Published: 06/17/2021 12:50:00 Updated: 06/17/2021
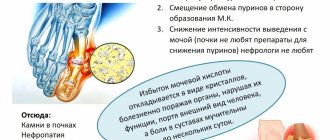
Gout is a systemic disease characterized by impaired purine metabolism in the body and the deposition of urate crystals in the joints. The main manifestation of the disorder is considered to be repeated attacks of arthritis with intense pain in the joints and the appearance of tophi - gouty nodules. Also, with this disease, accumulation of salts in the kidneys is possible with the development of urolithiasis and renal failure.
The prevalence of the disease among adults in Europe ranges from 0.9 to 2.5%, and in the United States reaches 3.9%. Gout is most often diagnosed in men over 40 years of age. Among women, the pathology occurs 6-7 times less often.
Causes of gout
The main cause of gout is considered to be a violation of uric acid metabolism, leading to its excess in the blood - hyperuricemia.
This condition occurs when the intake of purines from food increases, when their metabolites simply do not have time to be excreted in the urine, or when the excretory function of the kidneys is impaired, as well as in the case of accelerated destruction of cells containing the nucleosides adenine and guanine in RNA and DNA, from which urinary tract is formed. acid in the human body. In this case, uric acid salts (urates) accumulate in the soft tissues of various organs, mainly in the joints, provoking their inflammation. Gout is also known as the “disease of feasting kings.” It received this name because the main source of its occurrence was considered to be excess in food and alcoholic beverages.
Now the disease has been studied in more detail and the following factors predisposing to the development of the pathology have been identified:
- consumption of foods rich in purines - meat, fatty fish, legumes, caffeine-containing products, offal (kidneys, liver, brain), seafood, as well as alcohol in large quantities;
- consumption of carbonated, sweet drinks and fruit juices;
- obesity;
- hemolytic anemia, leukemia, lymphoma;
- genetic defects (usually in men) and reduction of enzymes involved in the metabolism of uric acid;
- the use of medications such as diuretics, cytostatics, salicylates.
In women, gout usually develops in postmenopause, so the disease is also associated with hormonal changes in the body.
Gout symptoms
From Greek, gout is translated as “foot in a trap,” since the patient is primarily bothered by intense pain in the first metatarsophalangeal joint of the foot, knee or ankle.
It is also possible to involve the hand in the form of oligo or polyarthritis (inflammation of two or many joints). In this case, the joint becomes swollen, the skin above it acquires a red tint and begins to shine. This form of the disease is called acute gouty arthritis. The first clinical manifestations of the pathology may be preceded by a long asymptomatic period of hyperuricemia, when disorders are detected only by laboratory blood tests.
The first attacks of pain occur suddenly, mainly in the early morning or at night, tend to increase on the first day and completely disappear within a few hours or within a day. During an exacerbation of gout, signs of intoxication may also be present - increased body temperature, chills, weakness. After inflammation subsides, gouty arthritis occurs again, usually within six months to two years. As the disease progresses, the duration of periods of its asymptomatic course decreases, joint pain occurs more often and is more difficult to tolerate.
When going into a chronic form, gouty arthritis is accompanied by deformation and limitation of movements in the joints, pain of varying intensity becomes constant. Subsequently, deposits of uric acid crystals become visible. Under the skin, most often in the joint area, tophi appear - white or yellow nodules with crumbly, curdled contents. The formation of ulcers and purulent wounds is possible over them.
Hyperuricemia is accompanied by frequent exacerbations of concomitant diseases - coronary artery disease, diabetes mellitus, arterial hypertension, atherosclerosis. If gout complications develop, their characteristic symptoms are added. For urolithiasis, this is nagging pain in the lower back, periodic appearance of blood in the urine, and frequent urination at night. With a prolonged course of chronic tophi gout, the articular cartilage is destroyed, limitation of joint mobility persists outside periods of exacerbation, and complete fusion of the joint space with the formation of ankylosis (complete immobility of the joint) is possible. Depression can also be a consequence of constant pain.
