3 decreased below 22 mmol/l before the dialysis procedure, a decrease in the content of valine in muscle tissue was noted. It is unclear what degree of acidosis is acceptable and what pH and bicarbonate levels must be maintained to eliminate or minimize negative nitrogen balance in uremia. Growth
Studies in patients with renal tubular acidosis (RTA) and experimental data indicate the effect of acidosis on growth rate.
Taking bicarbonate during PCA increases the growth rate. Conflicting data have been obtained on the effect of uremia on the level and activity of growth hormone in uremia. Bones
The bicarbonate content of bones exceeds 8000 mmol, their total buffering capacity is about 25,000 mmol.
In patients with acidosis on dialysis, about 22.4% of the bicarbonate contained in the bones is lost within 2.7 years. Unfortunately, there are no data obtained during 5-10-year or longer follow-up. But if we assume that the bone buffer neutralizes only 20 mmol of acid per day, then the entire buffer capacity of bone tissue in the absence of replenishment will be used up within 3.5 - 4 years. The effect of acidosis on bone depends on the cause of the acidosis and the level of calcium intake and excretion. Treatment of acidosis associated with uremia
Compensatory mechanisms make it possible for many years to neutralize many of the consequences of moderate acidosis at a pH of at least 7.25. At a pH above 7.25, severe complications such as cardiovascular disorders and growth retardation are not observed. The threat of complications occurs at a pH below 7.25 - 7.20. Currently, the most studied and obvious is the effect of acidosis on electrolyte balance and protein metabolism. Maintaining a high serum bicarbonate level (29.1 mmol after dialysis) leads to improved appetite and increased muscle mass. In patients not on dialysis, protein restriction reduces acid intake, and protein-rich foods increase acid excretion by the kidneys. However, protein restriction is not recommended for dialysis patients because it reduces serum albumin and increases mortality. Due to the high content of organic acids in foods of plant origin, the severity of acidosis may be reduced. However, these foods are usually rich in potassium, which is undesirable as kidney failure progresses. In addition, potassium aggravates the severity of acidosis. Calcium carbonate and calcium acetate, the most commonly used phosphate binders, reduce the severity of acidosis in dialysis patients. In the preuremic stage, under the influence of calcium carbonate, the excretion of acids decreases. And finally, far from the last place in the correction of acidosis is the use of bicarbonate, even if it causes gastrointestinal discomfort. The maximum level of pH and bicarbonate concentration in the dialysate is limited by the risk of formation of insoluble calcium carbonate and clogging of the dialysis machine pipes. Overcorrection of acidosis results in metabolic alkalosis at the end of the dialysis procedure. Since the bicarbonate concentration during the interdialysis interval decreases by 6–8 mmol/l, in order for its concentration before the dialysis procedure to correspond to 20–22 mmol/l, at the end of the procedure the serum bicarbonate concentration should reach 28–30 mmol/l.
Literature:
Bommer J, Keller C, Gehlen F, et al. Clin Nephrology 1996;46:280–5.
Excretory acidosis
The main reason is the loss of bases (alkalies) by the body as a result of certain pathologies.
For example, with renal failure, hypoxia of kidney tissue or nephritis, a renal type of excretory acidosis develops. With an open wound of the intestine and diarrhea, intestinal excretory acidosis develops.
Inflammation in a particular organ and oxidative stress are cyclical processes, because an increase in inflammatory mediators and the circulation of immune system cells provokes an increasing formation of ROS by activating enzymes that generate radicals.
Preventive actions
In order not to encounter the problem of disturbed acid-base balance, and therefore to avoid the consequences and symptoms of acidosis, you must adhere to a number of rules. First of all, it is recommended to eat a healthy and balanced diet, take care of the quality of the products consumed, spend more time in the fresh air, engage in active sports, and also give up bad habits, in particular, the abuse of alcoholic beverages and cigarettes. Also, to prevent acidosis, doctors give the following recommendations:
- pay attention to any metabolic disorders in a timely manner in order to take therapeutic measures to prevent further consequences;
- food should consist mainly of raw products of plant origin;
- play more sports, move actively, because this promotes improved blood supply to all organs and normalizes the functioning of the respiratory system;
- drink more liquid, but do not forget about clean water, its amount should be approximately 2 liters;
- to quickly relieve the unpleasant symptoms of poisoning, you can drink a soda solution;
- Monitor the quality of drinking water and the degree of its saturation with minerals and nutrients.
As it turned out, acidosis is not a serious disease or dangerous pathology, it is just a temporary condition of the body, so there is no need to panic. But the clinical picture of a disturbed acid-base balance can be extremely unpleasant, so if you experience discomfort, you should not leave things to chance. Do not self-medicate, consult a doctor immediately. Only a specialist can make the correct diagnosis, identify the type of acidosis and prescribe competent and effective treatment accordingly.
Mixed acidosis.
The same patient may exhibit signs of both gas and non-gas acidosis.
Examples of conditions in which mixed (combined) acidosis can develop: heart failure, brain injury, chronic broncho-obstructive pulmonary diseases, chronic gastroenteritis.
In any chronic disease, as a result of a constant, ongoing inflammatory process, the accumulation of markers of oxidative stress and the action of reactive oxygen species on tissue, blood acidification is observed.