Diagnosis of gout
The diagnosis and treatment of gout is carried out by a rheumatologist, internist or general practitioner (family doctor). Most often, the patient comes not at the onset of the disease, since the symptoms of acute gouty arthritis quickly disappear, which can be regarded by a person as recovery, but during one of the subsequent exacerbations. The doctor conducts a conversation with the patient, during which the fact of an acute joint attack in the past, the presence of characteristic complaints, as well as risk factors for the development of pathology are established. The phenomena of bursitis and tophi - the main objective manifestations of gout - are more often found on the leg. The diagnosis can be confirmed by a comprehensive examination of the body using laboratory and instrumental methods. If gout is suspected, the following tests are performed:
- Clinical blood test. During an attack of gouty arthritis, an acceleration of the COE, an increase in the number of leukocytes and a shift in the leukocyte formula to the left are possible.
- Determination of urate in blood serum. About 70% of patients with an exacerbation of gout have a high level of uric acid - more than 0.42 mmol/l in men and 0.36 mmol/l in women.
- Determination of uric acid in daily urine. The method is used to assess the risk of kidney stones.
- General urine analysis. It is carried out for the purpose of diagnosing diseases of the urinary system that contribute to the development of gout or are its complication.
- Examination of synovial fluid. Biomaterial is collected for analysis using puncture of the affected joint. The presence of monosodium urate crystals in the joint fluid makes it possible to confirm gout, and its sterility during bacterial culture excludes infectious causes of arthritis.
- Microscopic examination of a biopsy sample from tophi. When viewed through a polarizing microscope, urates appear as needle-shaped crystals with a pointed end. This method is also highly specific in diagnosing gout, like the previous one.
Instrumental diagnosis of gout may include research methods such as:
- Ultrasound of affected joints;
- MRI and CT;
- X-ray diagnostics.
X-rays at the onset of the disease are carried out for the purpose of differential diagnosis with other arthropathies, but there are no specific signs of gout. Only when the pathology becomes chronic can one see intraosseous tophi and marginal bone erosions in the photographs.
Ultrasound examination of joints before the development of chronic gout is effective only during exacerbations.
MRI makes it possible to detect tophi inside the joints even before they appear under the skin, making it possible to begin specific therapy for hyperuricemia earlier. The method is used in differential diagnosis with other diseases, as well as to monitor the effectiveness of treatment. CT, in comparison with other imaging methods, allows for more accurate differentiation of tophi masses.
Diagnosis and treatment of gouty arthritis
Gout is a chronic progressive disease associated with a disorder of purine metabolism, which is characterized by an increase in uric acid in the blood and deposition of sodium salt of uric acid (urate) in the tissues of the musculoskeletal system and internal organs with the development of recurrent acute arthritis and the formation of gouty nodules (tophi).
Gout is one of the “old” diseases and has been known since ancient times. The term gout comes from the Greek words pus, meaning foot, and agra, meaning grip. Thus, already in the name of the disease one of the cardinal manifestations of gouty arthritis is emphasized. Gout is considered not only as an ailment in which the pathological process is localized in the musculoskeletal system, but also as a systemic disease characterized by damage to vital organs, especially the kidneys. The prevalence of gout in different regions varies widely and is largely related to the nutritional habits of the population, averaging 0.1%. In the US, this figure is 0.84% (this figure may be overestimated).
Gout affects predominantly men (male/female ratio is 9:1). Men normally have higher levels of uric acid. In women of reproductive age, increased estrogen content increases the renal clearance of urate. In the postmenopausal period, their uric acid levels are the same as in men of the same age. Therefore, if the peak incidence in men falls at the age of 35–50 years, then in women it falls at 55–70 years. However, gout can develop at a younger age and is observed even in children.
As is known, uric acid is the end product of the breakdown of purines and is excreted from the body by the kidneys. In healthy individuals, 400–600 mg of uric acid is excreted in the urine in 24 hours. To understand the pathogenesis of gout, one should focus on the clearance of uric acid. It characterizes the volume of blood that can be cleared of uric acid in the kidneys in 1 minute. Normally, this figure is 9 ml/min. The source of uric acid formation in the body is purine compounds, which come from food or are formed in the body during nucleotide metabolism. In blood plasma, uric acid is found in the form of free sodium urate. Normally, the upper limit of this indicator for men is 0.42 mmol/l (7 mg%) and for women - 0.36 mmol/l (6 mg%). Uric acid levels above these numbers are regarded as hyperuricemia and are considered a high risk factor for the development of gout. Thus, according to the Framingham study, the development of gouty arthritis is observed in 17% of men and women with uricemia 7.0–7.9 mg%, in 25% with 8–8.9 mg% and in 90% with uric acid levels above 9.0 mg%.
If there is a persistent increase in uric acid in the blood serum above the level for a given individual, it begins to be deposited in the tissues in the form of free sodium urate, which is converted into uric acid in the urinary tract.
The following clinical variants of gout are distinguished:
- asymptomatic hyperuricemia (hyperuricosuria);
- interictal gout;
- acute gouty arthritis;
- chronic tophi gout.
Hyperuricemia can occur for a long time without any subjective or objective symptoms and is only accidentally diagnosed during examination of the patient. However, it is not as harmless as it may seem at first glance, and is often associated with disorders of fat and carbohydrate metabolism, and, even more seriously, leads to urate nephropathy. It should be noted that the definition of “asymptomatic gout” is conventional. To identify it, it is necessary to re-examine the level of uric acid, especially in the “gouty personality,” that is, in young men with an addiction to alcohol, obesity and arterial hypertension. In some cases, the period of asymptomatic (chemical) hyperuricemia lasts several years and only after this does the clinical presentation of gout occur. It should be borne in mind that hyperuricemia is usually preceded by hyperuricosuria. Therefore, in patients with uric acid diathesis, it is necessary to re-examine the level of uric acid not only in the blood, but also in the urine in order to timely detect gout.
The level of uric acid in the blood can increase under the influence of various factors, both internal and external. These factors contribute to either an increase in the formation of endogenous purines or a slowdown in their excretion by the kidneys. From this perspective, there are two types of hyperuricemia - metabolic and renal. The metabolic type is characterized by an increase in the synthesis of endogenous purines in the presence of high uricosuria and normal clearance of uric acid. In contrast, in the renal type there is low clearance of uric acid and, consequently, impaired renal excretion of uric acid. The types of hyperuricemia presented are of paramount importance in the selection of anti-gout disease-modifying drugs used in the treatment of this disease.
Reasons for increased purine biosynthesis
Hereditary factors:
- decreased activity of hypoxanthine-guanine phosphoribosyltransferase;
- high phosphoribosyltransferase activity;
- deficiency of glucose-6-phosphate.
Nosological forms and clinical syndromes:
- increased nucleotide metabolism (polycythemia vera and secondary erythrocytosis, acute and chronic leukemia, lymphoma, hemolytic anemia, hemoglobinopathies, pernicious anemia, etc.);
- tumors;
- psoriasis and psoriatic arthritis;
- systemic lupus erythematosus, systemic scleroderma;
- hyperparathyroidism;
- obesity;
- Gaucher disease;
- Infectious mononucleosis;
- tissue hypoxia.
Medicines, diet and chronic intoxication:
- ethanol;
- a diet high in purines;
- fructose;
- a nicotinic acid;
- cytotoxic drugs;
- warfarin;
- ethylamine-1,3,4-thiadiazole.
Reasons for slower excretion of uric acid by the kidneys
Nosological forms and clinical syndromes:
- chronic renal failure;
- kidney diseases with predominantly interstitial and tubular disorders (polycystic kidney disease, analgesic nephropathy, hydronephrosis);
- lead nephropathy;
- dehydration;
- diabetic ketoacidosis;
- hyperproduction of lactic acid;
- preeclampsia;
- obesity;
- hyperparathyroidism;
- hypothyroidism;
- sarcoidosis;
- chronic beryllium intoxication.
Medicines and chronic intoxications:
- thiazide diuretics;
- cyclosporine;
- low doses of salicylates;
- anti-tuberculosis drugs (pyrazinamide);
- ethanol;
- Levodopa.
There are also primary and secondary gout. With primary gout, there is no underlying disease that precedes its development. The basis of such gout is a family-genetic abnormality of purine metabolism, determined by several genes, or the so-called “constitutional dyspurism”. Studies of urate homeostasis have shown an autosomal dominant mode of inheritance of such anomalies. In particular, this is observed in congenital disorders in the content of enzymes that occupy a key position in purine metabolism. Thus, with a decrease in the activity of hypoxanthine-guanine phosphoribosyltransferase, an increase in the resynthesis of purines from nucleotides occurs, which contributes to the development of Lesch-Nyhan syndrome. This syndrome occurs only in children and young adults and usually results in urate nephropathy and death. With a high content of phosphoribosyl pyrophosphate, a metabolic type of hyperuricemia is also observed, since this enzyme is involved in the synthesis of uric acid precursors. As for secondary gout, it is one of the syndromes of another disease, a “second disease” that develops in many pathological processes and most often in chronic renal failure.