Symptoms of acidosis
Symptoms of acidosis are sometimes difficult to distinguish from other pathological conditions.
The main symptoms of acidosis are:
- Short-term nausea, vomiting;
- General malaise;
- Increased heart rate
- Dyspnea;
- Cardiac arrhythmias;
- Increased blood pressure;
- Disorder of the functions of the central nervous system (drowsiness, confusion, dizziness, loss of consciousness, lethargy);
- Shock states
Clinical picture
Obvious and erased forms of lactic acidosis are observed (hyperlactic acidemia without wedges, manifestations), turning into a hyperlactic acidemic coma with the addition of acute infectious or inflammatory diseases, various intoxications, and alcohol abuse.
Harbingers of coma may be dyspeptic disorders (anorexia, nausea, vomiting), increased breathing, apathy, drowsiness or agitation with insomnia, chest pain, muscle asthenia or muscle weakness during physical activity. load.
Most often, hyperlactic acidemic coma develops suddenly. Within a few hours, a symptom complex develops: drowsiness (rarely agitation), delirium, loss of consciousness, symptoms of dehydration, decreased body temperature, Kussmaul-type breathing, decreased blood pressure, collapse with oliguria or anuria. Some patients experience sharp pain in the chest similar to myocardial infarction, hemorrhagic necrosis of the tips of the fingers and toes, as well as the ears.
Characteristic biochemical changes in the blood: severe decompensated metabolic acidosis (blood pH drops to 7.0-6.8, standard bicarbonate to 2-5 mEq/l); The lactate concentration in the blood serum exceeds 16 mg% (normal 9-16 mg%).
Oxidative stress is a precursor to acidosis.
As is known, free radicals can damage almost all cell structures: membrane proteins and lipids, DNA, thereby disrupting the vital functions of cells.
The accumulation of under-oxidized products in the cell leads to a disruption of the redox potential of cell membranes, which complicates the supply of substances necessary for functioning, as well as the removal of decay products.
As a result, the cytoplasm of the cells becomes acidified and intracellular metabolic acidosis develops.
Diagnosis of acidosis
The following research methods are used to diagnose acidosis:
- Analysis of blood gas composition (for analysis, arterial blood is taken; analysis of venous blood will not accurately determine the pH level);
- Urine pH level analysis; Arterial blood analysis for serum electrolytes.
- Blood tests for basic metabolic parameters (blood gas composition) show not only the presence of acidosis, but also determine the type of acidosis (respiratory, metabolic).
Treatment of acid-base imbalance
To treat acidosis, it is necessary to determine what type of acidosis and set a goal to reduce the symptoms of this type. It is necessary to influence the root cause of acidosis, and not just alkalize the body with alkaline products, because alkalizing the body will only bring short-term benefits and will lead to acidosis again.
Respiratory acidosis
The main goal: to reduce the degree of respiratory failure in the first place, as well as symptomatic treatment aimed at eliminating unpleasant sensations: headache, overexcitement, severe cardiac dysfunction.
Non-gas acidosis
The main goal: eliminate excess acids from the body, restore the normal content of bicarbonates.
The first step is to eliminate the disease or pathological condition (for example, oxidative stress that causes inflammation) that causes acidosis.
Symptomatic treatment is aimed at eliminating heart rhythm disorders, gastrointestinal tract functions, and disorders of neuromuscular tone.
Treatment
In severe cases of metabolic acidosis (pH = 7.25 and below), an 8.4% sodium bicarbonate solution of 100-200 ml is administered. In other cases, it is recommended to enter 4.2; 2.1 and 1.05% sodium bicarbonate solutions.
The doses of sodium bicarbonate required to correct metabolic acidosis are calculated based on the deficiency of buffer systems (DBS). To do this, use the Mellemgard-Astrup formula:
DBS=0,Z × SBO × W,
where DBS is the deficiency of buffer systems (in meq/l), SBO is the shift of buffer bases (in meq/l), W is the patient’s weight (in kg).
In case of long-term metabolic acidosis, accompanied by pronounced disturbances in the electrolyte balance, the elimination of acidosis should take place within 2-3 days to allow the body to rearrange electrolytes under the conditions of the correction. The administration of sodium bicarbonate is necessarily controlled by determining the pH of the blood.
Patients with renal failure and dehydration are also given intravenous saline and 5% glucose solution (up to 200-500 ml). In the treatment of gas acidosis, which is most often observed in patients with respiratory failure, drugs are used that primarily improve pulmonary ventilation (bronchodilators, steroid hormones, antibiotics and sulfonamides during exacerbation of the inflammatory process, as well as cardiac glycosides and diuretics). Due to the depression of the respiratory center under the influence of severe hypercapnia, respiratory stimulants are used: cordiamine 1 ml up to 5-10 times a day intramuscularly or intravenously. It is advisable to administer a drip of 10 ml of a 2.4% solution of aminophylline to 200-300 ml of physiological solution. To stimulate the respiratory center, remeflin is used in a daily dose of up to 8-16 mg orally or intravenously. An important place in the treatment of both metabolic and gas acidosis is occupied by oxygen inhalation (see Oxygen therapy).
As an emergency aid for respiratory failure, assisted or artificial ventilation is used (see Artificial respiration).