The clinical picture of acute gouty arthritis is of great importance in recognizing gout, especially its early stages. It is well known, but the frequency of diagnostic errors in the first year of the disease reaches 90%, and after 5–7 years the correct diagnosis is made only in half of the cases. Late diagnosis is associated with underestimation of the classic early signs of the disease, as well as with the diversity of the onset and course of gout. Its diagnosis is based on the characteristics of the clinical picture of the disease, increased levels of uric acid in the blood and the detection of sodium urate crystals in tissues. In practice, the following so-called Rome diagnostic criteria for gout are widely used:
- acute attack of arthritis affecting the metatarsophalangeal joint of the big toe;
- gouty nodes (tophi);
- hyperuricemia (the level of uric acid in the blood serum is higher than the physiological norm);
- detection of urate crystals in synovial fluid or tissues.
The diagnosis of gout is considered reliable if any two of the four criteria are met.
Less common are the diagnostic criteria for gout proposed by the American College of Rheumatology (ACR) in 1977, which characterize acute inflammatory arthritis or its recurrent attacks rather than gout in general. According to these criteria, a reliable diagnosis is made if 6 out of 12 signs are present:
- more than one attack of acute arthritis;
- development of the most acute inflammatory process during the first day;
- monoarthritis;
- redness of the skin over the affected joint;
- pain or swelling of the first metatarsophalangeal joint;
- asymmetrical lesion of the first metatarsophalangeal joint;
- asymmetrical damage to the tarsal joints;
- the presence of formations resembling tophi;
- asymmetric swelling within the joint (radiological sign);
- subcortical cysts without erosions;
- hyperuricemia;
- sterile joint fluid.
In the first 3–4 years, gout occurs as a recurrent acute inflammatory monoarthritis with complete regression and restoration of joint function, while the interictal period lasts from several months to 1–2 years. Subsequently, this period is shortened, more and more new joints are involved in the process, and inflammatory phenomena are localized not only in the joints of the feet, but spread to the joints of the upper extremities, which usually coincides with the formation of tophi. Tophi are deposits of uric acid crystals. They appear on average 6 years after the first attack of gout, but sometimes after 2-3 years. Urates are most often deposited on the surface of the articular cartilage, in the synovial membrane, synovial sheaths, tendons, and also in the subcortical region of the epiphyses of bones. Most often they are located on the ears and on the back of the elbow joints. Tophi are divided into single and multiple, and are also classified according to their size, with small tophi being up to 1 cm in diameter, medium - from 1 to 2.5 cm and large - more than 2.5 cm. Gouty nodes with localization in the musculoskeletal system are the main element in the formation of chronic gouty arthritis. Tophi can be located in the kidneys and other visceral organs.
The chronic course of gout is not limited only to the involvement of joints and the formation of tophi, but is also characterized by damage to internal organs. Gouty nephropathy is the most important manifestation of gout from a prognostic point of view and the most common cause of death in this disease. Some variants of gouty nephropathy include acute uric acid blockade of the renal tubules, uric acid nephrolithiasis caused by the deposition of uric acid salts in the calyces and pelvis of the kidneys, chronic urate nephropathy and diffuse glomerulonephritis. Acute uric acid blockade of renal tubules occurs, for example, when a tumor disintegrates as a result of massive drug or radiotherapy. Chronic urate nephropathy is associated with the deposition of urate in the interstitium of the kidneys, and the development of diffuse glomerulonephritis is associated with immune disorders in individuals with dysregulation of purine metabolism. Such glomerulonephritis, in its immunomorphology, is most often mesangioproliferative and with it deposits of IgG and complement are detected. It should be borne in mind that gout is often associated with such pathological conditions as arterial hypertension, obesity, hyperlipidemia, fatty liver, atherosclerosis, cerebrovascular accidents, and alcohol dependence.
The course of gout is characterized by a variety of rates of disease development. A relatively benign course with rare attacks, slight hyperuricemia and uricosuria and long-term persistence of functional insufficiency of the musculoskeletal system is possible. In other cases, on the contrary, from the very beginning of the disease there are frequent attacks of acute arthritis with severe pain or continuous attacks with multiple joint damage over several weeks or months (gouty status). Gout refractory to therapy leads to the rapid development of functional failure of the joints and kidneys.
The basis for identifying variants of the course of gout are: the number of attacks of arthritis during the year, the number of affected joints, the severity of osteochondral destruction, the presence of tophi, kidney pathology and its nature.
Variants of the course of gout
Mild: attacks of arthritis 1–2 times a year and involve no more than 2 joints, there is no kidney damage or destruction of joints, there are no tophi or they are single and do not exceed 1 cm in diameter.
Moderate severity: 3–5 attacks per year, damage to 2–4 joints, moderately severe osteoarticular destruction, multiple small tophi, kidney damage is limited to nephrolithiasis.
Severe: frequency of attacks more than 5 per year, multiple joint damage, multiple large tophi, severe nephropathy.
The main goals of therapy for gouty arthritis are:
- relief of acute attacks of the disease;
- reduction of urate content in the body;
- treatment of chronic polyarthritis;
- impact on extra-articular pathology.
Relief of acute gouty arthritis is carried out with anti-inflammatory drugs. For these purposes the following are used: colchicine - colchicine, colchicum-disperse; non-steroidal anti-inflammatory drugs (NSAIDs) - Voltaren, Diclovit, Dicloran, Celebrex, Movalis); corticosteroids - polcortolone, prednisolone, methylprednisolone; or a combination of NSAIDs and corticosteroids - Ambien. Both colchicine and NSAIDs reverse acute arthritis within hours, whereas in untreated patients it can last several weeks. Until recently, it was believed that the best drug for relieving an attack of acute arthritis due to gout was colchicine. The pronounced and rapid (within 48 hours) effect of colchicine was considered as one of the diagnostic signs of this disease. Colchicine can prevent further development of an acute attack of gout when administered in the first 30–60 minutes of an attack. Its therapeutic effect is due to the inhibition of the phagocytic activity of neutrophils. In an acute attack of gout, monosodium urate salts, phagocytosed by neutrophils, due to their membranolytic action, lead to the death of these cells and the release of lysosomal enzymes, which are responsible for the development of acute inflammation.
Among NSAIDs, preference is given to indomethacin (Indotard, Methindol) and diclofenac (Voltaren, Dicloran, Diclofen). These drugs are prescribed at a dose of 200–250 mg/day, with most of the daily dose used in the first hours of an attack. Controlled studies have not revealed a higher effectiveness of traditional NSAIDs compared to selective COX-2 inhibitors (nimesil, nimulid, Celebrex), such as celecoxibs (coxib, celebrex). However, a final judgment on the comparative effectiveness of these drugs can only be made through further research. The daily dose of colchicine is 4–6 mg/day, with the patient taking 2/3 of this dose before 12 noon on the first day of the attack. Typically, a single dose is 0.6 mg and is taken every hour until there is a clear reduction in gouty inflammation. After a significant reduction in inflammation, the dose of colchicine begins to be reduced by 0.6 mg 2 times a day, until complete withdrawal. Often, patients are unable to increase the daily dose to the optimal dose due to the occurrence of adverse reactions. The main side effects of colchicine are nausea, vomiting, diarrhea, hemorrhagic gastroenteritis, leukopenia, and neuropathy are also possible. In case of gouty status, characterized by continuous attacks of acute arthritis, refractory to NSAID therapy, intravenous administration of colchicine is possible.
Anti-gout therapy (basic, disease-modifying) is aimed at preventing relapses of acute arthritis, reducing the level of uric acid in the blood, preventing further formation of tophi and their reverse development. All anti-gout drugs are divided into two large groups: uricodepressors (uricostatics) and uricosurics. Uricodepressors inhibit the synthesis of uric acid by inhibiting the enzyme xanthine oxidase, which converts hypoxanthine into xanthine and xanthine into uric acid. Uricosurics increase the excretion of uric acid by inhibiting the reabsorption of urate by the renal tubules.
The drugs of the first group include allopurinol (allopurinol, allopurinol-egis, allupol, purinol, remid, thiopurinol, milurite), which occupies a leading position among other anti-gout drugs. Indications for the use of allopurinol are metabolic gout, high hyperuricemia, frequent acute attacks of arthritis, uric acid disease, genetically determined deficiency of hypoxanthine-guanine phosphoribosyltransferase. The use of allopurinol is also possible in patients with gouty nephropathy with initial manifestations of chronic renal failure and slight azotemia. The initial dose of allopurinol is 300 mg/day. If this dose is ineffective, it is increased to 400–600 mg/day, and when a clinical effect is achieved, it is gradually reduced. The maintenance dose is determined by the level of hyperuricemia and is usually 100–300 mg/day.
Allopurinol promotes the disappearance of acute arthritis attacks or their noticeable weakening, the reverse development of tophi and their distinct softening, the reduction of uric acid levels to subnormal levels, the normalization of urinary syndrome indicators, the reduction of renal colic and renal excretory function. In some patients, it initially causes an increase in uric acid levels and an exacerbation of gouty arthritis, so in the first stage of therapy it is combined with anti-inflammatory drugs, in particular low doses of colchicine or NSAIDs. For this reason, it should not be taken for acute gouty arthritis. When treating with allopurinol, adverse reactions often develop, which are manifested by gastrointestinal toxicity, allergic reactions (skin rash, eosinophilia), hepatotoxicity with an increase in serum aminotransferases.
Uricosuric drugs, which are weak organic acids, are less important in the treatment of gout than uricostatics. They should not be prescribed for high levels of uric acid in the blood, as well as for nephropathy, even with initial manifestations of renal failure. Among the uricosuric drugs, sulfinpyrazone and probenecid are especially widely used in the United States. Sulfinpyrazone (sulfinpyrazone, apo-sulfinpyrazone, anturan) is prescribed at 200–400 mg/day in two doses. It, like other uricosuric drugs, is taken with a large amount of fluid, which should be alkalized to prevent nephrolithiasis. Adverse reactions are relatively common and include gastric and intestinal dyspepsia, leukopenia, and allergic reactions. Contraindications to the use of sulfinpyrazone are gastric ulcers and, of course, gouty nephropathy.
Probenecid (benemid) is a derivative of benzoic acid. The drug is prescribed at 1.5–2.0 g/day. Benzoic acid is found in cranberries, as well as lingonberry berries and leaves. Therefore, decoctions and fruit drinks from the berries and leaves of these plants are indicated for patients with gout and, to a greater extent, for patients with gouty nephropathy, especially since in addition to benzoic acid they contain hippuric acid, which has uricoseptic properties. Uricosuric activity is inherent in the angiotensin II receptor blocker and fenofibrate (grofibrate, nofibal). The most effective are benzbromarone derivatives, which have not only uricosuric properties, but also uricodepressor properties. They are used as monotherapy or in combination with allopurinol. Such a combination drug is allomaron. Allomaron contains 20 mg benzbromarone and 100 mg allopurinol, and is usually taken 1 tablet 2 times a day.
An integral part of complex therapy for gout are alkalizing drugs and alkalizing solutions, which can reduce the risk of developing nephropathy and, in particular, urolithiasis. These drugs include Magurlit, Blemaren and Uralit. Their use should be regularly monitored by urine pH. In addition to these remedies, you can take baking soda 2-4 g per day or alkaline mineral water.
If the symptoms of arthritis are pronounced, it is also necessary to carry out local treatment (dolobene, finalgon, dicloran plus, doltit cream, nemulide gel, bischofite gel).
Diet for gout is given the greatest importance compared to other rheumatic diseases. It involves reducing the total caloric intake of food, especially since with gout, increased body weight is usually observed. It is necessary to reduce the intake of exogenous purines and animal fats into the body. Fats reduce the excretion of uric acid by the kidneys. You should be extremely careful when consuming any alcoholic beverages, including beer and red wine. Exclude liver, kidneys, fatty meats, meat broths, smoked meats, peas, beans, lentils, spinach, cauliflower, sprats, and herring from the diet. You should limit your meat consumption to 2-3 times a week, and it is better to eat it boiled.
The combination of a strict diet with long-term use of anti-gout drugs, as well as active influence on diseases that increase the level of uric acid in the blood, can significantly slow down the rate of progression of osteochondral destruction, prevent further formation of tophi and maintain the functional state of the musculoskeletal system and kidneys.
V. V. Badokin, Doctor of Medical Sciences, Professor of the Russian Medical Academy of Postgraduate Education, Moscow
Treatment of gout
The main principle of treating the disease is to reduce hyperuricemia. This is facilitated by changing the patient’s lifestyle and reviewing the diet. Correction of hyperlipidemia, arterial hypertension, hyperglycemia, weight loss and smoking cessation are recommended. A diet for gout involves eliminating foods that are rich in purines or retain them in the body:
- sweet carbonated drinks;
- seafood;
- meat;
- spicy, hot and smoked foods;
- chocolate, ice cream;
- salty cheeses;
- caffeine, cocoa, strong tea;
- alcohol.
Endogenous purines increase when consuming large amounts of animal protein, so the daily requirement for a patient with gout is considered to be no more than 1.5 g per 1 kg of the patient’s body weight. Dairy products with low fat content, such as kefir, cottage cheese, yogurt, help remove uric acid from the body. Polyunsaturated fatty acids, for example, those found in olive oil, have a similar effect. Also, if you have gout, you must follow a drinking regime - at least 2.5 liters of water per day.
It is advisable to begin drug treatment of gout on the first day of the onset of acute arthritis. To stop an attack, non-steroidal anti-inflammatory drugs, colchicine and glucocorticosteroid drugs are used locally or systemically. Hypouricemic therapy is prescribed after laboratory and instrumental confirmation of the diagnosis. It is aimed at:
- reduction of uric acid to the target level - 0.36 mmol/l;
- reduction of foci of urate accumulation in the body;
- reduction in the size of tophi;
- reduction in the frequency of attacks of gouty arthritis.
Allopurinol is considered the drug of choice for this. If it is intolerant, it is possible to use a selective xanthine oxidase inhibitor, febuxostat. Losartan, Amlodipine and Fenofibrate also have a moderate uricosuric effect. Diuretics are used with great caution, only for health reasons. Surgical removal of tophi is carried out only when severe complications occur, for example, carpal tunnel syndrome, spinal compression.
Author:
Baktyshev Alexey Ilyich, General Practitioner (family doctor), Ultrasound Doctor, Chief Physician
Correction
Drug treatment
To correct hyperuricemia, it is necessary to eliminate the etiological factor. If, during the collection of anamnesis, it turns out that the patient is taking a drug that causes hyperuricemia, one should consider either reducing the dosage or replacing the drug with an alternative one that does not have such side effects. Treatment tactics are largely determined by the presence of clinical symptoms, as well as their severity.
- Diet.
People suffering from gout should definitely stop drinking alcohol and exclude high-purine foods (red meat, liver, smoked meats) from their diet, as they can provoke an increase in symptoms. - Colchicine alkaloids.
The main group of drugs for the relief of acute gout attacks. Reduction of symptoms occurs within 12 hours after the first dose. - NSAIDs.
Prescribed in addition to colchicine alkaloids to relieve arthritis symptoms. Preference is given to drugs with the most pronounced analgesic and anti-inflammatory effect. - Glucocorticosteroids.
Hormonal drugs are used to eliminate symptoms in case of individual intolerance to colchicine and NSAIDs or if the patient has strict contraindications to their use. - Uricodepressors.
Drugs in this group reduce the content of UA by suppressing the activity of the enzyme involved in the synthesis of UA (xanthine oxidase). Long-term use helps reduce the incidence of gout symptoms, slow down the deposition of crystals and the formation of tophi. - Selective xanthine oxidase inhibitors.
The main difference from uricodepressors is the lower incidence of unwanted side reactions, such as upper and lower respiratory tract infections, hepatotoxicity, and hypersensitivity reactions. - Uricosuric drugs.
These drugs inhibit the reabsorption of sUA in the renal tubules, thereby increasing the excretion of sUA. - Anti-gout drugs of mixed action.
There are also combination drugs that simultaneously act on 2 levels of urinary magnesium metabolism. They contain a uricodepressive component and a uricosuric agent. - Alkalizing drugs.
To prevent the development of urate nephropathy and the deposition of urate stones, the main urate-lowering therapy should be supplemented by taking drugs to alkalinize urine - sodium citrate, sodium bicarbonate.
Experimental therapy
Development and clinical trials of new drugs to combat hyperuricemia continue. Rasburicase, a drug containing recombinant uricase (an enzyme that destroys uric acid), has shown high effectiveness in the treatment of tumor lysis syndrome. This drug is also distinguished by the possibility of resorption of deposited sodium monourate crystals.
Promising is the use of genetically engineered biological drugs - an antagonist of interleukin-1 receptors (anakinra), fully humanized monoclonal antibodies to IL-1-beta (canakinumab), soluble protein IL-1 (rilonacept). Their use has demonstrated sufficient effectiveness in preventing exacerbations of symptoms of chronic gouty arthritis.